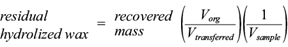INTRODUCTION
Polyhydroxyalkanoates (PHA) are natural, renewable and biocompatible biopolymers, produced intracellularly in bacteria, with great importance due to environmental issues regarding accumulation of non biodegradable plastic waste. PHA are stereospecific isotactic polymers synthesized by many microorganisms. They are classified according to the length of the side chain of their monomers: short chain (scl-PHA), with three to five carbon atoms and medium chain length (mcl-PHA), six to 14 carbon atoms (Liu et al. 2011). The composition and amount of intracellular PHA are related to the carbon source, the C/N ratio, microorganisms used and the synthesis process (Jiang 2010).
Several studies have evaluated various renewable carbon sources, such as canola oil (Rathinasabapathy et al. 2013) and used cooking oil, which have demonstrated great biotechnological potential for the production of mcl-PHA (Cruz et al. 2015). During the biosynthesis of PHA by Cupriavidus necator (formerly Wautersia eutropha > Ralstonia eutropha > Alcaligenes eutrophus) from fatty acids, López-Cuéllar et al. (2011), Rathinasabapathy et al. (2013) and Inomata et al. (2014) demonstrated that C. necator was able to synthesize mcl-PHA using fructose and canola oil as substrates in a three stages fed-batch system, although it has been reported that the PHA synthase of C. necator is strictly specific for scl-PHA monomers (Slater et al. 1992).
The synthesized mcl-PHA presented three medium chain length comonomers, 3-hydroxyvalerate (3HV), 3-hydroxyoctanoate (3HO), and 3-hydroxydecanoate (3HD) bound to 3HB. This copolymer showed improvement in its thermal and mechanical properties (melting temperature [Tm] = 176 ºC) compared to pure PHB (Tm = 179 ºC). Loo and Sudesh (2007) synthesized the copolymer poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) [P(3HB-co-3HHx)] with incorporation of 5 mol % of 3-hydroxyhexanoate (3HHx) units that reduce the melting point from 180 ºC to less than 155 ºC. Due to this characteristic, PHA have attracted much attention as a biodegradable alternative to traditional petrochemical plastics and can replace these materials in many applications.
Budde et al. (2011) described a method for the development of R. eutropha cultured in vegetable oil using emulsifying agents. They reported that Triton X-100 at concentrations of 0.05 % (v/v) in presence of vegetable oil can emulsify 1 % (v/v) palm oil crop, rendering it into an available carbon source. Like vegetable oils, beeswax is a renewable carbon source that has a great demand on the world market. Beeswax has a wide range of uses in cosmetics manufacturing, candle processing, agriculture, and pharmaceutical and food industries (Zhang and Xiao 2013). These manufacturing processes require first-class beeswax that has not been overheated. Unpurified raw wax and its waste may be potential substrates for mcl-PHA synthesis. In terms of cost of substrates for fermentation, the average price of unpurified organic beeswax is 3 to 8 USD/kg (Bradbear 2004), which is comparable to the 6.5 to 7.8 USD/kg cost of sodium octanoate/sodium caprylate (Alibaba 2017).
Beeswax is a mixture of wax acid esters, and it has not been reported as a carbon source for PHA synthesis. When beeswax is methanolyzed with alkali, it generates hydrolysates rich in fractions of fatty alcohols and acids (Maia and Nunes 2013). Some studies suggest that fatty alcohols can be converted to fatty acids providing precursors for synthesis of mcl-PHA by β-oxidation (Jiang 2010). This study evaluated the effect of Hw as an additional carbon source in the culture medium, on the chemical and thermal properties of scl-mcl-PHA obtained during the accumulation phase of C. necator in a three-stages fed-batch system.
MATERIALS AND METHODS
Microorganism and medium
C. necator ATCC 17699 was propagated in Luria Bertani broth (LB) and incubated at 30 ºC. The inoculum for all assays was prepared by transferring 100 mL of seed flask cultivation with LB medium, incubated for 36 h at 150 rpm and 30 ºC on a rotary shaker (Excella E24, New Brunswick Scientific). The fermentation medium of stage 1 (batch culture), first fed-batch culture in stage 2 (biomass production), and second fed-batch culture in stage 3 are described in table I (synthesis of mcl-PHA fractions). The trace element solution was defined by Shang et al. (2004).
TABLE I COMPOSITION OF THE CULTURE MEDIUM
| Reagents | Batch culture (g/L) | First fed-batch (biomass production) (g/L) | Second fed-batch (synthesis of mcl-PHA fractions) (g/L) |
| Glucose | 20 | 30 | 0 |
| (NH4)SO4 | 4.0 | 0.35 | 0 |
| MgSO4(7H2O) | 1.2 | 1.20 | 1.2 |
| KH2PO4 | 4.5 | 4.50 | 4.5 |
| Citric acid | 1.7 | 1.70 | 1.7 |
| Trace element solution (mL/L) | 10 | 10 | 10 |
| Hydrolized wax | 0 | 0 | 5 |
mcl-PHA: medium chain length polyhydroxyalkanoates
Fermentation
The cultures were done in triplicate, in a 5 L fermentation unit (FA-5000, VICHI). The content of the seed flask (10 % v/v) was transferred to a fermentation medium under the same conditions described above, pH 7.0 controlled with 2 M NaOH and 0.47 M HCl. Samples were taken every 3 h to quantify glucose, ammonium sulphate, biomass and PHA. The fermentation was done in a modified three stages method (López-Cuéllar et al. 2011):
Stage 1: Initial batch cultivation with C/N = 14, and 3.9 L volume.
Stage 2: Biomass production by a fed batch system. A glucose solution of 30 g/L was fed at 50 mL/h (MasterFlex L/S Model 77200-62) (Chakraborty et al. 2012) to achieve a high density culture.
Stage 3: Synthesis of scl-mcl-PHA under nitrogen limitation in the culture medium, using Hw as a co-substrate (together with remaining glucose from the previous stages) at a concentration of 5 g/L, in presence of 0.43 g/L Triton X-100 (Sigma-Aldrich), three times its CMC (Budde et al. 2011) added at 0.33 L/h.
Analytical techniques
Dry weight (X) was determined by gravimetry, defining residual biomass (rX) as the concentration of X without PHA (Budde et al. 2011). Glucose concentration was determined by the DNS method (Barbosa et al. 2005). Quantification of ammonium sulphate was carried out with the Weatherburn method (López-Cuéllar et al. 2011). The amount of residual beeswax hydrolysates (rHw) was defined as the concentration of recovered Hw, subtracting the centrifuged sample concentration determined by the modified equation described by Budde et al. (2011):
where V org is the volume of the organic phase after the extraction. Because the solvent mixture includes methanol, not all the solvent remains in the organic phase after contact with the aqueous medium. We measured V org to be 15 mL when using chloroform/methanol 10:5. V transferred was 5 mL and V sample (volume of medium taken from the culture) was 10 mL.
PHA purification
PHA was purified using chloroform extraction during 10 min with reflux and then it was precipitated with cold hexane after filtering cellular residues (Nurbas and Kutsal 2004). This procedure was repeated three times to carry out the purification of the polymer and avoid the presence of debris cells and possible residual metabolites present in beeswax. The exceeding solvent was removed by evaporation (R-210, BUCHI).
PHA characterization
Nuclear magnetic resonance (NMR) was carried out to elucidate the structure of PHA (Avance PDX-300, Bruker). Deuterated chloroform (CDCl3) was used as solvent. The spectra corresponding to 1H and 13C were analyzed with specialized software (MestReNova V. 6.0). Experiments were performed using a differential scanning calorimeter (DSC) (Mettler-Toledo DSC822e). The samples (8 mg) were encapsulated in aluminum pans and heated in temperature range from -80 to 220 ºC using a heating rate of 10 ºC/min, taking the second run as valid to determine glass temperatures (Tg) and melting temperatures (Tm) (Nurbas et al. 2004).
scl-mcl-PHA synthesis
The profile of biomass and PHA production in fed-batch fermentation are shown in figure 1. C. necator produced 13.64 g/L of total biomass at the end of the fermentation, when 0.43 and 5 g/L of Triton X-100 and Hw respectively were aggregated and ammonium sulphate was exhausted (Fig. 2c).
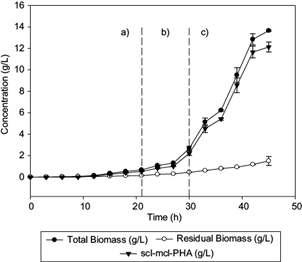
Fig. 1 Profiles of total biomass, residual biomass, and polyhydroxyalkanoate, observed in (a) batch culture, (b) first fed-batch (biomass production), and (c) second fed-batch (synthesis of medium-chain-length polyhydroxyalkanoates (mcl-PHA) fractions, during fermentation of C. necator with glucose and beeswax as carbon sources. Lines represent the standard deviation
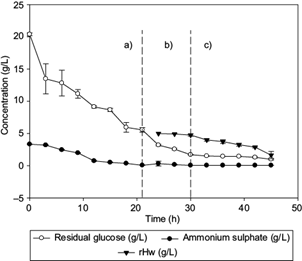
Fig. 2 Profiles of residual ammonium sulphate concentration, residual glucose, and hydrolyzates (Hw) consumption (residual hydrolyzates [rHw inside the fermentor]), observed in (a) batch culture, (b) first fed-batch (biomass production), and (c) second fed-batch (synthesis of medium-chain-length polyhydroxyalkanoates [mcl-PHA]) fractions, during fermentation of C. necator with glucose and Hw as carbon sources. Lines represent the standard deviation
The lag phase lasted 12 h, similar to Volova et al. (2014), and the maximum specific growth rate (µ) in the fed batch stage was 0.20/h, as reported by Chakraborty et al. (2012). The initial ammonium sulphate and glucose concentrations were 3.3 and 20.39 g/L, respectively (Fig. 2a), with a consumption rate of 0.52 and 0.7 g/L/h, respectively, for 21 h (first stage of fermentation). After 21 h of culture, 0.3 g/L of ammonium sulphate concentration was simultaneously fed with the waxy carbon source (Fig. 2b), having a consumption rate of 0.13 g/L/h in order to maintain the metabolism of C. necator and stress conditions necessary for intracellular accumulation of PHA (Loo and Sudesh 2007).
At this stage, the feeding of fresh culture medium with Hw (C/N = 200) as co-substrate was started. An increase of the intracellular PHA concentration from 1.08 to 13.64 g/L was observed (Fig. 1c), which is attributed to inhibition of the cell growth and accumulation of the waxy carbon source and the remaining glucose from previous stages (Khanna and Srivastava 2007). The Hw concentration decreased 3.35 g/L in the culture medium during the last 21 h of culture, with a consumption rate of 0.45 g/L/h (Fig. 2c).
Shang et al. (2004) registered the same consumption rate for R. eutropha NCIMB 11599 when feeding it with < 1.20 g/L of valeric acid and glucose to produce P(3HB-co-3HV). The Yp/s of Hw at the end of fermentation was 0.33 gp/gs. Barbosa et al. (2005) reported a Yx/s of 0.1785 gx/gs for the production of PHB using glucose. The Yx/s obtained in this work was higher (0.225 gx/gs). This indicates that Hw is a co-substrate (together with the remaining glucose in the previous stages) that accumulates PHA quickly under stress conditions (Fig. 1c).
Loo and Sudesh (2007) suggested that concentrations of NH4Cl lower than 0.5 and 5 g/L of palm kernel oil promote the production of 87 % w/w of PHA. During the PHA biosynthesis, it was observed that intracellular production of the polymer is favored as the concentration of Hw decreases in the reactor (Fig. 2c). In our work, the amount of synthesized scl-mcl-PHA obtained after 45 h during the third stage of the fed-batch culture, corresponds to 65.3 % w/w of the total biomass generated (13.64 g/L).
In this regard, hydrolyzed beeswax ans its wastes are an attractive carbon source for the mass production of sustainable biopolymers. At the end of experiments, 95 % w/w of Triton X-100 was recovered. The remaining 5 % w/w should have been left in cell package (Budde et al. 2011).
Nuclear magnetic resonance (NMR)
The spectra of 1H NMR of scl-mcl-PHA isolated from C. necator when grown on Hw as carbon source (third stage fed-batch culture) are shown in figure 3a. The corresponding signal to methine of the monomeric fraction of 3HB is observed as a sextet 5.24 ppm, which is a stereogenic center bonded to oxygen, methyl and methylene of PHA (Nurbas and Kutsal 2004, Impallomeni et al. 2011, Liu et al. 2011). This shift to low field is exerted by the deprotection effect caused by the electronegativity of the oxygen atom.
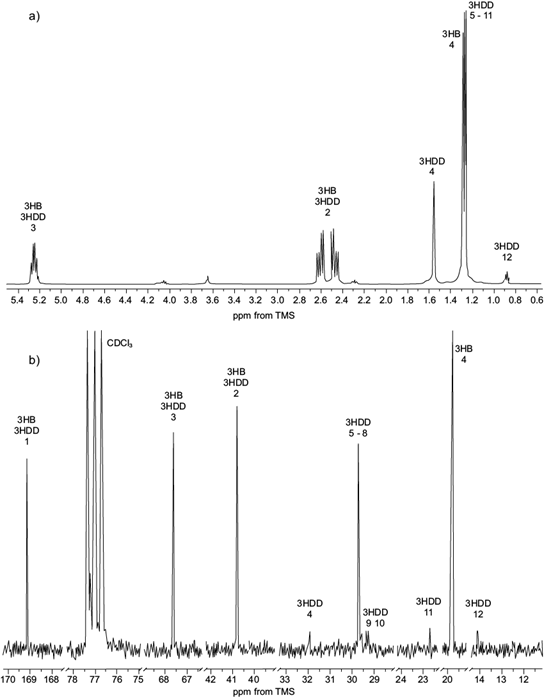
Fig. 3 Nuclear magnetic resonance (NMR) spectra at 300 MHz of short- or medium-chain-length polyhydroxyalkanoates (scl-mcl-PHA) obtained by C. necator: 3-hydroxybutyrate (3HB), 3-hydroxydecanoate (3HD) in deuterated chloroform (CDCl3). Chemical shifts are in parts per million downfield from tetramethylsilane (TMS). (a) 1H spectra, (b) 13C spectra
The methine groups of mcl-PHA monomers overlap with the signal of the methine of 3HB. However, it should be seen as a quintuple signal, as a result of links with both methylene groups of the main chain and the aliphatic chain. The characteristic signals of 3HB and mcl-PHA monomers methylene groups are observed as an ABX system, because methylene protons are diastereotopic, and these are attached to the methine group from the main chain. The center of the signal for both monomers is observed at 2.50 ppm, as reported by several studies on PHA (Nurbas et al. 2004, Impallomeni et al. 2011, Liu et al. 2011, López-Cuéllar et al. 2011).
An additional signal was observed in the aliphatic region of the spectra with a displacement of 0.88 ppm, which is characteristic for terminal methyl groups of mcl-PHA monomers (Impallomeni et al. 2011, Liu et al. 2011, López-Cuéllar et al. 2011). The signal of 3HB methyl group observed at 1.32 ppm. However, it appears as a triplet due to signal overlaping with the signals corresponding to the methylene group of mcl-PHA monomers (Impallomeni et al. 2011, Liu et al. 2011).
The signal is a chemical shift of 1.56 ppm corres- ponding to the methylene of the aliphatic chain attached to the stereogenic center of mcl-PHA monomers (Liu et al. 2011) originating from fatty acids contained as esters in the beeswax. The 13C NMR spectra of the obtained biopolymer is shown in figure 3b. The signals corresponding to the carbon, the methine, the methine and the methylene were visualized in 169.0, 67.6 and 40.7 ppm, respectively, for the monomers. The chemical shift for the methyl group is observed at 19.8 ppm for HB while mcl-PHA monomers are observed in 14.1. ppm.
The remaining signals of the methylene groups of the aliphatic chain suggest the presence of 3HD monomer, observed between 22 and 35 ppm (López-Cuéllar et al. 2011). Liu et al. (2011) reported the chemical shift of C-5 to 3-hydroxydodecanoate (3HDD) in 32 ppm; in this study, it was observed at 29.7 ppm, confirming the presence of 3-hydroxydecanoate (3HD) together with other monomers of mcl-PHA that do not appear in the 13C NMR spectra. By 1H NMR spectra, performing the integration of signals of the methyl, methylene and methine groups it was determined that the mcl-PHA monomers were in a proportion of 4.6 mol % over the 3HB. The monomer composition of the esters of fatty acids obtained in the mcl-PHA are consistent with those reported for biodegradable plastics.
Thermal characterization
DSC for the obtained scl-mcl-PHA (Fig. 4b) showed an endothermic transition at 79.8 ºC with a DHm of 45.6 J/g. This δHm is similar to that reported by López-Cuéllar et al. (2011) (δHm 41.44 J/g) for a mcl-PHA with 3HV, 3HO, 3HDD and 3HB monomers. Liu et al. (2011) obtained a PHA with 3HD monomers using a strain of Pseudomonas putida KTQQ20 inhibiting the β-oxidation, which consumed decanoic, dodecanoic, and tetradecanoic acids.
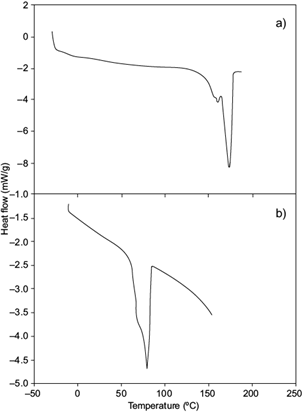
Fig. 4 Thermogram of short- or medium-chain-length polyhydroxyalkanoates (scl-mcl-PHA) synthesized by Cupriavidus necator using glucose and hydrolyzates (Hw) as carbon source. (a) Poly-3-hydroxybutyrate (P3HB), (b) poly-3-hydroxybutyrate-3-hydroxydecanoate (P3HB-co-3HDD)
The obtained polymer had 3HD and 3HDD monomers, P(16 mol % 3HD-co-84 mol % 3HDD) with a Tm of 78 ºC, attributed to the presence of 3HDD and 3HD monomers, P(16 mol % 3HD-co-3HDD 84 mol %) and a δHm of 41 J/g, similar to that obtained in this study and reported for mcl-PHA (López-Cuéllar et al. 2011).
The Tm in this mcl-PHA was 79.82 ºC, lower than the one reported by López-Cuéllar et al. (2011); 133 ºC. This result suggests that the scl-mcl-PHA obtained may contain a percentage of medium-chain monomers. Also, the monomer length presented fractions of 3-hydroxydodecanoate (3HDD), which reduce the recrystallization of polymeric material and consequently its melting point (Khanna and Srivastava 2007), with respect to PHB (Fig. 4a).
Volova et al. (2014) reported this thermal phenomenon, realizing that as the molar fraction of 3HB, 3HV and 3HHx was modified (92.6, 1.3, 6.1 to 71.4, 26.1, 2.5 mol %, respectively) in the terpolymer P(3HB/3HV/3HHx), the degree of crystallinity of the polymer decreased (from 62 to 53 mol %), but the Tm increased (from 173 to 175 ºC respectively). When mcl-PHA was obtained by gradually heating (-80 ºC to 220 ºC) Tg was observed at -52 ºC. Impallomelli et al. (2011) reported similar values for mcl-PHA (Tg -52 to -43 ºC), with similar characteristics to elastomeric materials, due to the crystallization of comonomers of medium chain length into the PHA.
During the cooling of scl-mcl-PHA, a sequence reverse transition with hysteresis (exothermic peaks) was observed at 112.33 ºC with a δHc of 36.13 J/g (data not shown). We suggest that this decline of δHc in the cooling process can be described as recrystallization by the possible presence of PHB crystals dispersed among medium chain length monomers (López-Cuéllar et al. 2011). Impallomeni et al. (2011) hypothesize that such biodegradable polymeric materials crystallize with the participation of both the main chain and the side chains in a layered order, similarly to what happens with polymers carrying long side chains.
CONCLUSION
C. necator was able to synthesize scl-mcl-PHA using Hw as carbon source under N limiting conditions in a three stages fed batch fermentation system. The increase in the consumption rate of Hw showed that the carbon source is assimilated by C. necator, favoring the production of scl-mcl-PHA inclusions. Adding hydrolyzed beeswax in the presence of Triton X-100 did not affect the growth of C. necator, which makes it a promising strategy that facilitates an economically feasible process of PHA production.
The 1H and 13C RMN analysis determined that the obtained polymer has a relationship of 4.6 mol % of mcl-PHA monomers due to the incorporation of Hw fractions during the biosynthesis. The thermal properties of these materials are affected by the addition of medium chain length monomers, which reduce their melting point. The scl-mcl-PHA synthesized in this study may be used in agriculture, radio electronics, medicine, and pharmacology as an environmentally friendly material to replace petrochemical plastics.











 nueva página del texto (beta)
nueva página del texto (beta)