INTRODUCTION
The microbial degradation of polycyclic aromatic hydrocarbons (PAHs) is the main route for decomposition and mineralization of these compounds, even though physicochemical interactions and environmental features participate in this process (Cerniglia 1992, Haritash and Kaushik 2009). Among many bacterial species Pseudomonas aeruginosa, Pseudomonas fluorescens, Mycobacterium spp., Haemophilus spp., Rhodococcus spp. and Paenibacillus spp. are some usually studied PAH-degrading bacteria, which have been generally isolated from contaminated soil or sediments (Mrozik et al. 2003, Haritash and Kaushik 2009). This ability of bacteria to degrade PAHs is due to a multicomponent enzyme system that generates hydroxylated metabolites followed by a series of reactions to achieve the final mineralization of these compounds (Kanaly and Harayama 2000, Haritash and Kaushik 2009, Zhang et al. 2011). In the case of P. aeruginosa, its ability to synthesize rhamnolipids in addition to its oxygenase/dehydrogenase system stands out (Rahman et al. 2003, Jouanneau et al. 2006, Kumara et al. 2006, Zhang et al. 2011, Zhao et al. 2011, Cao et al. 2012, Gai et al. 2012). In low and high molecular weight PAHs, the first step of PAH biotransformation performed by some bacteria is the generation of dihydrodiols through their oxygenase activity (Kanaly and Harayama 2000) as it occurs in fish. In fish, this process is performed by cytochrome P450 isoenzymes (CYP450), particularly by oxygenases as is the case of the isoform 1A1 (CYP1A1) (Whyte et al. 2000). This similarity in the initial processes of PAH biotransformation between some bacteria and fish suggest possible toxicokinetic and toxicodynamic symbiotic interactions between intestinal bacteria and fish, besides those well documented endosymbiotic relationships (Austin 2002, Kuzmina and Skvortsova 2002, Palm et al. 2003, Verner-Jeffreys et al. 2003). In this regard, it has been demonstrated that some intestinal bacteria of fish, such as Aeromonas allosaccharophila, Aeromonas eucrenophila, Aeromonas media and Pseudomonas flavescens degrade PAHs (Voverienė et al. 2002). The density of PAH-degrading bacteria in the digestive track of fish is higher than in water, suggesting a role of bacteria in the adaptation and survival of fish chronically exposed to PAHs (Mickéniené and Šyvokienė 2008). Accordingly, hydrocarbon-degrading bacteria were found in the liver and bile of some fish, such as Carangoides fulvoguttatus and Plectropomus maculatus, which were related to PAH levels (King et al. 2005). The number of hydrocarbon-degrading bacteria regarding the total heterotrophic bacteria in the digestive tract of molluscs and anostracan, are increased in more PAH-polluted localities with regard to a reference site (Šyvokienė and Mickėnienė 2004). Changes of intestinal P. aeruginosa in the juvenile African catfish (Clarias gariepinus) were related to intramuscular doses of benzo[a]pyrene (BaP), as well as some biomarkers in the liver of the fish (ethoxyresorufin-O-deethylase and glutathione S-transferase) were inversely correlated with the bacterial population (Karami et al. 2012). Despite these studies about the capacity of some bacteria to degrade PAHs, a lack of information prevails about the possible protective role of Pseudomonas spp. against toxic effects elicited by PAHs in vivo in fish. Thus, the aim of this study was to evaluate the relationships of Pseudomonas spp. strains with specific biomarkers of exposure to PAHs in wild Chirostoma jordani from three lakes in Mexico, and to assess the capacity of these intestinal bacteria to biotransform PAHs.
MATERIALS AND METHODS
Fieldwork
Three bimonthly sampling campaigns were performed in Lago Mayor (Lake Mayor) and in Lago Menor (Lake Menor) in the second section of Chapultepec Park, Mexico City. In Lake Zumpango, State of Mexico, two bimonthly sampling campaigns in three sites were performed. Water samples were collected with a Van Dorn bottle from the water column on the surface, middle, and bottom the same day at midday. Mezquital silverside (Chirostoma jordani) specimens were collected (n = 15 in each lake and sampling campaign), sacrificed through fast freezing in ice and then in dry ice, and transported to the laboratory according to the Mexican protocol for the production, protection and welfare of experimental animals (SAGARPA 2001). In the laboratory, the fish were measured with a vernier caliper to form homogeneous groups (52.51 ± 0.73 mm) and weighed to within 0.1 mg (1.14 ± 0.01 g) in analytic balance. Necropsy was performed immediately to obtain the liver and the intestinal tract; both organs were weighed to within 0.1 mg. The liver was homogenized 1:5 (w/v) in phosphate-buffered solution (PBS1X) containing protease inhibitor (aprotinin, 3 mg/mL, Sigma™) by using a Glas-Col GKH homogenizer at 4000 rpm with Teflon pestles. The intestinal tract was processed under sterile conditions by using sterile PBS1X with protease inhibitor to perform the first dilution, which was also employed for the biomarkers assay. The homogenates were centrifuged (9000 × g and 4 ºC/15 min) to obtain S9 fraction and stored at -70 ºC until the biomarker assay was done (less than two weeks). Control fish born in laboratory within the age of 8-10 months were used to compare the enzymatic catalysis with the wild fish.
Evaluation of PAHs in water samples
For the evaluation of PAHs, extraction was performed with 10 mL of dichloromethane (HPLC grade) using 100 mL of water samples. The organic extract was purified in C18 cartridges activated with methanol-milli Q water (1:2 v/v), and the supernatant was evaporated to dryness under a gentle stream of nitrogen. The residue was reconstituted with 500 μL of methanol HPLC grade for PAHs analysis using standard curves of PAH mixtures (Sulpeco™) according to a previous report (Vega-López et al. 2013).
Populations levels of Pseudomonasspp. in C. jordaniintestinal tract and in water samples
The CFU of Pseudomonas spp. of the intestinal tract of the Mezquital silverside, and in water was estimated by dilution plate technique on a cetrimide agar base (Difco™). Positive Pseudomonas colonies (blue, blue-green, green or yellow-green fluorescence colonies on cetrimide agar under UV light) were automatically counted with the Quantum-Capt software system 12-640322 in a Vilber Lourmat dark chamber. Pseudomonas estimation was reported as CFU/100 g tissue or CFU/100 mL of water. Additionally, strains were also cultured in peptone broth for 24-48 h to perform the indole test by using Kovac’s reagent according to Bergey’s Manual (Palleroni, 2015).
Molecular identification and phylogenetic analysis of Pseudomonasspp. strains
Five colonies were isolated from each fish (n = 750 colonies), and individually cultured (33 ºC/48 h) in 5 mL of Dibico™ nutritive broth (3% beef extract, 5% gelatin peptone) until a growth of 9.0 × 108 CFU/mL was reached (tube 3 of McFarland’s nephelometer). The culture was centrifuged at 11 180 × g/4 ºC/15 min to obtain the pellet intended for DNA extraction. Total DNA was isolated by the salt-extraction method (Aljanabi and Martinez 1997). The DNA quality was estimated by electrophoresis in 1.2% (w/v) agarose gel in a 1X TBE buffer (89 mmol Tris, pH 8.3, 89 mmol boric acid, 2 mmol EDTA). Staining was performed with 0.5 µg/mL ethidium bromide solution. DNA quantity was determined spectrophotometrically; an A260/A280 nm ratio of 1.8:2.1 was considered to be acceptable. PCR amplifications of the 16S rRNA gene were performed with universal bacterial primer as previously described by Relman (1993). Reaction mixtures contained 5 ng of template DNA, 3μL of 10X reaction buffer (15 mmol MgCl2), 2μL 25 mmol MgCl2, 0.25 mmol of each dNTP, 10 pmol of each primer and 5U of Taq polymerase (Invitrogen™) adjusted to 30 μL reaction volume. PCR conditions were used as follows: an initial denaturation step at 94 ºC for 5 min; followed by 30 cycles at 94 ºC (1 min 30 s), 58 ºC (1 min 30 s) and 72 ºC (1 min 30 s) with a final extension step at 72 ºC for 5 min. Amplicons were purified with a NucleoSpin gel and PCR clean-up kit (Macherey-Nagel™) according to manufacturer directions. Purified 16S rRNA was analyzed by the PCR- restriction fragment length polymorphism method (PCR-RFLP) according to Barsotti et al. (2002), with modifications: 6 μL of 16S rRNA + 2 μL CutSmart buffer + 1 μL Mbo1 restriction enzyme + 1 μL Alu1 restriction enzyme (New England BioLabs™) + 12 μL of ultrapure H2O. This mixture was subjected to overnight incubation at 37 ºC and the products of digestion were visualized in agarose gels (2.5%) stained with an ethidium bromide solution. The undigested 16S rRNA of the strains were sequenced by Macrogen Co. using an Illumina HiSeq2500 (HCS 2.0.12/RTA 1.17.21.3/SAV 1.8.20 software) sequencing system. The 16S rRNA gene sequences were compared with the GenBank nucleotide databases (from the National Center for Biotechnology Information) by using BLASTN and BLASTX algorithms (Altschul et al. 1990). Best matched (query cover age with lower score to the top) database from the GenBank information was selected to construct the molecular phylogenetic analysis by the maximum likelihood method (Tamura and Nei 1993). The molecular phylogenetic analysis was conducted using MEGA6 software (Tamura et al. 2013).
Evaluation of Pseudomonasstrain capacity involved in the biotransformation of PAHs
With the aim to evaluate the activities of some enzymes involved in the biotransformation of PAHs, of each isolated strain (9.0 × 108 CFU/mL) 0.1 mL was cultured in nutritive broth enriched with three environmentally relevant concentrations of PAHs that were selected based on the real environmental PAH levels found in the lakes under study. Sulpeco™ PAH mixture was used to reach 0.1, 1.0 and 10 µg/L; the strains were cultured for 48 h at 33 ºC protected from the light. At the end of the incubation time, the culture was centrifuged at 11 180 × g/4 ºC/15 min to obtain the pellet that was re-suspended in 1 mL of PBS1X; the cells were disrupted by sonication in three bursts of 20 s at 50% power at 4 ºC. The sonicated fraction was centrifuged at 9000 × g and 4 ºC/15 min and stored at -70 ºC until the biomarker assay was performed.
Evaluation of biomarkers
The metabolism of CYP1A1 (EC 1.14.14.1) in the liver and viscera, including the intestinal tract of the Mezquital silverside was evaluated by EROD activity according to the method of Whyte et al. (2000) with modifications for microplates. Fluorescence was evaluated in a Biotek Synergy Mx spectrofluorometer at 520 nm of excitation and 585 nm of emission. Activity was calculated based on a calibration curve of resorufin (0.05-0.25 μmol) and expressed as millimol per minute per milligram of protein (mmol/min/mg protein).
The activity of the naphthalene dioxygenase system (NDO) controlled by NDO genes, which is responsible for oxidizing PAH substrates (Peng et al. 2008) was measured. The activity of this system was evaluated in the PAH-cultured strains based on the method discussed in the report of Jouanneau et al. (2006), which considers that the NADH consumption is involved in the hydroxylation of low and high molecular weight PAHs. For this purpose, a modification of Whyte method (2000) was performed. Results are expressed as mmol/min/mg protein.
The activity of epoxide hydrolase (EH) (EC 3.3.2.10): microsomal (EH1) plus soluble epoxide hydrolase (sEH) in the liver and viscera of the fish was assessed according to Thomaeus et al. (2008) method by using trans-stilbene oxide (TSO) as a substrate (4 mmol). The molar extinction coefficient of TSO (15 mmol-1/cm) was used. Results are expressed as mmol/min/mg protein. In bacterial strains the activity of the EH was assessed in the centrifuged fraction of bacteria exposed to the PAH mixture with TSO as a substrate as stated in Thomaeus’ report (2008).
Total glutathione-S-transferase (GST) (EC 2.5.1.18) in the liver and viscera of the fish was evaluated by the spectrophotometric method found in Boryslawskyj et al. (1998) using 2,4-dichloronitrobenzene as the substrate. The activity of GST in the bacterial strains was evaluated by using the same substrate as previously reported (Zablotowicz et al. 1995, Santos et al. 2002). The total protein content was determined with a Thermo Scientific Pierce® 660 nm protein kit assay.
The bioconcentration factor (BCF) was estimated for PAHs in the liver of the Mezquital silverside by the same spectrofluorometric method, as described for water samples, by taking 50 μL of non-centrifuged fraction diluted with 50 μL of methanol, vortexed (1 min) and centrifuged at 9000 rpm.
Statistical analysis
Biomarker results for wild specimens were compared by two-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test by using GraphPad Prism version 5.00 for Windows. In the same way, the activity of biomarkers involved in biotransformation of PAHs in bacterial strains exposed to PAH mixture was compared with the same biomarkers in fish by using the former statistical methods. The possible toxicokinetic and toxicodynamic symbiotic interactions among intestinal bacteria and fish with environmental variables (water PAH content) and with endogenous levels of PAH (liver BCF of PAH) were estimated through the redundancy analysis (RDA). Monte Carlo permutation was used to assess statistical significance of the canonical axes. For all statistical tests performed, the significance level was set at ≤ 0.05. RDA was calculated with XLSTAT software (Addinsoft SARL).
RESULTS
Molecular identification and phylogenetic analysis of Pseudomonasstrains isolated from fish intestinal tract
Three of the four isolated bacterial strains from the intestinal tract of the wild C. jordani identified by nucleotides sequence of 16S rRNA clearly belong to the Pseudomonadales cluster (Fig. 1). Therefore, in our study these bacterial strains were named as Pseudomonas spp., PM1 strain (KY056727.1), Pseudomonas spp., PM2 strain (KY056728.1) and Pseudomonas spp. PM3 strain (KY056729.1). In contrast, the identity of PM4 strain (KM232744.1) remains unknown; however, this bacterium belongs to the Aeromonadales cluster. Nonetheless, the PM4 strain is able to growth on cetrimide agar base and is a pyocyanin producing microorganism.
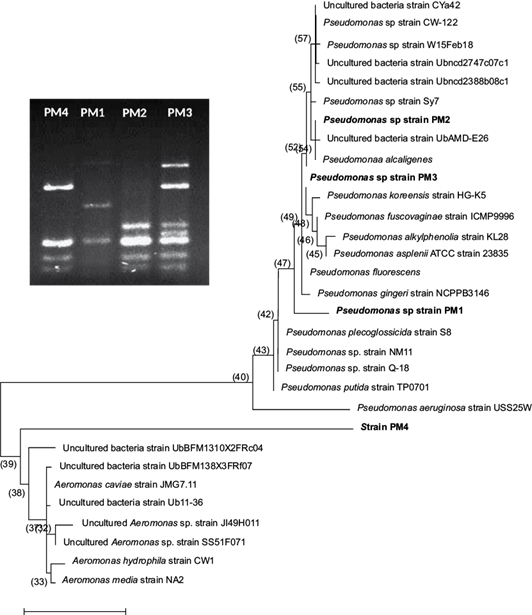
Fig. 1 Molecular phylogenetic analysis of Pseudomonas spp. strains isolated from the intestinal tract of the wild C. jordani by the maximum likelihood method. The evolutionary history was inferred by the Tamura and Nei model (1993) with the MEGA6 software (Tamura et al., 2013). Pseudomonas spp. strains were analyzed by the PCR-RFLP method using Mbo1 and Alu1 restriction enzymes as shown.
CFU number of Pseudomonasspp. strains in water and in the intestinal tract of C. jordani
In the water from the three lakes under study, the CFU/100 mL of Pseudomonas spp. was lower than 10 CFU/100 mL in all sampling campaigns (data not shown). In contrast, in the intestinal tract of the fish, the CFU number of Pseudomonas spp. was higher in specimens from both lakes of Chapultepec Park with an exponential value (E) of 109 than in fish from Lake Zumpango (E5 to E6) (Fig. 2). Interestingly, in fish from the two lakes of Chapultepec Park, only the PM4 strain was detected in all sampling campaigns. However, in fish from Lake Zumpango the three Pseudomonas strains (PM1 = 11.43%, PM2 = 22.85% and PM3 = 11.43%) and the PM4 strain (54.28%) by using PCR-RFLP method were found.
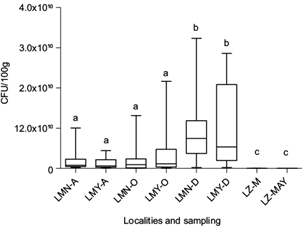
Fig. 2 CFU/100 g of Pseudomonas spp. strains isolated from the intestinal tract of the wild C. jordani from three lakes in the Valley of Mexico. LMN: Chapultepec Lake Menor; LMY: Chapultepec Lake Mayor; LZ: Lake Zumpango. A: August 2012. O: October 2012. D: December 2012. M: March 2013. MAY: May 2013. Bars with the same letter denote lack of statistical differences between them.
Comparison of the capacity of biotransformation and detoxification of PAHs by Pseudomonas spp. strains with the wild C. jordani
The catalysis of naphthalene dioxygenase system (NDO) of PM1 and PM4 exposed under laboratory conditions to 10 µg PAHs/L was significantly different from the mean activity of CYP1A1 of the wild C. jordani (Fig. 3a). In contrast, the activity of EH involved in the detoxification of epoxides by all bacterial strains treated with PAH mixtures was significantly lower than EH activity of the fish from the three lakes under study (Fig. 3b). In the liver, GST activity was higher than in four bacterial strains treated with the three PAH mixtures (p ≤ 0.05). Oppositely, the GST activity of bacterial strains treated with 10 µg PAHs/L was higher than in the viscera of the wild Mezquital silverside (Fig. 3c).
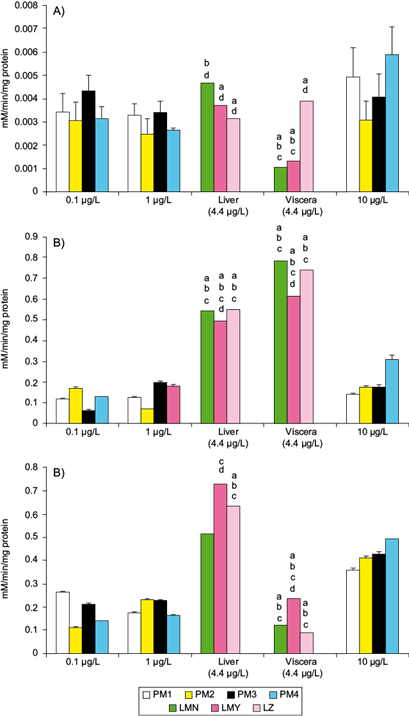
Fig. 3 Mean activities of some enzymes involved in the biotransformation and conjugation of PAHs in Pseudomonas spp. strains isolated from the intestinal tract of the wild C. jordani exposed in vitro to three PAH mixtures (0.1, 1.0 and 10 µg/L) in nutritive broth, and in the liver and viscera of the wild C. jordani from three lakes in the Valley of Mexico. (a) CYP1A1 activity in the liver and viscera of fish and NDO system activity in Pseudomonas spp. Strains. (b) EH activity in the liver ad viscera of fish and in Pseudomonas spp. strains. (c) GST activity in the liver and viscera of fish and in Pseudomonas spp. strains. The fish enzymatic activities were compared (p ≤ 0.05) with regard to aPM1, bPM2, c PM3 and dPM4 exposed to 10 μg PAHs/L. The mean activity of fish enzymes is shown as mean PAH levels in the lakes under study (4.4 µg PAHs/L). LMN: Lake Menor; LMY: Lake Mayor; LZ: Lake Zumpango; PM1: Pseudomonas PM1 strain; PM2: Pseudomonas PM2 strain; PM3: Pseudomonas PM3 strain; PM4: Pseudomonas-like PM4 strain, unidentified bacteria
Taking into account the activities of the Mezquital silverside, the mean activity of hepatic CYP1A1 was higher in fish from Lago Menor than the one observed in Lago Mayor and Lake Zumpango; however, in the viscera, CYP1A1 was greater in Lake Zumpango specimens (Table I). Interestingly, higher activity of CYP1A1 in the viscera of Lake Zumpango specimens coincided with the highest diversity of Pseudomonas spp. (PM1, PM2 and PM3) and Pseudomonas-like (PM4) strains and with a low CFU number (E+5 to E+6). In C. jordani the mean EH activity in both the liver and viscera was similar in specimens from Lago Menor and Lake Zumpango that was higher than in the fish from Lago Mayor. The hepatic and visceral GST activity was higher in fish from Lago Mayor than those from Lago Menor and Lake Zumpango (Table I).
TABLE I ACTIVITY OF CYP 1A1 (EROD), OF EPOXIDE HYDROLASE (EH) AND OF GLUTHATHIONE-S-TRANSFERASE (GST) IN THE LIVER AND VISCERA OF Chirostoma jordani. MEAN ± STANDARD ERROR. CONTROL FISH BORN IN LABORATORY WERE OBTAINED AS IN DZUL-CAAMAL et al. (2012).
| Liver | Viscera | ||||||
| CYP 1A1 (nmol/min/mg protein) |
EH1 (μmol/min/mg protein) |
GST (μmol/min/mg protein) |
CYP 1A1 (nmol/min/mg protein) |
EH1 (μmol/min/mg protein) |
GST (μmol/min/mg protein) |
||
| CONTROL FISH | |||||||
| 9.79E-03 ± 1.03E-03 | 1.25E-02 ± 1.49E-03 | 2.85E-02 ± 3.03E-03 | 8.10E-03 ± 3.86E-04 | 4.36E-02 ± 3.03E-03 | 1.38E-02 ± 1.96E-03 | ||
| LAKE MENOR | |||||||
| August 2012 | 9.14E-03 ± 5.89E-03 | *2.84E-01 ± 2.53E-02 | *9.61E-01 ± 1.81E-01 | *1.76E-03 ± 3.23E-04 | 2.05E-01 ± 4.41E-02 | 1.49E-01 ± 5.47E-02 | |
| October 2012 | *4.51E-03 ± 3.34E-03 | 1.14E+00 ± 3.76E-01 | 2.73E-01 ± 4.95E-02 | *1.08E-03 ± 1.17E-04 | *1.03E+00 ± 2.36E-01 | 1.50E-01 ± 3.27E-02 | |
| December 2012 | *2.20E-04 ± 1.55E-05 | *1.98E-01 ± 3.43E-02 | 3.08E-01 ± 5.15E-02 | *2.93E-04 ± 3.53E-05 | *1.11E+00 ± 4.24E-01 | *5.96E-02 ± 1.07E-02 | |
| Mean value | 5.3E-03 ± 3.6E-03 | 1.9E-01 ± 3.2E-02 | 5.1E-01 ± 3.9E-02 | 1.9E-03 ± 2.6E-04 | 1.4 ± 3.7E-01 | 2.9 ± 3.3E-02 | |
| LAKE MAYOR | |||||||
| August 2012 | 7.63E-03 ± 3.94E-03 | *3.20E-01 ± 1.43E-01 | 1.77E+00 ± 5.92E-01 | *2.45E-03 ± 2.65E-04 | 3.45E-01 ± 1.40E-01 | 2.71E-01 ± 2.57E-02 | |
| October 2012 | *3.14E-03 ± 2.02E-03 | *6.97E-01 ± 1.59E-01 | 2.21E-01 ± 7.67E-02 | *1.22E-03 ± 1.22E-03 | *9.78E-01 ± 4.13E-01 | 3.71E-01 ± 1.22E-01 | |
| December 2012 | *3.41E-04 ± 8.05E-05 | *4.65E-01 ± 1.50E-01 | 1.93E-01 ± 4.62E-02 | *2.56E-04 ± 5.07E-05 | 5.19E-01 ± 1.33E-01 | *7.41E-02 ± 2.77E-02 | |
| Mean value | ‡4.7E-03 ± 4.7E-04 | 4.9E-01 ± 1.5E-01 | 1.9E-01 ± 6.0E-02 | 2.1E-03 ± 2.9E-04 | ‡6.1E-01 ± 2.3E-01 | ‡4.6E-01 ± 2.2E-02 | |
| LAKE ZUMPANGO | |||||||
| March 2013 | *3.16E-03 ± 3.38E-04 | *5.73E-01 ± 1.01E-01 | *6.66E-01 ± 4.44E-02 | *3.84E-03 ± 3.27E-04 | *7.99E-01 ± 1.44E-01 | *8.82E-02 ± 1.51E-02 | |
| May 2013 | *3.29E-03 ± 1.68E-03 | 2.60E-01 ± 3.86E-02 | 3.17E-01 ± 1.25E-01 | *4.57E-03 ± 1.49E-03 | *2.72E-01 ± 1.08E-01 | *8.11E-02 ± 2.53E-02 | |
| Mean value | ‡₤ 3.2E-03 ± 2.5E-03 | ‡₤ 4.2E-01 ± 2.08E-02 | ‡₤ 4.9E-01 ± 2.8E-01 | ‡₤ 4.2E-03 ± 2.4E-04 | ₤ 5.4E-01 ± 1.3E-01 | ‡₤ 8.5E-02 ± 2.0E-02 | |
*Statistically different to control fish p ≤ 0.05; ‡statistically different to the Lake Menor fish p ≤ 0.05; ₤statistically different to the Lake Mayor fish p ≤ 0.05
In contrast, the activity of the NDO system and EH activity of Pseudomonas PM1 strain and PM4 strain was induced by higher PAH concentration, but in Pseudomonas PM2 and PM3 strains an irregular concentration response was noted (Fig. 3a, b). The activity of GST in Pseudomonas PM2 and PM3 strains and in PM4 strain treated with PAH mixture was induced in a concentration-dependent manner; nevertheless, in Pseudomonas PM2 strain the response was irregular with a peak detected at 10 µg PAHs/L (Fig. 3c).
Toxicokinetic and toxicodynamic symbiotic interactionsamong intestinal Pseudomonasspp. strains with biotransformation and conjugation processes in wild C. jordani and with environmental variables
The symbiotic interaction among Pseudomonas spp. strains with metabolic activity of the Mezquital silverside showed clear differences between the liver and viscera. The biotransformation process (CYP1A1 and EH) carried out by fish showed a clear association with PAH water concentration in all sampling campaigns and lakes with no seasonal differentiation. In the liver, the activity of CYP1A1 and EH was induced by water concentration of naphthalene and benzo[a]anthracene (Fig. 4a); however, in the viscera only CYP1A1 activity was induced by the same hydrocarbons (Fig. 4b).
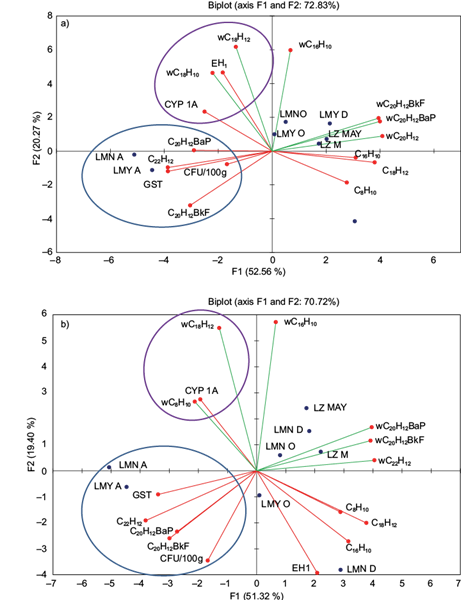
Fig. 4 Toxicokinetic and toxicodynamic symbiotic relationships between Pseudomonas spp. strains and the wild C. jordani collected from three lakes in the Valley of Mexico with environmental variables (water PAH content) and endogenous levels of PAH (BCF of PAH in the liver), were estimated using redundancy analysis. (a) Liver. (b) Viscera
Alternatively, the conjugation process evaluated by GST in fish from Chapultepec Park lakes was related to CFU of the PM4 strain, and with endogenous levels of PAHs only in the rainy season. GST activity was related to CFU and with endogenous levels of benzo[a]pyrene, benzo[k]fluoranthene and indeno[1,2,3- cd]pyrene in the liver and viscera (Fig. 4A and 4B). Interestingly, these associations agreed with the lower intestinal CFU numbers in fish from both lakes of Chapultepec Park. Nevertheless, in specimens from Lake Zumpango the activity of GST was not related to CFU or to endogenous levels of some PAHs.
DISCUSSION
The Pseudomonas spp. strains isolated from the intestinal tract of the wild C. jordani were able to carry out the biotransformation and conjugation processes of PAHs, as occurs in this fish species.
The number of CFUs of Pseudomonas spp. strains in the intestinal tract of the Mezquital silverside presented seasonal variations with a peak during winter and spring in specimens from Chapultepec Park and Lake Zumpango, respectively. However, in all sampling campaigns CFU number of Pseudomonas spp. strains was higher in fish from Chapultepec Park (109) than in Lake Zumpango (105 to 106) specimens. Although there are not many studies about this topic, it has been found that abundance and dynamics of autochthonous and allochthonous bacterial microbiota of the digestive tract of fish involved in PAH degradation depended on the fish species, nutrition habits and intensity of feeding, as well as on the season (Mickéniené and Syvokiené 2008, Šyvokienė et al. 2011). In the current study, differences in the number of CFUs of Pseudomonas spp. strain and Pseudomonas-like strain (PM4) could be explained by the depth of the lakes under study, in the case of Chapultepec Park, the depth was lower (Vega-López et al. 2013) than in Lake Zumpango. This assumption is likely to be true in the fish species and in the lakes under study because the depth of the water bodies allows greater contact with the sediments, which are usually the main reservoirs of toxicants and pollutants, including bacteria. Nevertheless, levels of PAHs in water were not related with CFU number of Pseudomonas spp. strains, indicating that the symbiotic relationship between host and bacteria is the key factor, even more than the environment levels of PAHs as suggested in other fish species (Mickéniené and Syvokiené 2008, Šyvokienė et al. 2011).
Many studies exist about the capacity of Pseudomonas spp. to biotransform PAHs in bioreactors, as well as in polluted environments; however, a lack of information prevails about the capacity of endosymbiotic bacteria to biotransform PAHs with regard to their fish hosts. The results of this study indicate the second highest capacity of the NDO system of Pseudomonas PM1 strain involved is the biotransformation of PAHs; nonetheless, it has been considered that P. fluorescens apparently did not have the same capacity as other Pseudomonas spp. to degrade PAHs (Arino et al. 1998, Haritash and Kaushik 2009, Leneva et al. 2010, Abbasnezhad et al. 2011). Pseudomonas PM2 strain was close to P. alcaligenes. In this regard, inoculation of phenanthrene- and fluoranthene-contaminated soil microcosms with P. alcaligenes resulted in the removal of significant amounts of the PAHs (Gordon and Dobson 2001, Alemayehu et al. 2004, O’Mahony et al. 2006, Hickey et al. 2007). Notwithstanding, Pseudomonas PM2 strain isolated from the intestinal tract of C. jordani showed an independent PAH-concentration response of the NDO system, which was low regarding the mean CYP1A1 activity of the Mezquital silverside. Differences with previous reports suggest that Pseudomonas PM2 strain requires certain environmental conditions to degrade PAHs (O’Mahony et al. 2006, Hickey et al. 2007) which are not present in the intestinal lumen of C. jordani as occurs in bioreactors. Although, the NDO system capacity of Pseudomonas PM3 strain isolated from the intestinal tract of C. jordani was the highest at low and median PAH concentrations, activity which did not present a PAH-concentration dependent response. Thus, further studies aimed at exploring the regulation of PAHs biotransformation in these Pseudomonas strains are necessary. The identity of the PM4 strain isolated from the intestinal tract of C. jordani using cetrimide agar base remains unknown. The phylogenetic analysis revealed a close similarity of PM4 strain to uncultured bacteria strains by using pairwise distances estimated with the maximum composite likelihood approach. However, the topology of the cluster showed that PM4 apparently belongs to Aeromonas genus. It is possible to provide some explanations about the topic: firstly, the cetrimide agar base is not completely selective; secondly and most relevant, the horizontal gene transfer (HGT) in Pseudomonas is likely to contribute to their adaptation. The HGT is documented as a mechanism involved in the acquisition of genes that undoubtedly contribute to strain- and species-specific activities (Silby et al. 2011). Despite the lack of conserved phenotypic differences in the PM4 strain isolated from the intestinal tract of C. jordani, current results suggest advantageous genes acquisition involved in PAH biotransformation. The PM4 strain exposed in vitro to high PAH concentration showed the highest NDO system activity among the other Pseudomonas spp. strains. Comparing to mean CYP1A1 activity in the liver and viscera of the wild Mezquital silverside specimens, NDO system activity of PM4 strain was greater. Interestingly, in fish that solely possess the PM4 strain in their digestive tract, lower mean CYP1A1 in their viscera was detected, but in the livers high mean CYP1A1 activity was noted. These responses could indicate a differentiated symbiotic relationship between Pseudomonas spp. strains and C. jordani allowing an organ-specific response in the fish aimed at biotransforming PAHs.
In all cases, the mean EH activity in the liver and viscera of fish from the three lakes under study was higher than in Pseudomonas spp. strains exposed to PAH mixture. The activity of EH in Pseudomonas spp. in removal of the epoxides has been studied (Jacobs et al. 1991, Li et al. 2003, Teufel et al. 2012), as well as the biodegradation of PAHs by bacteria (Cerniglia 1992, Zhang et al. 2011) as a likely source of possible epoxide generation. Despite a lack of information comparing the EH activity in Pseudomonas spp. with EH in fish, the current results show, for the first time, a higher rate of epoxide formation during PAH biotransformation performed by fish in contrast with Pseudomonas spp. strains. Differences between both organisms could be explained by complete mineralization of PAHs carried out by bacteria (Peng et al. 2008). Oppositely, in fish the cytochrome P450 isoenzymes, such as CYP1A1, are responsible, in some cases, for the formation of epoxides (Padrós et al. 2003, Shailaja et al. 2006). Comparing the activity of EH of the strains, the PM4 strain exposed to a higher PAH mixture showed the highest activity of EH with regard to the other strains. This response is in agreement with the higher NDO system activity of the PM4 strain as a source of epoxides. Nonetheless, intermediate PAH-derived metabolites which arise during the metabolism of PAHs could be conjugated with glutathione by GST activity.
The GST activity in all isolated Pseudomonas strains was lower than the mean GST activity in the livers of fish; however, GST activity of these bacteria exposed to 10 µg PAHs/L was higher than in viscera of the wild Mezquital silverside specimens. Diols, dihydrodiols and tetradiols derived of PAH biotransformation are conjugated with glutathione via GST activity to be excreted through the bile (Oliva et al. 2010, Fonseca et al. 2011, Kammann et al. 2014). Although it is not a completely suitable comparison, in C. gariepinus exposed intramuscularly to BaP, liver GST activity showed an inverse response with regard to the CFU concentration of PM4 strain suggesting that the hydrocarbon induced some physiological changes in the fish allowing altered intestinal bacterial populations (Karami et al. 2012). Differences between GST activities of Pseudomonas strains with regard to hepatic GST in the fish could be explained by bile production favoring the excretion of glutathione-conjugated metabolites. Nonetheless, the activity of GST of Pseudomonas spp. strains exposed to 10 µg PAHs/L was higher than the mean activity of GST in the viscera of the fish collected in the three lakes under study. This response evidences two possible scenarios: (i) in fish viscera a greater extent of PAHs are biotransformed by bacteria, and PAH-derived metabolites are conjugated with glutathione, and (ii) the influence of enterohepatic shunt (Jaeschke 2008) possibly allows the recirculation of glutathione-conjugated metabolites between the intestine and the liver of the Mezquital silverside by reducing the activity of GST. Nevertheless, other studies are required to demonstrate this phenomenon in this fish species.
The toxicokinetic and toxicodynamic symbiotic interactions among Pseudomonas spp. strains with metabolic activity of the wild Mezquital silverside environmentally exposed to PAHs and analyzed by using RDA showed two independent response patterns. In the liver, the activity of CYP1A1 and EH were induced by water concentration of naphthalene and benzo[a]anthracene, independently of the season and lakes. However, in the viscera only the CYP1A1 activity was augmented from the same hydrocarbons. Current results suggest that the simple structure of naphthalene composed by two rings allows its biotransformation by CYP1A1 activity to form 2-naphthol (Whyte et al. 2000). In addition, during the hydroxylation of both hydrocarbons it is possible that epoxides are detoxified by EH. The juvenile trout (Oncorhynchus mykiss) exposed to heavy fuel oil, rich in naphthalene, suffers a strong induction of CYP1A1 activity (Adams et al. 2014). In contrast, the adult rainbowfish (Melanotaenia fluviatilis) and Nile tilapia (Oreochromis niloticus) treated with naphthalene under controlled conditions, displayed no effect or inhibition of EROD (Pollino et al. 2009, Pathiratne and Hemachandra 2010). In the rainbow trout liver cell line, it was found that anthracene could not be considered as an inducer of CYP1A activity (Behrens et al. 2001). Differences with preceding reports denote a particular sensitiveness of the wild C. jordani, because the activity of this CYP450 isoenzyme and EH were related to an environmental concentration of two hydrocarbons, independently of the season and lakes. However, it is necessary to stress that in fish species the PAH toxicity is widely oversimplified because each PAH is a toxicologically active compound with distinct associated damage (Incardona et al. 2006).
In contrast, the conjugation process evaluated by GST in the liver and viscera of fish from the Chapultepec Park lakes was related to the amount of CFU of the PM4 strain and with the endogenous levels of benzo[a]pyrene, benzo[k]fluoranthene and indeno[1,2,3-cd]pyrene only in the rainy season. There are no preceding reports on this topic; however, it could be feasible to infer some explanations. The visceral levels of these hydrocarbons were directly related to the intestinal numbers of the PM4 strain. Supposing that HGT by conjugation was a favorable phenomenon that permits a higher capacity for PAH hydroxylation, the gene transfer in PM4 strain, probably from Aeromonadales, was probably a key factor for protecting their hosts. This hypothesis is likely because the linear relationship and ordination of the data obtained from the RDA showed a clearer ordination of endogenous levels of these hydrocarbons with the CFU number of PM4 in the viscera than in the liver. Accordingly, it is well known that P. aeruginosa and Aeromonas spp. are able to use PAHs as a sole carbon source (Cerniglia 1992, Arino et al. 1998, Mrozik et al. 2003, Cui et al. 2008, Santos et al. 2008, Zhang et al. 2011, Zhao et al. 2011). In fish, the dominant hydrocarbon-degrading bacterium is Aeromonas spp., which prevails in the intestinal tract over Pseudomonas/Shewanella (Mickéniené and Syvokiené 2008). In addition, bacteria associated with hydrobionts participate in the process of self-purification of PAHs (Šyvokienė and Butrimavičienė 2013). The ratio of hydrocarbon-degrading bacteria to total heterotrophic bacteria is considered dependent on the species, specifically their nutrition habits and intensity, and on the season (Šyvokienė et al. 2011). In the same way, it has been proposed that hydrocarbon-degrading bacteria in the intestinal tract of fish participate in purification processes of PAHs in Rutilus rutilus, Gasterosteus aculeatus and Alburnus alburnus (Mickéniené and Syvokiené 2008). Nevertheless, by using RDA in the current study, we found that metabolites derived from benzo[a]pyrene, benzo[k]fluoranthene and indeno[1,2,3- cd]pyrene generated by the PM4 strain through the NDO system were conjugated with glutathione by GST activity of the fish. In this way, renal excretion of these conjugated metabolites is possible in the wild C. jordani.
CONCLUSIONS
The symbiotic relationship between Pseudomonas spp. strains with their host confers protection in vivo of fish against the effects of PAHs, a response named in this study toxicosymbiosis. However, this is a quite complex process not easily discernible based on the metabolic machinery of the fish and bacteria, because both organisms are able to biotransform and detoxify PAHs, and to conjugate the PAH-derived metabolites with endogenous substrates. Nevertheless, the diversity of these bacteria and their protective capacity depends on several factors (such as the density of the intestinal microbiota and the interspecific differences of the bacteria), which stresses the need to provide insights related to the challenge that involves the exposure to toxicants between hosts and their microbiota.











 nova página do texto(beta)
nova página do texto(beta)


