INTRODUCTION
Finding more efficient methods to extract metals from polymetallic sources, such as electronic and mine wastes, has become a very important topic, since current deposits are far from matching the increasing demand (Watling 2015).
Some metals, such as manganese oxides, act as refractory agents, which unfortunately are common components in silver ores mainly found in the southeastern USA, Latin America and China (Clevenger and Caron 1925).
In the presence of manganese oxides in ores, silver resists attacks by cyanide, since manganese minerals occlude silver by forming a structural bound. This is due to the fact that silver grains are very finely disseminated as isomorphism in the crystal lattices of manganese minerals.
Manganese itself may cause excessive consumption of cyanide as a consequence of complexes formation (Qiu et al. 2014). Pyrolusite (MnO2) is the most abundant manganese compound found in silver ores.
Usually, manganese is surrounding the dispersed silver compounds, making them inaccessible to the leaching solution. In addition, silver-manganese ores possess some silver compounds that are leaching-resistant, resulting in an increase of this ore’s refractoriness. Silver mineral is primarily found with higher oxides, which makes difficult its isolation and subsequent identification (Gandhi and Sarkar 2016). Furthermore, the recalcitrant nature of MnO2 limits conventional mineral recovery methods.
Other metals like As, Co, Pb and Zn are getting increasingly scarce, owing to a decreasing tendency of ore grades, insufficient environmental-friendly technologies and the increasing demand from developing countries (UNEP 2009, Graedel and Cao 2010). Some authors expect a depletion of Mn, Pb, Zn, As and Ag in approximately 50 years (Backman 2008, Dodson et al. 2012).
An option to minimize this problem is to take advantage of alternative sources of metals such as waste ores and tailings, which are usually abandoned at mine sites. Bioleaching technology is an environmental-friendly technology used to extract metals from complex mineralogical sources, such as tailings.
Bioleaching has several advantages: (i) short construction times, (ii) low cost, (iii) there is no need for a specialist, since bioleaching systems are easy to operate and maintain, (iv) it is possible to use native bacteria that are adapted to the specific complexity of polymetallic materials, and (v) it is possible to regenerate leaching media with the aid of microorganisms, which therefore minimize wastes (Akinci and Guven 2011, Johnson 2014, Watling 2015, Fonti et al. 2016).
Some authors observed simultaneous bioleaching of several metals from polymetallic matrixes (Ijadi Bajestani et al. 2014, Nguyen et al. 2015a, b). In order to take advantage of bioleaching in mine tailings, an important issue to consider is the relative high pH (9-11) of such matrices.
As wastes of a cyanide silver recovery process, the tailings were amended to a pH around 9-11, which is necessary for an efficient silver extraction (Li et al. 1992, Li and Wadsworth 1993, Sun et al. 1996, Jiang et al. 2015, Olyaei et al. 2016). Setting the pH to optimal values for bioleaching bacteria (pH ≤ 2) increases economic costs and restricts this technology to tailings with sufficient metals concentrations to make this option profitable (Mireles 2015).
Reports of polymetallic bioleaching are scarce, especially regarding polymetallic matrices at similar conditions to those commonly observed in the mining industry, i.e., high tailing concentrations (> 10 %) and high pH (> 2) (Akcil and Gahan 2015). Some authors reported that using native microorganisms is more efficient than many effective consortia constructed in the laboratories (Mousavi et al. 2007, Lavalle et al. 2008, Ghosh and Das 2017, Marchevsky et al. 2017). In addition, the exploitation of such microorganisms would eliminate the need to use standard laboratory cultures, particularly for large-scale studies (Keeling et al. 2005).
The aim of this research was to evaluate at flask level the effects of bioleaching processes using native strains with the following sets of variables: (a) pH (2.0, 4.0 and 6.0), tailings concentrations (10, 20 and 30 %, w/v), and two types of culture medium (9K and nonN); and (b) continuous supplementation of biogenic H2SO4 synthetized with two culture mediums (9K and nonN) at different tailings concentrations (10, 20 and 30 %, w/v).
MATERIALS AND METHODS
Tailings characterization
Tailings were kindly donated by First Majestic Silver Corp and obtained from La Encantada mine, located northeast of Torreón, Coahuila, Mexico. Mineral samples for bioleaching testing were obtained after the cyanidation process. The samples were taken from the tailings dump of La Encantada mine, then homogenized and stored in polyethylene containers at ambient temperature.
Tailings were characterized by X-ray diffraction (XRD, Rigaku Miniflex 600 Benchtop) as follows: 100 g of sample were taken and dried at 50 ºC for 24 h. After that, the sample was pulverized in an agate mill for 3 min and sieved to obtain a particle size of 50 μm. Then they were placed in a circular holder to be analyzed from theta 3.0 to 2-theta 90, 0.7 deg/min with steps of 0.02 deg. The X-ray (Cu K-alpha) was operated at tube voltage and current of 40 and 15 mA.
For the analysis in the inductively coupled plasma optical emission spectrometry (ICP-OES, Perkin Elmer 5300DV), 100 g of sample were dried at 50 ºC for 24 h. After that, the sample was pulverized in an agate mill for 3 min, then 2 mL of HNO3 were added and the sample was heated at 70 ºC for 30 min. Then 6 mL of HCl were added and heated again at 70 ºC for 2 h. Afterwards, 17 mL were taken and centrifuged at 3000 rpm. Finally, the supernatant was analyzed in the ICP-OES. The target metals were Mn, As, Pb and Zn.
Bacterial strain genetic characterization and culture conditions
Native strains were isolated from La Encantada mine. Bacteria isolation was performed from the wet tailings directly taken from the tailings dump. From this sample, 10 g were taken and incubated with 90 mL of 9K liquid medium at 160 rpm and 31 ºC by 30 days. The microorganisms suspended in the 9k liquid medium were used as inocula for subsequent cultures with 9K fresh liquid medium (Liu et al. 2007).
Bacterial native strains were spread in 9K liquid medium, with the following initial concentrations: 3.0 g/L (NH4)2SO4; 0.5 g/L MgSO4·7H2O; 0.1 g/L KCl; 0.5 g/L K2HPO4; 0.01 g/L Ca(NO3)2, and 44.22g/L FeSO4·7H2O.
Acclimation to the tailings was not performed; we assumed that the strains were already acclimatized to them. The pH was adjusted to 2.0 with H2SO4 2 N and incubated at 30 ºC and 160 rpm, in 500 mL baffled Erlenmeyer flasks.
Nitrogen-free medium (nonN) was used as an alternative to cultivate the isolate native strains. The nonN medium contained 44.22 g/L FeSO4·7H2O and 0.5 g/L K2HPO4; pH was adjusted to 2.0 with H2SO4 2 N.
Bacterial DNA was extracted following the procedure proposed by Tian et al. (2012).
One milliliter was taken from an overnight culture (1 × 108 cel/mL) and centrifuged at 13 000 rpm for 2 min. Cell pellet was resuspended in 300 mL of lysis buffer (EDTA, 0.05M; NaCl, 0.1M; pH 7.5) by gentle vortexing for 30 s; afterwards, 100 mL of lysozyme solution (10 mg/mL) and 30 µL of SDS solution, 20 % (w/v), were added.
The mix was incubated at 37 ºC for 5 min. DNA was purified using a mix of phenol-chloroform-isoamyl alcohol (25:24:1, vol/vol/vol). Extracted DNA was used as a template to amplify a conserved 16S ribosomal DNA (rDNA) region for total bacteria by polymerase chain reaction (PCR), using primer pair, forward 5′-CCGTCAATTCCTTTGAGTTT-3′ and reverse 5′-GTGCCAGCAGCCGCGGTAA-3′.
Approximately 20 ng of purified DNA were used per reaction combined with 2.5 μL of 10X PCR buffer, 1 μL of MgCl2 (25 mmol), 1 μL of dNTP mixture (20 mmol), 1 μL for each primer (10 mmol), 0.25 μL (5 U/μL) of GoTaq DNA polymerase (Promega); all dissolved in 18.25 μL of sterile milliQ water.
PCR was programed as follows: initial DNA denaturation at 95 ºC for 5 min, followed by 10 cycles of nesting (denaturation 30 s at 94 ºC, annealing from 65-55 ºC for 30 s, lowering temperature by 0.5 ºC for each cycle and a final extension of 30 s at 72 ºC). In addition, 30 cycles of 30 s at 94 ºC, 30 s at 55 ºC, 30 s at 72 ºC and a final extension at 72 ºC for 10 min were performed (T100TM Thermal Cycler, BIO-RAD).
The PCR product was sequenced and compared using the basic local alignment search tool through its official website (http://www.ncbi.nlm.nih.gov) (Altschul et al. 1990). The PCR products were sent for sequencing to the University Unit of Massive Sequencing and Bioinformatics of the National Autonomous University of Mexico.
A Genome Analyzer IIx (Illuminia) was used for sequencing. The Illumina protocol (Illumina 2007) used for the sample preparation is briefly described as follows:
Add ‘A’ bases to the 3’ end of the DNA fragments (adds an “A” base to the 3’ end of the blunt phosphorylated DNA fragments, using the polymerase activity of Klenow fragment (3’ to 5’ exo minus). This prepares the DNA fragments for ligation to the adapters, which have a single ‘T’ base overhang at their 3’ end).
Ligate adapters to DNA fragments (ligates adapters to the ends of the DNA fragments, preparing them to be hybridized to a flow cell).
Purify ligation products (purifies the products of the ligation reaction on a gel to remove all un-ligated adapters, remove any adapters that may have ligated to one another, and select a size-range of templates to go on the cluster generation platform).
Enrich the adapter-modified DNA fragments by PCR (uses PCR to selectively enrich those DNA fragments that have adapter molecules on both ends, and to amplify the amount of DNA in the library. Only 14 cycles of PCR are employed, to avoid skewing the representation of the library.)
Experimental design
All experiments were carried out for 17 days to improve the target metals solubilization, in accordance with some authors (Gericke et al. 2008, Lavalle et al. 2008, Nguyen et al. 2015b).
For the first set of experiments, the effects on metals removal of the following parameters were evaluated: initial pH (2.0, 4.0 and 6.0), tailings concentration (10, 20 and 30 %, w/v [111.11, 250.00 and 428.57 g/L, respectively]) and (NH4)2SO4 addition (9K and nonN medium).
Experiments were performed in 500 mL baffled Erlenmeyer flasks. In the flasks, 100 mL of the respective medium was inoculated with 10 % v/v of native bacterial culture supernatant and incubated at 30 ºC and 160 rpm. Initial pH and tailings concentration were adjusted following the experimental design previously described.
The purpose of the second set of experiments was to evaluate the effect of: (i) biogenic H2SO4 medium continuous supplementation, (ii) tailings concentration, and (iii) (NH4)2SO4 in pre-biogenic H2SO4 mediums on metals removal. This set of experiments was performed as follows:
First, pre-biogenic H2SO4 mediums were obtained from cultures grown in 9K and nonN mediums. The cultures were performed in 500 mL baffled Erlenmeyer flasks: 100 mL of the respective medium were added and inoculated with 10 % v/v of native bacterial culture supernatant at pH of 2.0, which was adjusted with H2SO4 2 N.
Cultures were incubated in a rotatory shaker at 160 rpm and 30 ºC for 2-5 days until Fe2+ was completely oxidized. After the precipitate was removed, the solutions were analyzed to determine the concentrations of Fe3+.
Next, 111.11, 250.00 and 428.57 g/L (10, 20 and 30 %, w/v) of tailings were added to 500 mL baffled Erlenmeyer flasks and volume was adjusted to 100 mL using their respective oxidized media (9K or nonN).
Experiments were carried out by triplicate for 17 days. Initial pH of 6.0 was adjusted with H2SO4 2 N in all experiments.
Continuous supplementation of the fresh biological oxidized medium was added to the respective tailings concentration every 24 h for 17 days (Fig. 1). The medium was separated by centrifugation at 1500 rpm for 15 min. Later, the fresh biological oxidized medium was added to the respective tailings concentration and incubated at 160 rpm and 30 ºC.
Monitoring and control techniques
Changes in pH and oxidation-reduction potential (ORP) were monitored regularly at intervals of 24 h for 17 days, using a Hanna pH/ORP Meter HI 2114P.
The concentration of Fe2+ was determined by titration of potassium dichromate with N-phenylanthranilic acid as an indicator (Welcher 1963). The concentration of Fe3+ was determined by titration of EDTA at pH 2.0 with sulfosalicylic acid as an indicator (Davis and Jacobsen 1960). Responses were Mn, As, Pb and Zn removal, quantified by ICP-OES as described in the tailings characterization section. All determinations were performed by triplicate.
Statistical analysis
Full factorial design was used to evaluate: (i) the effects of initial pH (2.0, 4.0, and 6.0), tailings concentration (10, 20 and 30 %, v/w) and two types of culture medium (9K and nonN) for the first set of experiments, and (ii) tailings concentration (10, 20 and 30 %, v/w) with continuous supplementation of two biogenic acids mediums (9K and nonN) for the second set of experiments. The response was the removal of Mn, As, Pb and Zn. The design was developed and analyzed using commercial software Minitab 17® (Minitab, State College, PA, USA).
RESULTS AND DISCUSSION
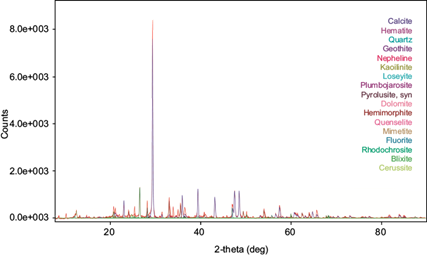
The XRD characterization of tailings (Fig. 2) indicated that calcite is the mayor component (56.2 %), followed by the minor components (2-10 %) Fe, Si, Mn and Zn in the forms of hematite, goethite, quartz, nepheline, kaolinite and loseyite, and finally by the trace components (< 2 %), represented in general by compounds with Pb, Mn, Zn and As, such as rhodochrosite, plumbojarosite, pyrolusite, hemimorphite and mimetite among others. Tailings composition and abundances are shown in table I.
TABLE I TAILINGS COMPOSITION
| Element | Abundance (%) | Compound formula | Abundance (%) |
| Mn | 2.93 | Calcite: CaCO3 | 56.2 |
| Pb | 2.35 | Hematite: Fe2O3 | 7.6 |
| Zn | 1.96 | Goethite: FeO(OH) | 7.5 |
| As | 0.30 | Quartz: SiO2 | 6.1 |
| Ag | 142 g/t | Nepheline: (Na,K)AlSiO4 | 3.8 |
| Kaolinite: Al2Si2O5(OH)4 | 2.9 | ||
| Loseyite: (Mn,Zn)7(OH)10(CO3)2 | 2.7 | ||
| Rhodochrosite: MnCO3 | 1.9 | ||
| Plumbojarosite: Pb(Fe3(OH)6(SO4)2)2 | 1.7 | ||
| Pyrolusite: MnO2 | 1.7 | ||
| Dolomite, CaMg(CO3)2 | 1.6 | ||
| Hemimorphite: Zn4(Si2O7)(OH)2·H2O | 1.5 | ||
| Quenselite: PbMnO2(OH) | 1.4 | ||
| Mimetite: Pb5(AsO4)3Cl | 1.1 | ||
| Fluorite: CaF2 | 1 | ||
| Blixite: Pb 2Cl(O,OH)2-x | 0.9 | ||
| Cerussite: PbCO3 | 0.4 |
Note: All data are averages from replicates and standard deviation was lower than 5 %
Certain components in tailings can affect the performance of native bioleaching strain, specifically CaCO3 (Fig. 2), since it neutralizes H2SO4 produced by the bacteria, thus decreasing acid leaching (Lombardi and García 2002).
Calcite is present in tailings as a waste from silver extraction by cyanidation, and, as mentioned above, tailings were previously exposed to this process. For an efficient cyanidation it is very important to set pH to alkaline conditions (Li et al. 1992, Li and Wadsworth 1993, Sun et al. 1996).
Additionally, bioleaching microorganisms able to oxidize either inorganic sulfur compounds and/or iron (II) are extremely acidophilic (pH < 3.0) (Rohwerder et al. 2003). Nevertheless, bioleaching activity was observed in all the experimental units (Table II).
TABLE II METALS REMOVALS EFFICIENCIES OBSERVED IN ALL EXPERIMENTAL UNITS
| Tailings concentration (%, w/v) |
Batch Initial pH |
Semicontinous Initial pH |
|||||||
| 2 | 4 | 6 | 6 | ||||||
| 9K | nonN | 9K | nonN | 9K | nonN | 9K | nonN | ||
| Manganese removal (%) | |||||||||
| 10 | 93.49ab | 90.21bc | 37.17e | 35.95e | 37.01e | 36.02e | >99a | >99a | |
| 20 | 83.49cd | 80.37d | 36.80e | 36.18e | 33.24e | 32.12e | 96.23a | >99a | |
| 30 | 20.94f | 18.04<f | 17.31f | 16.05f | 15.86f | 14.11f | 90.98ab | 95.52ab | |
| Arsenic removal (%) | |||||||||
| 10 | 13.92f | 12.11fg | 9.60gh | 8.88gh | 9.04gh | 8.54gh | >99a | 85.33b | |
| 20 | 7.63h | 7.32h | 7.12h> | 7.18h | 7.86gh | 6.85h | 53.12c | 48.00d | |
| 30 | 6.03h | 5.01h | 5.96h | 5.10h | 6.08h | 5.44h | 53.10c | 42.66e | |
| Lead removal (%) | |||||||||
| 10 | >99a | 95.54abc | >99a | 96.32ab | >99a | 98.21a | >99a | >99a | |
| 20 | 95.74abc | 94.28abcd | >99a | 94.25abcd | 88.51bcde | 85.56def | >99a | 95.23ab | |
| 30 | 80.43ef | 78.58f | 80.85ef | 78.29f | 86.6cdef | 84.22cdef | >99a | 85.13cdef | |
| Zinc removal (%) | |||||||||
| 10 | 66.45de | 61.45efg | 69.44bcd | 65.23def | 77.99a | 74.14ab | 68.39bcd | 67.43cd | |
| 20 | 20.78j | 19.52j | 58.97fg | 55.28gh | 77.78a | 74.52abc | 67.42cd | 49.83hi | |
| 30 | 20.59j | 17.21j | 57.91g | 55.44gh | 61.54efg | 60.98efg | 60.58f | 45.92i | |
Note: All data are averages from replicates, standard deviation was lower than 5 %. Different letters indicate significant differences (p < 0.05)
Bioleaching bacteria produce the adherence of extracellular polymeric substances (EPS) to minerals and provide a site between the metal sulfide surface and the cells in which iron (III)-ions are accumulated (Bellenberg et al. 2014). This site creates microenvironments with conditions for bioleaching processes (Yu et al. 2008, 2011).
Isolated native strains showed 99 and 97 % of DNA similarity with Leptospirillum ferriphilum and Leptospirillum ferrooxidans, respectively. Isolated native strains also showed proliferation, Fe2+ oxidation and medium acidification in nonN medium, similar to the strain in reported by Corbett (2011).
However, no authors reported the presence of genes involved in N2 fixation in L. ferriphilum (Levicán et al. 2008, Corbett 2011), suggesting that this microorganism can use alternative mechanisms to assimilate nitrogen such as the ATP supplied by Fe-S clusters for fixing nitrogen (Tyson et al. 2004, Ram et al. 2005). The genes responsible of the Fe-S cluster assembly for nitrogen fixation are nifS-nifU-hesB-hscB-hscA-fdx-orf1 (Parro and Moreno-Paz 2003).
Additionally, L. ferrooxidans is able to fix atmospheric nitrogen and can act as a nitrogen fixer for both isolated strains. For N2, L. ferrooxidans can use the nifHDKENX operon to encode the structural component of molybdenum-iron nitrogenase, make modifications to their chemotaxis, and use a copper-transporting ATPase (Parro and Moreno-Paz 2003, Tyson et al. 2005, Sato et al. 2009).
The mediums 9K and nonN showed similar behavior in ORP and pH-time ratio at the same tailings concentrations (Figs. 3, 4). The highest removals of metals (93.49, 13.92 and > 99 % for Mn, As and Pb, respectively) were found at initial pH 2.0 and tailings concentrations of 10 %. The final pH was < 4.0 and ORP > 300 mV.
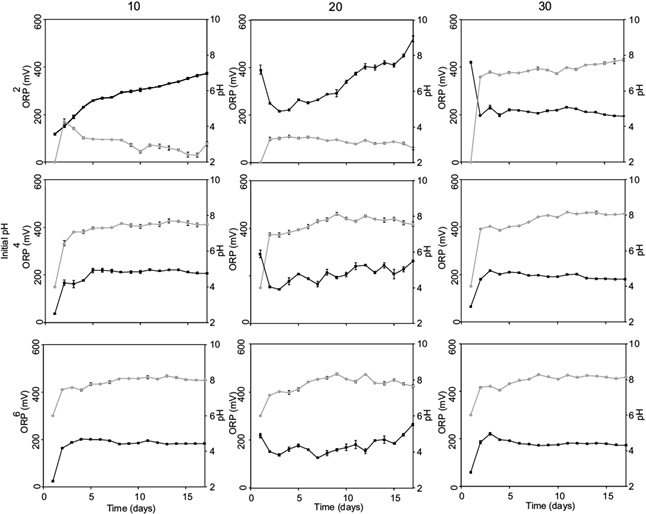
Fig. 3 Oxidation-reduction potential (ORP) and pH time course at three tailing concentrations, three initial pH and medium with nitrogen source (9K). Oxidation-reduction potential: ■; pH: 
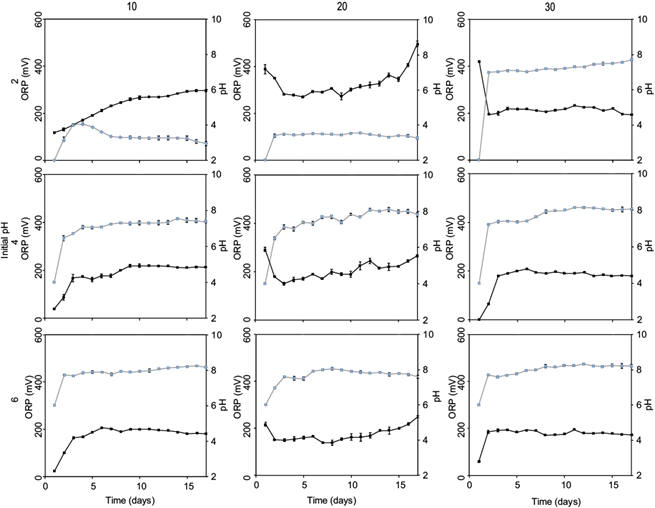
Fig. 4 Oxidation-reduction potential (ORP) and pH time course at three tailing concentrations, three initial pH and medium without nitrogen source (nonN). Oxidation-reduction potential: ■; pH: 
Several authors have reported the highest metals removal at low solids concentration and pH (Peng et al. 2008, Gericke and Pinches 1999, Zhang et al. 2010, Tian et al. 2012, Yan-Fei et al. 2013, Yang et al. 2015).
Ahmadi et al. (2015) reported bioleaching of copper (55 %), nickel (98.2 %) and cobalt (59.5 %) after 30 days at 5 % (w/v) of tailings density. Tailings were a low-grade sulfide ore from the Golgohar iron mine, Iran. Experiments were carried out at flask level with a stirring rate of 150 rpm at 45 ºC and inoculated with a mixture of moderately thermophilic culture. The medium contained a nitrogen source and yeast extract (0.02 % [w/w]). Under our conditions, bioleaching was probably driven by biogenic H2SO4, evidenced by low pH values (Figs. 3, 4).
Askari-Zamani et al. (2005) evaluated rhenium bioleaching from molybdenite concentrate. Maximum rhenium extraction (7.3 %) was obtained with a native strain of Acidithiobacillus ferrooxidans isolated from the same mine where the concentrate was extracted. The experiment was performed at flask level with 10 % of molybdenite concentrate, 200 mL of iron-free 9K medium and 9 g of energy source for bacteria growth (ferrous sulfate, pyrite or elemental sulfur powder). Flasks were incubated in an orbital shaker at 120 rpm and 35 ºC.
On the other hand, the lowest removals of metals were found at high initial pH (4.0 and 6.0) and high concentrations of tailings (20 and 30 %): 14-36, 5-7, and 92-78 % for Mn, As and Pb, respectively (final pH was > 7.0 and ORP < 300 mV).
High tailings concentrations decreased Mn, As and Pb removal, probably by the lower availability of oxygen and CO2 dissolved in the aqueous phase, resulting in a restricted H2SO4 of both biogenic production and bacterial Fe2+ ion oxidation.
Therefore, bacterial activity (pH and redox) at high pulp concentrations needs more time to achieve similar performances as the ones obtained using low pulp concentrations. Low bacterial growth reduces H2SO4 production, and, consequently, it reduces the Mn, As and Pb solubilization rate, as observed in this research.
Also, this tendency is probably enhanced by the presence of CaCo3 in tailings. High CaCo3 concentration was observed in tailings (56.2 %), acting as an alkalinizing agent and consuming H2SO4 biosinthetized by L. ferriphilum and L. ferrooxidans.
In reference to the potential (Eh)-pH diagram (Takeno 2005), highest Mn, As and Pb removals were obtained when Fe2+ iron was the predominant component (final pH < 3.0 and ORP 200-500 mV).
The lowest Mn, As and Pb removals were observed when Fe2O3 was the predominant component (final pH 7.0-8.0 and ORP around 200 mV). The highest Zn removal was observed at initial pH of 6.0. ORP and pH were 200 mV and 8.0 in average, respectively. According to Takeno (2005), Zn can be present as Zn2+ or ZnOH.
H2SO4 by itself is incapable to solubilize MnO2. the presence of a reducing agent (such as H2O2 or Fe2+ ion) is necessary to catalyze the Mn solution (Jiang et al. 2004, Bafghi et al. 2008, Tian et al. 2012), as shown in reactions 1 and 2.
Ferrous ion is added to the medium as an electron source for L. ferriphilum. H2O2 formation is mediated by the reaction of ferrous and ferric iron with dissolved oxygen in water. As Cohn et al. (2006) reported: (i) H2O2 synthesis with superoxide (O2•)- acts as an intermediate species mediated by dissolved O2 and Fe(II) via the Haber-Weiss reaction mechanism, and (ii) Fe(III) extracts an electron from a water molecule, resulting into a hydroxyl radical and then combining two hydroxyl radicals into an H2O2 formation.
Initial pH, tailings concentration and type of medium showed a significant effect on Mn, As and Zn removal (p < 0.05).
Carbonated mineral species observed in tailings, such as rhodochrosite (MnCO3) and loseyite [(Mn,Zn)7(OH)10(CO3)2], can be dissolved by direct protonation catalyzed by biogenic H2SO4 (Luo and Millero 2003).
Steger (1976) reported the complete dissolution of rhodochrosite in 8 % of H2SO4 (pH ≈ 0.85), whilst Macías et al. (2012) observed high dissolution of Zn and Mn (77 and 86 %, respectively) in loseyite by using an oxidizing medium (8.8 mol/L H2O2 and pH 2.0), compared to Zn and Mn dissolution (12 and 10 %, respectively) by aqua regia digestion (12 mol/L HCl and 15.8 mol/ HNO3 in a 3:1 ratio).
For all the media evaluated in this report with high pH and tailings concentrations, probably the removal of metals obtained was mainly influenced by chemical lixiviation catalyzed by Fe2+ and biogenic H2SO4 (reaction 2) (Nguyen et al. 2015b).
However, Lombardi and García Jr. (2002) found the highest Mn removal (80 %) in medium at 300 mV of redox potential and pH of 3.0 inoculated with A. ferrooxidans (formerly Thiobacillus ferrooxidans). L. ferriphilum and L. ferrooxidans probably improve the metals leaching by EPS.
Pseudomonas putida strain MnB1 EPS can enhance the dissolution of rhodochrosite in mediums with pH ranging from 5.0 to 8.0. This effect can be attributed to N-H, C=O and C-H groups found in EPS (Wang and Pan 2014).
Tailings concentration and type of medium presented a significant effect on Pb removal (p < 0.05). Mimetite (Pb5(AsO4)3Cl) and hemimorphite (Zn4(Si2O7)(OH)2·H2O) can be dissolved by protonation.
Turek et al. (2015) observed in mimetite five and 11 times more As and Pb dissolved at pH of 2.5 compared to 3.75, respectively. McPhail et al. (2003) reported hemimorphite solubilization at pH 3.0-6.0 and 50 ºC.
Plumbojarosite (Pb(Fe3(OH)6(SO4)2)2) is a recalcitrant mineral. Patiño et al. (2014) observed its solubilization at pH 11.20, and Viñals and Núñez (1988) at pH 0.75, demonstrating that only extreme pH values can solubilize this compound. High redox and pH < 3 probably drive the leaching in the experiments with low pH and tailings concentration.
Results obtained from the first set of experiments showed three significant positive correlations (Table III):
TABEL III PEARSON´S CORRELATION MATRIX OF BATCH REGIME EXPERIMENTAL UNITS (P-VALUE IS REPORTED IN PARENTHESIS)
| Variable | Initial pH | Tailing concentration | Mn removal | As removal | Pb removal | Zn removal |
| Initial pH | 1 | 0.000 (1.000) | -0.568 (0.000) | -0.243 (0.077) | -0.013 (0.927) | 0.735 (0.000) |
| Tailing concentration | 1 | -0.593 (0.000) | -0.842 (0.000) | -0.849 (0.000) | -0.467 (0.000) | |
| Mn removal | 1 | 0.759 (0.000) | 0.609 (0.000) | -0.148 (0.286) | ||
| As removal | 1 | 0.725 (0.000) | 0.379 (0.005) | |||
| Pb removal | 1 | 0.338 (0.012) | ||||
| Zn removal | 1 |
Mn-As removal correlation presumably reveals simultaneous dissolution of mimetite and loseyite, since these minerals are dissolved at pH 3.0-2.0; Zn removal-initial pH correlation is associated to high Zn removal found at initial pH 6.0; and As-Pb removal correlation probably indicates dissolution of mimetite.
Pb and As removal had the highest negative correlation with tailings concentration, which could be explained by the abatement of biogenic leaching agent (H2SO4).
Bioleaching in continuous supplementation of biogenic H2SO4, synthetized in two culture media at different concentrations of tailings are shown on table II and figure 5.

Fig. 5 Oxidation-reduction potential (ORP) and pH time course of biogenic ferric ion oxidized with nitrogen source (9K) and oxidized without nitrogen source (nonN) at three tailing concentrations and two biogenic ferric ion source. Oxide reduction potential: ■; pH: 
Continuous supplementation of acid medium can catalyze metals solubilization at high tailings concentrations, since results similar to the highest values in the first experiment were obtained. It is evident that the production and sustentation of leaching media are essential to achieve an effective process (Nguyen et al. 2015b).
The media 9K and nonN showed similar pH and ORP. However, as tailings concentrations increased, removals of metals were significantly lower (p > 0.05) in experiments with nonN medium compared to 9K medium.
High concentrations of tailings require more leaching agents since high abundance of mineral compounds can consume protons from the continuous biosynthesis of H2SO4 while reducing Fe3+ ion.
In general, removals of metals in continuous supplementation of biogenic H2SO4, synthesized in two types of culture medium (9K and nonN) at different tailings concentrations (10, 20 and 30 %, w/v) were higher or similar than the first experimental set.
Zn removal obtained in our experiments did not show a significant difference (p > 0.05) compared to the first experimental set. In the same way, treatments with a 9K acid medium were similar compared to a nonN acid medium.
These results are in agreement with Nguyen et al. (2015b), who reported 90 and 30 % of Mn and Zn solubilization, respectively, in a medium without nitrogen sources (experiments were carried out at 5 % of sediment and inoculated with Acidithiobacillus ferrooxidans).
Continuous supplementation of fresh medium, rich in Fe3+ ions and H2SO4, resulted in an improved leaching of Mn, As, and Pb.
CONCLUSIONS
In the first set of experiments, at low tailings concentration (< 20 %) and low initial pH (2), the main effects over metals removals was the acid medium in combination with high redox. It is interesting to note that the the highest Zn removal was observed at an initial pH of 6.
The second set of experiments showed that a medium without nitrogen added can achieve similar metals removal (Mn and Pb) in all conditions as compared to 9K medium.
The use of a medium without nitrogen source opens an opportunity to increase benefit-cost ratio by removing metals from tailings that would otherwise remain neglected, thus demonstrating the feasibility of using extra-cellular culture supernatants to catalyze mining bioleaching at competitive rates and efficiencies.











 nueva página del texto (beta)
nueva página del texto (beta)