INTRODUCTION
Gold has been a subject of fascination since ancient times. It is well known that this metal had also a religious and even mythical significance due to its remarkable stability. Ancients used gold to produce jewelry, sculptures, vessels, coinage as well as decoration for monuments and buildings, among others (Akcil 2002, Akcil 2003). Gold was used as commodity money during centuries and its accumulation was correlated to “social power” and even triggered wars. On this field, Schoenberger (2011) published an interesting discussion on the historical value of gold. In modern times, the importance of gold has not declined and is a well-known fact that this commodity supports the world economy. Taking into account that gold still plays a fundamental role in modern society and economy, huge projects around the world have focused on the exploitation of low-grade gold ores. To extract and concentrate gold, some processes were developed and typically been used in gold mining worldwide. The most important process currently used to separate gold from the ores is cyanidation.
Cyanidation, which was developed in Scotland in 1887 (Habashi 1987), is widely used in the USA, Canada, Turkey as well as in several developing countries (Akcil 2002, Eisler and Wiemeyer 2004, Veiga et al. 2014). It consists in the dissolution of gold from the ore in an alkaline dilute cyanide solution. Later, gold is recovered from the solution using either activated carbon, zinc or ion-exchange resins (Eisler and Wiemeyer 2004) and important amounts of cyanide remain in solution in the form of free cyanide or as complexes of other metals (Donato et al. 2007). It is difficult to establish the average consumption of water for the operation of gold mining companies since this depends on a variety of factors including the type of ores, the degree of water recycling, the technology used for the extraction and even the characteristics of the site, among others (Mudd 2007). However, there is a consensus of opinion among the scientific community and society that gold mining activities have a very strong environmental impact, and most of these impacts are due to wastewaters. Both quality and quantity are problems when it comes to evaluating the wastewater issue of gold mining activities since huge amounts of cyanide-polluted water are generated and must be adequately treated. Considering that cyanidation has gained a bad reputation in the eyes of the public, alternative lixiviants for gold ores were explored (Hilson and Monhemius 2006). However, although these remarkable efforts, it seems that cyanidation dominance in the gold mining industry will prevail in years (or decades) to come. Therefore, treatment processes to remove cyanide from the water were (and are still) subject of intense study in the last decades.
Some well-known treatment processes to remove cyanide from gold mining wastewaters are natural cyanide degradation (in tailings ponds), biological processes, chemical processes, electrolytic oxidation and other methods such as evaporation (applied in arid regions) (Kuyucak and Akcil 2013). Probably the most applied process, due to its simplicity, is the natural cyanide degradation in tailings ponds. This process basically involves the storage of wastewaters in ponds exposed to the environment and the degradation of cyanide takes place through different natural mechanisms (volatilization, photodecomposition, chemical oxidation, microbial oxidation, hydrolysis and precipitation). The great disadvantage of this treatment is the low degradation rate, therefore enormous volumes of polluted water must be stored for long periods of time with the subsequent possibility of incidents and accidents. In fact, countless big accidents involving tailings dams happened in the past, data from some of them were compiled in earlier publications (Mudder and Botz 2004, Rico et al. 2008). However, not only incidents and accidents are responsible for negative environmental impacts; Donato et al. (2007) have extensively discussed the impact of gold cyanide-bearing tailings solutions on wildlife and their arguments suggest that wildlife deaths due to “correctly” operated and controlled tailings ponds could be underestimated.
Concerning biological processes, many researchers reported in the past the application of different microorganisms for the removal of cyanide from wastewaters (specially, from gold mining) (Evangelho et al. 2001, Akcil and Mudder 2003, Ebel et al. 2007, Mekuto et al. 2015, Mekuto et al. 2016). Although biological systems have interesting advantages, as described by Akcil (2003), there are also limitations related to operational conditions. For instance, biological systems are often sensitive to load fluctuations in both flow rate and pollutant charge. Therefore, under certain circumstances, chemical processes are preferred, and many options were studied (Mudder and Botz 2004, Kuyucak and Akcil 2013).
The oxidation of cyanide with hydrogen peroxide is a widely used chemical method because it does not produce dangerous by-products or harmful environmental pollutants (Chergui et al. 2015). Some studies showed that higher performances in the removal of cyanide can be reached by the combined action of hydrogen peroxide and other materials such as activated carbon (plain and metal-impregnated) (Deveci et al. 2006, Yeddou et al. 2010, Yeddou et al. 2011), activated alumina (Chergui et al. 2015) and copper-impregnated pumice (Kitis et al. 2005). The combined effect of hydrogen peroxide and ozone on the oxidation of cyanide was also tested and remarkable results were reported by Monteagudo et al (2004). The results reported by these authors showed that cyanide removal was improved when hydrogen peroxide and ozone were combined for the treatment. Such improvement was attributed to the formation of hydroxyl radicals (•OH), a very strong oxidant agent. Processes using •OH radicals for the degradation of pollutants are known and grouped as advanced oxidation processes (AOPs) and have several applications in wastewater treatment (Oturan and Aaron 2014). Considering the mentioned aspects, a treatment in which an activated carbon, hydrogen peroxide and ozone are combined could improve the treatment of wastewaters containing cyanide.
In this work, the removal of cyanide was tested using activated carbon, hydrogen peroxide and ozone. The performance of treatments combining hydrogen peroxide and ozone, activated carbon and ozone, as well as ozone, hydrogen peroxide and activated carbon, was evaluated and discussed. These results can provide insights to perform improvements in existing systems and/or the development of new systems for the treatment of wastewaters containing cyanide.
EXPERIMENTAL AND METHODS
Reagents
Synthetic solutions of NaCN (400 mg/L, 15.37 mM) were prepared using NaCN (> 95 %, Sigma-Aldrich). The pH values of the NaCN solutions were adjusted with a 0.1 N aqueous solution of NaOH (99.0 %, Merck). H2O2 (30 % w/w, Scharlau) and commercial activated carbon (PanReac AppliChem, ref: 121237, d80 < 38 µm) were used during the treatments in combination with O3 (see ozonation system description below). For the measurement of O3, 2 % w/w KI (99.0 % PanReac AppliChem), 2N Na2S2O3•5H2O (99.0 %, Baker) and 2N H2SO4 (97 %, Merck) aqueous solutions were prepared. All the solutions were prepared with double distilled water.
Measurement of cyanide concentration
The concentration of cyanide (CN¯) was measured using a colorimetric method (Standard Method 4500-CN-E, in a range from 0.02 to 0.2 mg/L CN¯) in a Hach DR2800 spectrophotometer. In addition, a cyanide - selective electrode method (4500-CN-F, in a range from 0.05 to 10 mg/L CN¯) with a Thermo Orion 9606BNWP electrode, was also used.
Ozonation system description
Ozone (O3) was produced from dry oxygen (99.999 %, AGA) using a Philaqua BMT 802M generator at an approximate dose of 2.51 g O3/h ([O3]gas-phase = 6.9x10-2 g/L). O3 was transferred to 90 mL samples of cyanide solutions through a porous diffuser. The concentrations of the O3 feed (at the inlet of the reactor) and the O3 not consumed (at the outlet of the reactor) were measured by the iodometric titration method (Standard Method 2350-E). The amount of consumed O3 was calculated at different times by applying a mass balance into the reactor. All the tests were carried out at room temperature (~20 ºC).
Influence of activated carbon and H2O2 doses and pH on cyanide removal and O3 consumption
90 mL samples of synthetic cyanide solutions (400 mg/L, 15.37 mM) were treated using O3 alone and the combinations O3/H2O2, O3/AC and O3/H2O2/AC at two pH values (10 and 11). Consumed O3 and CN¯ removal were determined at different times for 7.5 min (the experiment was carried out separately for each time). All runs were carried out in triplicate. Additionally, the statistical software Statgraphics Centurion XVI (version 16.1.03) was used to compare the treatments.
The treatment using the combination H2O2/O3 was performed at different ratios of H2O2/O3 feed (2.0, 5.0 and 10.0 mg H2O2/mg O3). When the combination AC/O3 was employed, three different AC dosages of 0.3, 0.5 and 1.0 g/L were used. The surface area, pore diameter and pore volume of the activated carbon were measured using a Quantachrome Instruments Nova4200e surface area and pore size analyzer by the Brunauer-Emmett-Teller (BET) method. The point of zero charge (PZC) of the activated carbon was determined using the method reported by Newcombe et al. (1993).
The conditions of the treatments with O3, H2O2/O3 and AC/O3 that allowed the highest cyanide removal were selected for the treatment with the combination O3/H2O2/AC. Additionally, tests with H2O2 and activated carbon alone were carried out for comparative purposes.
Characterization and treatment of samples from cyanidation effluents
In order to assess if the treatment (O3, O3/AC, O3/H2O2, or O3/H2O2/AC) that showed the highest cyanide removal for the synthetic solutions can degrade free cyanide in an industrial effluent, experiments with effluents from a cyanidation process were carried out applying the best treatment. The samples of effluents were characterized, before and after the treatment application, by measuring the following parameters: pH, cyanide concentration (Standard Methods 4500-CN-E and 4500-CN-F), chemical oxygen demand (COD, Standard Method 5220-B), total organic carbon (TOC, Standard Method 5310-B) and turbidity.
RESULTS AND DISCUSSION
Influence of AC, H2O2 doses, and pH on cyanide removal and O3 consumption
The results of cyanide removal at different times under all conditions tested are shown in table I. According to the results of the ANOVA test of the treatment using only O3, the pH value had a significant effect on cyanide removal (p < 0.05). Moreover, the higher cyanide removal was achieved at pH 11.0. As mentioned by von Gunten (2003)), the pH value plays an important role because ¯OH ions can initiate the O3 decomposition into •OH radicals. Previous studies have reported that the treatment of cyanide using O3 is better at alkaline conditions (Andreozzi et al. 1999, Mert et al. 2014). In addition, Soto et al. (1995)) found that the oxidation of the protonated form of cyanide (HCN) using O3 is slower in comparison with the oxidation of cyanide ions (CN¯). Since at pH values higher than the pKa of HCN (pH = 9.2) the proportion of CN¯ ions is higher in comparison with HCN (Stavropoulos et al. 2015), the removal of cyanide could increase at high pH values. Furthermore, working at alkaline conditions avoid the generation of the volatile species HCN which is a compound known for its high respiratory toxicity (Chan et al. 2010).
TABLE I CYANIDE REMOVAL USING THE TESTED COMBINATIONS: O3; O3/H2O2; O3/CA AND O3/H2O2/CA ([CN¯] o = 15.37 mM)
| pH | Treatment | H2O2* (mM) | AC** doses (g/L) | Cyanide removal (%) | ||
| 4.5 min | 6.0 min | 7.5 min | ||||
| pH = 10 | O3 | - | 79.8 | 90.8 | 100.0 | |
| O3/H2O2 | 0.18 (2 mg H2O2/mg O3) | - | 84.4 | 98.8 | 100.0 | |
| O3/H2O2 | 0.49 (5 mg H2O2/mg O3) | - | 87.0 | 98.2 | 100.0 | |
| O3/H2O2 | 1.02 (10 mg H2O2/mg O3) | - | 94.6 | 99.6 | 100.0 | |
| O3/CA | - | 0.30 | 69.4 | 97.0 | 100.0 | |
| O3/CA | - | 0.50 | 78.8 | 97.6 | 100.0 | |
| O3/CA | - | 1.00 | 79.3 | 99.2 | 100.0 | |
| pH = 11 | O3 | - | - | 84.6 | 93.3 | 100.0 |
| O3/H2O2 | 0.18 (2 mg H2O2/mg O3) | - | 88.2 | 98.6 | 100.0 | |
| O3/H2O2 | 0.42 (5 mg H2O2/mg O3) | - | 83.0 | 96.1 | 100.0 | |
| O3/H2O2 | 1.04 (10 mg H2O2/mg O3) | - | 95.9 | 99.8 | 100.0 | |
| O3/CA | - | 0.30 | 72.7 | 89.8 | 100.0 | |
| O3/CA | - | 0.50 | 80.6 | 98.0 | 100.0 | |
| O3/CA | - | 1.00 | 79.3 | 92.5 | 100.0 | |
| H2O2 | 1.04 | - | 66.7 | 80.8 | 90.0 | |
| CA | - | 0.50 | 12.5 | 29.2 | 20.8 | |
| O3/H2O2 /CA | 1.02 (10 mg H2O2/mg O3) | 0.50 | 83.5 | 99.3 | 100.0 | |
* The values in parenthesis are expressed in milligrams of H2O2 per milligram of O2 feed
**AC = activated carbon
When H2O2 was included in the treatment, the highest free cyanide removal was achieved using 10 mg H2O2/mg O3. In addition, there were not found statistically significant differences between 2 mg H2O2/mg O3 and 5 mg H2O2/mg O3. On the other hand, the removal of cyanide was not affected by the pH values tested (10.0 and 11.0) (Fig. 1b). As reported previously, the addition of H2O2 could increase the generation of •OH radicals because H2Osubspan>2 anions (HO2¯) react rapidly with O3 which is decomposed in •OH radicals and O2 (see eqs. (1)-(4)) (von Sonntag and von Gunten 2012). Furthermore, as shown in figure 1, an increment of the O3 consumption was observed when the H2O2/O3 ratio increased. This consumption may be related to a scavenging effect of O3, since it is known that O3 reacts rapidly with •OH radicals (von Gunten 2003). In other words, the reaction of O3 with •OH competes with the reaction between cyanide and •OH (Gurol and Bremen 1985). Thus, more O3 is needed in order to achieve the removal of cyanide.
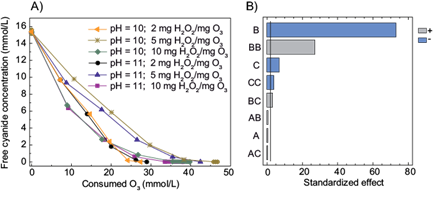
Fig. 1 a) Cyanide removal as a function of the O3 consumed (mM) at different H2O2/O3 ratios (mg H2O2/mg O3 feed) and pH values b) Pareto chart of the main effects obtained from the cyanide removal using the treatment O3/ H2O2 (A = pH, B = time, C = H2O2/O3 ratio)
The characterization of the activated carbon and the results of the cyanide removal using the combination of O3/AC are depicted in table II and figure 2, respectively. According to the Pareto chart of the main effects obtained using this combination (Fig. 2b), the cyanide removal was not affected by the activated carbon dosage and the pH value (10.0 and 11.0). Furthermore, the addition of activated carbon did not increase the cyanide removal in comparison with O3 and O3/H2O2. This is in good agreement with previous results for the treatment of other compounds, for instance, Zaror (1997) reported that the addition of AC did not affect the oxidation of phenolic compounds. Moreover, Sanchez-Polo et al. (2005) mentioned that activated carbon could act as an initiator of the O3 decomposition reaction rather than a catalyst. This behavior is explained because activated carbon loses its reactivity during the process due to a decrease of basic functional groups responsible for the promotion of O3 decomposition. In this study, an activated carbon dose of 0.5 g/L was chosen to carry out the comparison between all the treatments (Fig. 3).
TABLE II CHARACTERIZATION OF THE EMPLOYED COMMERCIAL ACTIVATED CARBON (AC)
| SBET (m2/g) | Vmicro (cm3/g) | Φmicro (Å) | pHPZC |
| 934.15 | 0.3843 | 3.68 | 7.40 |
(PanReac AppliChem, ref: 121237)
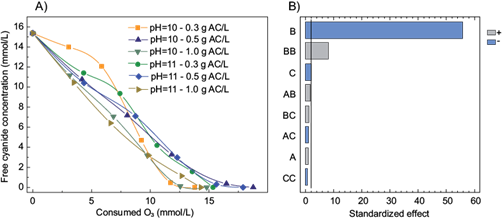
Fig. 2 a) Cyanide removal as a function of the O3 consumed (mM) at different activated carbon doses (g AC/L) and pH values b) Pareto chart of the main effects obtained from the cyanide removal using activated carbon (A = pH, B = time, C = activated carbon dose)
A comparison between all treatments applied (O3, O3/AC, O3/H2O2 and O3/H2O2/AC) and the individual effect of H2O2 and activated carbon on the cyanide removal was carried out at the conditions which presented the best cyanide removal for each combination. As shown in figure 3a, the higher cyanide removal was obtained using the combination O3/H2O2 (10 mg H2O2/mg O3, pH = 11.0). Also, the combination O3/H2O2/AC (10 mg H2O2|/mg O3, 0.5 g AC/L, pH = 11.0) exhibited a better cyanide removal in comparison with O3 alone (pH = 11.0).
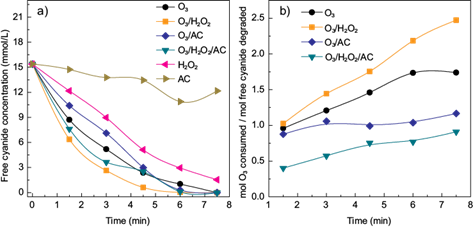
Fig. 3 a) Cyanide removal as a function of time using the tested combinations: O3; O3/H2O2 (10 mg H2O2 /mg O3); O3/CA (0.5 g AC/L) and O3/H2O2/CA (10 mg H2O2 /mg O3 - 0.5 g AC/L) and b) ozone consumption as function of time. ([CN¯]o = 15.37 mM, pH =11)
Regarding O3 consumption (Fig. 3b), the treatment using O3/H2O2 showed the highest values. In this case, the quantity of ozone consumed increased during the process. A similar trend was obtained for the treatment using only O3. These behaviors may be related to the scavenging effect of O3 (von Gunten 2003). As mentioned before, the reaction of O3 with •OH competes with the reaction between cyanide and •OH (Gurol and Bremen 1985). When the concentration of CN¯ decreases the probability of O3 to react with •OH radicals could increase. As a result, more O3 needs to be consumed to degrade the residual CNˉ. When activated carbon was added, the O3 consumption decreased. For the treatment using O3/AC (0.5 g AC/L, pH = 11.0) the O3 consumption was approximately 1 mol O3/mol CN¯, while for the treatment using O3/H2O2/AC the consumption was lower than 1 mol O3/mol CN¯. Zaror (1997) also found that the presence of AC in the ozonation process decreased the ozone consumption. In the case of the combination O3/AC, this reduction may be attributed to the generation of •OH radicals at specific sites over the large surface of the activated carbon. It seems that these radicals produced are less available to react with the incoming O3. Thus, the selectivity of •OH radicals (i.e., the reaction of •OH radicals with target components) is improved, since there is a decrease of the scavenging effect of O3. The same principle could apply to the combination O3/H2O2/AC with the difference of the increase of •OH production by the addition of H2O2 with a subsequent decrease in the consumption of O3. Future studies should be focused on the reaction mechanisms when O3, H2O2 and activated carbon are combined in order to have a better understanding of this phenomena.
Characterization and treatment of samples from cyanidation effluents
Samples of cyanidation effluents from gold ore processing were treated using the combination O3/H2O2 (10 mg H2O2/mg O3, pH =11.0) which presented the highest cyanide removal. Figure 4 shows the results of the cyanide removal from the samples of effluents ([CN¯]o = 172.5 mg/L). Moreover, the cyanide removal of synthetic solutions ([CN¯]o = 400.0 mg/L) treated under the same conditions is reported for comparative reasons. Almost a total removal of cyanide from the effluents was achieved at 3 min. This time was lower than the time needed to achieve the same conversion in the synthetic samples because the initial free cyanide concentration was lower in the effluent.
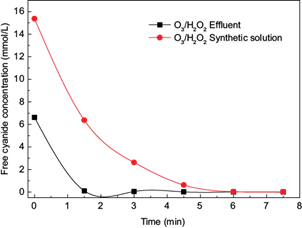
Fig. 4 Cyanide removal [CN-] as a function of time using the combination (O3/H2O2) in synthetic cyanide solutions and cyanidation effluents samples (10 mg H2O2 /mg O3, pH =11)
The results of the characterization of the effluents before and after the treatment are provided in table III. As shown in the results, the removals of TOC and COD were 82.2 % and 76.11 %, respectively. Since TOC represents the degree of mineralization (oxidation of organic carbon content to CO2), the removal values suggested that the treatment can trigger the formation of less toxic species. Also, the final cyanide concentration (0.08 mg/L) complies with the drinking water regulation of the Environmental Protection Agency (EPA) of the US which is 0.2 mg/L. The results suggested that the treatment could be applied for the removal of cyanide in cyanidation effluents. It should be noted that this study was only focused in the removal of free cyanide, although in these effluents cyanide can be in the form of various species such as free cyanide and cyanide complexes. Moreover, there are some reports of the capability of O3 to oxidize also cyanide complexes (Kuyucak and Akcil 2013). Therefore, it is important to mention that future studies should also focus on the combination of the processes studied to remove this kind of compounds.
CONCLUSIONS
The highest cyanide removal was achieved at pH 11.0 for the ozonation process. When H2O2 was added, the cyanide removal was improved with the increase of the H2O2/O3 ratio. This result was attributed to a possible increase in the generation of •OH radicals caused by the reaction between H2O2 anions (HO2¯) and O3. The highest removal was obtained with 10 mg H2O2/mg O3. Moreover, the O3 consumption increased with the increment of H2O2/O3 ratio which was attributed to a scavenging effect of O3. For the combination O3/AC, the statistical analysis showed that the cyanide removal was not affected by the activated carbon dosage. Also, the addition of AC did not improve the cyanide removal in comparison with O3 alone.
According to the comparison between all the treatments applied (O3, O3/AC, O3/H2O2 and O3/H2O2/AC), the best cyanide removal was reached with the combination O3/H2O2 followed by the combination O3/H2O2/AC. Furthermore, the O3 consumption increased during the process using the combinations O3/H2O2 and O3. When activated carbon was added, the O3 consumption decreased during the process. For the combination O3/AC the O3 consumption was approximately 1 mol O3/mol CN¯, while for the combination O3/H2O2/AC the consumption was lower than 1 mol O3/mol CN¯.
The treatment of cyanidation effluents was carried out using the treatment with O3/H2O2 (10 mg H2O2/mg O3, pH = 11.0). According to the results, almost a total removal of free cyanide (CN¯) was achieved after 3 min of treatment. Moreover, the final cyanide concentration was 0.08 mg/L. This value complied with the drinking water regulation of the Environmental Protection Agency of the US which is 0.2 mg/L.











 nova página do texto(beta)
nova página do texto(beta)


