INTRODUCTION
Soil contamination with polycyclic aromatic hydrocarbons does not only affect soil processes, but might damage a whole ecosystem as the pollutant might end up in rivers, ground water and aquifers (Shaheen et al. 2014). In 2015, the Mexican inventory of contaminated sites by oily sludges, solid wastes (tows, batteries, paints, cleaning plants, etc.) and spent caustic sodas were about 1161.93 ha (PEMEX 2016). Oil and its derived products contain aromatic hydrocarbons, such as benzene, toluene, ethylbenzene and xylenes (BTEX). These compounds might cause cell damage, cancer and leukemia and they are genotoxic and neurotoxic (Deziel et al. 2014, Gui-Zhen et al. 2015). Toluene is especially hazardous for the environment and humans as it is toxic and its solubility in water might pose a human health risk (Daghio et al. 2016).
The rate of removal of toluene from soil is important so as to limit the damage to the environment. Autochthonous soil microorganisms are known to degrade toluene. For instance, Jindrova et al. (2002) reported that bacteria, such as Pseudomonas sp., P. mendocina KRI, P. paucimobilis and Burkholderia cepacia G4, contribute to the degradation of BTEX in a gasoline-contaminated soil and groundwater. Zhang et al. (2013), using 14C-labeled toluene, found that phylotypes belonging to Paenibacillus, Microbacterium, Rhodococcus mineralized 43 to 49 % of it, confirming their ability to metabolize this compound. While Yadav et al. (2012) and Zhang and Bouwer (1997), reported between 98 % and a complete degradation of toluene respectively.
In pre-Hispanic times the lakes that surrounded Tenochtitlan (now Mexico City) covered 2000 km2. Lake Texcoco, the largest of those lakes, covered 1000 km2 and was divided by dikes so that brackish and freshwater were kept separated. From the 17th century onwards, the original lakes were drained to prevent flooding of Mexico City (Luna-Guido et al. 2003). The former lakebed is alkaline saline (pH 9-10.5 and sometimes EC > 100 dS/m) and a drainage system has been installed to wash the salts from the soil. In the 70s, the Comisión Nacional de Agua (CNA, the Mexican national water commission) started a program of drainage of saline soils and to establish vegetation. The most saline parts were flooded with effluents from a nearby treatment plant, decreasing salinity for several years, achieving near normal soil conditions and in reducing the salt content and pH substantially from electrolytic conductivity (EC) > 50 dS/m and pH > 10 to EC < 10 dS/m and pH < 8.5 (Luna-Guido et al. 2000). A unique ecosystem has been formed that allows to study how soil processes respond to high alkalinity. It can be speculated (Fernández-Luqueño et al. 2016) that degradation of hydrocarbons might be different in an alkaline soil with a high organic matter content than a more neutral arable soil with a low organic matter content.
As part of the study into the removal of hydrocarbons from soil, an arable soil and an alkaline soil were collected at two locations: Otumba and the former Lake Texcoco, respectively, both in the State of Mexico (Mexico). The two soils were contaminated with three different concentrations of toluene (100, 200 or 500 mg/kg), while dynamics of toluene were monitored in the soil and the headspace of the 120 cm3 glass flasks for 20 days. The objective of this study was to investigate the removal of toluene from two contrasting soils.
MATERIALS AND METHODS
Soil was collected from the 0-15 cm layer of two different locations in State of Mexico (Mexico). A first sampling site was located in the former Lake Texcoco in the Valley of Mexico City. The details of the sampling site and soil characteristics can be found in Dendooven et al. (2010). The sampling site is located in a drained part of the former lake Texcoco, which lies at an altitude of 2240 masl (19º30′48.00″N - 98º59′25.14″W). The site was not contaminated with hydrocarbons upon sampling and is flooded irregularly with sewage effluent that might contain hydrocarbons. The mean annual temperature at the sampling site is 16 ºC with a mean annual precipitation of 705 mm mostly from June until October. The pH in the saturated soil extract was 8.3, particle size distribution in the loamy sand soil was clay 96 g/kg, silt 93 g/kg and sand 811 g/kg, the electrolytic conductivity (EC) 2.68 dS/m, the water holding capacity (WHC) 860 g/kg and the organic carbon content of 48 g/kg all on a dry matter base.
The second sampling site was located in Otumba, State of Mexico (19º41′23.52″N - 98º43′14.28″W). The details of the sampling site and soil characteristics can be found in Aguilar-Chávez et al. (2012). Briefly, its average altitude is 2349 masl and characterized by a sub-humid temperate climate with a mean annual temperature of 14.8 ºC and average annual precipitation of 577 mm mainly from June through August. The area is mainly cultivated with maize and common bean, receiving a minimum amount of inorganic fertilizer without being irrigated. The pH in the saturated soil extract was 7.6 and the particle size distribution in the loamy sand soil was clay 90 g/kg, silt 40 g/kg and sand 870 g/kg. The EC in the soil extract was 1.15 dS/m, the WHC 650 g/kg and the organic carbon content 6.3 g/kg all on a dry matter base. The field based replication (three soil samples from both sites) was maintained in the laboratory experiment.
The soil (560 g of each soil) was adjusted to 40 % of WHC and pre-incubated separately for 7 days in a drum containing a beaker with 100 mL of water to avoid desiccation and one beaker with 100 mL of 1 M NaOH solution to trap CO2 evolved. Seven different treatments were applied to the six soil samples, i.e. three from Otumba and three from Texcoco. Sterilized and unsterilized soil was amended with 100, 200 or 500 mg C7H8/kg dry soil, while unsterilized and unamended soil samples served as control.
Forty-two subsamples of 10 g of each soil (n = 2) and replicate (n = 3) were added separately to 120 cm3 glass flasks. Half of the subsamples were first sterilized for three consecutive days at 120 ºC for 30 min. Seven subsamples of the sterilized and unsterilized soil of each soil and replicate were amended with 100 mg C7H8/kg dry soil (equivalent to 91.25 mg C/kg dry soil), 200 mg C7H8/kg dry soil (equivalent to 182.5 mg C/kg dry soil) or 500 mg C7H8/kg dry soil (equivalent to 456.25 mg C/kg dry soil). Seven samples of each soil (n = 2) and replicates (n = 3) were left unamended and served as control. A 2 cm3 vial containing 1 cm3 2 M NaOH was placed in the flasks and the flasks were stoppered airtight. After 0, 1, 2, 3, 6, 10 and 20 days, three flasks from each treatment (a flask from each soil sample) were selected at random. The headspace was analyzed for toluene on a gas chromatograph, the flasks were opened, the vial with NaOH removed and the CO2 trapped was quantified by automatic titration with 0.1 M HCl on an auto-titration system Metrohm SM 702 Titrino (Herisau, Switzerland) (Jenkinson and Powlson 1976a). The soil was removed from the flasks and extracted for toluene as described below.
The soil samples were analyzed for particle size distribution by the hydrometer method (Gee and Bauder 1986). While total organic carbon was measured with a total organic carbon analyzer TOC-VCSN (Shimadzu, Canby, USA). Total nitrogen (N) was measured by the Kjeldahl method using a digestion made with concentrated H2SO4, K2SO4 and HgO to digest the sample and the solution is then distilled, which converts the ammonium salt to ammonia. The amount of nitrogen present in the sample is determined by back titration (Bremner 1996). The water holding capacity (WHC) was measured as described by Jenkinson and Powlson (1976b). Briefly, the WHC was determined by subtracting a given mass of a dry soil sample from the mass of the same saturated with water, left to drain overnight throughWhatman No. 42 filter paper and covered with aluminum foil to avoid evaporation. The EC was measured in a saturated solution extract and pH in 1:2.5 soil-H2O suspension using a glass electrode. The toluene concentration in the soil was determined by a modified technique as described by Song et al. (1995). Briefly, 1 g soil was added to a Pyrex tube and 5 cm3 acetone was applied. The tubes were placed in an ultrasonic bath at 25 ºC for 30 min, mechanically shaken on a vortex for 10 sec, and sonicated again for 30 min at 130 W in a FS30H Ultrasonic Cleaner (Fisher Scientific, Suwanee, GA, USA). The extracts were separated from the soil by centrifugation at 3500 rpm and 4 ºC for 10 min. This process was repeated twice. The extracts were combined and adjusted to 10 cm3 with acetone. The concentration of toluene in the volatile organic compounds was quantified on a flame ionization detector (FID) fitted with 15 m a HP-5 column, with injection temperature of 150 ºC, oven temperature 40 ºC and detector temperature 280 ºC. The flow rate of He was 16.7 cm3/min.
Significant differences between the emission of CO2 and concentration of toluene in the soil and the headspace as a result of the different treatments applied were determined by analysis of variance and based on the least significant difference using the general linear model procedure PROC GLM (SAS Institute 1989).
RESULTS AND DISCUSSION
In the sterilized Texcoco soil spiked with 500 mg C7H8, the concentration of toluene did not change significantly over time (Fig. 1a). Approximately 310 mg C7H8/kg dry soil was extracted from the sterilized soil applied with 500 mg C7H8, 113 mg C7H8/kg dry soil when applied with 200 mg C7H8 and 60 mg C7H8/kg dry soil when applied with 100 mg C7H8. In the unsterilized Texcoco soil, nearly all toluene (< 20 mg remained) was removed within one day, independently of the amount added.
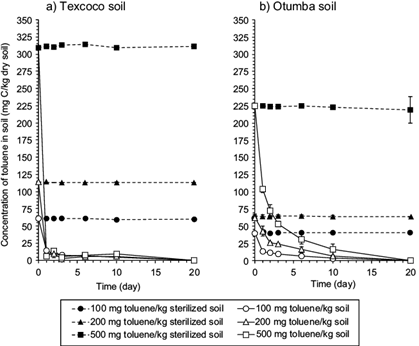
Fig. 1 Concentration of toluene (mg C/kg dry soil) in a) sterilized or unsterilized soil of the former Lake Texcoco and (b) the Otumba soil. Sterilized soil amended with 100 (●), 200 (▲) or 500 mg toluene/kg dry soil (■) and unsterilized soil amended with 100 (○), 200 (∆)or 500 mg toluene/kg dry soil (□)
In the sterilized arable soil spiked with 500 mg C7H8, the concentration of toluene did not change significantly over time as occurred in the Texcoco soil (Fig. 1b). Approximately 225 mg C7H8/kg dry soil was extracted from the sterilized soil applied with 500 mg C7H8, 63 mg C7H8/kg dry soil when applied with 200 mg C7H8 and 40 mg C7H8/kg dry soil when applied with 100 mg C7H8. In the unsterilized arable soil, the removal of toluene was slower than in the Texcoco soil, independently of the amount added (Fig. 1a). For instance, > 100 mg/kg dry soil remained in soil amended with 500 mg C7H8 after one day. Even after 10 days, the amount of C7H8 extracted from soil amended with 500 mg was still 16 mg C7H8/kg dry soil.
In this work the concentration of toluene did not change significantly over time in the sterilized soil. This indicated that no abiotic factor affected the removal of toluene from soil and sequestration of toluene was limited. Tsao et al. (1998) stated that 14 % of the added 737 mg C7H8/kg was not extractable from a soil after 30 days, that is, it was sequestered. Differences in the amount of toluene extracted might be due to the length of the incubation and the extraction technique and the soil characteristics (Davis and Madsen 1996). The incubation time of the sterilized soil was short, so that the sequestration of toluene was minimal, but it can be speculated that an increased contact between the contaminant and the soil will increase the fixation of hydrocarbon on the soil matrix and thus reduce the mineralization of toluene (Yang et al. 2010a, Woods et al. 2011).
The concentration of toluene in the headspace of the sterilized arable and alkaline soil did not change significantly over time (Fig. 2a). However, the amount of toluene in the headspace of the flasks incubated with the Texcoco soil was lower than in the headspace of the arable soil. The concentration of toluene in the headspace of the Texcoco soil dropped and within 2 days nearly all toluene was removed from the headspace, independently of the amount added to soil (Fig. 2b). In the flasks with arable soil, some toluene was still detectable in the headspace after two days.
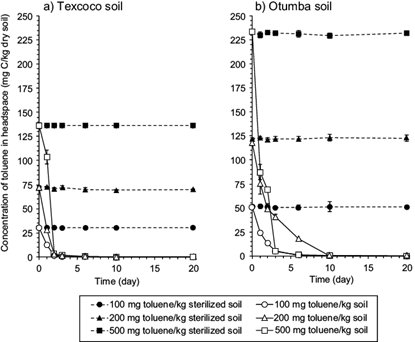
Fig. 2 Concentration of toluene (mg C/kg dry soil) in the headspace of a) sterilized or unsterilized soil of the former Lake Texcoco and (b) the Otumba soil. Sterilized soil amended with 100 (●), 200 (▲) or 500 mg toluene/kg dry soil (■) and unsterilized soil amended with 100 (○), 200 (∆) or 500 mg toluene/kg dry soil (□)
Toluene is highly volatile and a large amount of it added to soil was detected in the headspace. It can be assumed that some toluene got lost during application, some was not extracted or some got lost through the stoppers. In a separate experiment, (flasks without soil and NaOH) the amount of toluene lost through the stoppers was < 1.5 mg/kg. Overall, 98 % of the 500 mg toluene added to soil was recovered (sum of the toluene in the headspace and in the sterilized soil). Similar percentages were recovered when 100 or 200 mg toluene was added to the Texcoco and the arable soil.
It is well known that soil microorganisms degrade hydrocarbons from soil and even polyaromatic hydrocarbons, such as benzo(a)pyrene, are removed from soil. Most of the toluene was removed within two days. In different studies, different removal rates of toluene from soil have been reported. Tsao et al. (1998) found that nearly 60 % of 14C-labelled toluene added to a sandy loam soil at 0.003 mL/g was removed from soil within 7 days and all of it within 25 days. Zhang and Bouwer (1997) reported that in pre-equilibrated soil-water slurries with pH ranging from 6.8 to 8 and amended with 200 mg C7H8/L; the contaminant was completely removed from soil within 14 h. Lee et al. (2012) reported that in a soil with pH 6.7 and amended with 500 mg C7H8/L removal of the contaminant took approximately 10 days. The removal of toluene from the arable soil was similar to that described by Lee et al. (2012), while in the Texcoco soil it took only 3 days.
The two soils used in this experiment were selected as they are contrasting. On one hand, an extreme alkaline saline soil flooded irregularly with effluent that might contain hydrocarbons and on the other hand an arable soil never contaminated with hydrocarbons (Luna-Guido et al. 2000). These two contrasting soils allow us to determine if degradation of hydrocarbons would be affected by the different ecosystem characteristics. In this study, the removal of toluene was faster from the alkaline soil than from the arable soil. Different explanations are possible for this phenomenon. First, the microorganisms in the Texcoco soil metabolized the toluene more efficiently than those in the arable soil. However, it would be difficult to explain why microorganisms in the arable soil were less capable of removing toluene than those from the alkaline soil. Second, a higher microbial activity will stimulate the removal of a contaminant from soil. However, the emission of CO2, an indicator of microbial activity was larger from the arable soil than from the Texcoco soil, although the removal of toluene was larger from the latter than from the first. Third, the availability of the toluene in the arable soil was lower than in the alkaline soil so that the removal was lower in the first than in the latter. The bioavailability of toluene was indeed larger in the Texcoco soil than in the arable soil, as its removal was faster and the percentage mineralized larger in the first than in the latter. The bioavailability of a contaminant is affected by different soil characteristics. First, an increase in organic matter is known to decrease the removal of contaminants from soil as they might get fixed on it (Yang et al. 2010b). However, the soil organic matter was lower in the arable soil than in the alkaline soil. Second, clay particles are known to fix organic material rendering them unavailable for degradation. Davis and Madsen (1996) reported that an increase in the percentage of silt and clay in the soil increases the residence time of toluene, indicating that these two components adsorbed toluene reducing its bioavailability. However, the clay content was similar in the Texcoco and the arable soil. Third, a high salt content in soil is known to disperse soil particles and reduce the amount of soil aggregates thereby limiting the amount of organic material that can protected physically within the aggregates. Indeed, the EC of the Texcoco soil was higher than that in the arable soil and might thus have increased bioavailability and degradation of toluene.
Most of the toluene in the headspace was mineralized within 10 days. The amount of toluene in the headspace of flasks incubated with the arable soil was larger than in the alkaline soil. As such, more toluene volatilized from the arable than from the alkaline soil, although the removal of toluene was faster from the alkaline than the arable soil. Once again soil characteristics will define how much of the added toluene is fixed on the soil matrix and how much will volatilize. It appears, however, that the soil characteristics that controlled bioavailability were different from those that define volatilization. Voutsas et al. (2005) reported that the amount of water, organic matter content, porosity, vapor pressure, solubility of the contaminant, absorption coefficient, air flow, moisture and temperature are factors that control the volatility of an organic compound from soil. In this study, incubation conditions were similar for both soils. Consequently, soil water content, porosity, the coefficient of adsorption and organic matter content, defined the amount of toluene volatilized.
The organic C content was lower in the arable soil than in the alkaline soil although emission of CO2 was larger from the first than from the latter (Fig. 3a, b). The increase in emission of CO2 in soil amended with toluene compared to the unamended soil showed a lag of two days in both soils. As such the degradability of the soil organic matter was lower in the Texcoco soil than in the arable soil. The maize crop residue retained in the arable soil is easily mineralizable, but the soil organic material in the Texcoco soil was more resistant to degradation. Considering no priming effect (Kuzyakov et al. 2000), then 60 % of the toluene applied to the arable soil was mineralized, while 80 % in the Texcoco soil after 20 days. Mineralization of easily decomposable organic material (toluene) was not inhibited in the Texcoco soil.
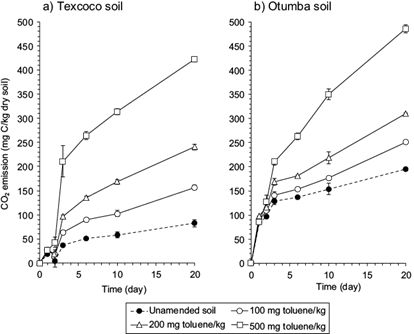
Fig. 3 Emissions of CO2 (mg C/kg dry soil) from a) unsterilized soil of the former Lake Texcoco and (b) the Otumba soil. Unamended soil (●) or soil amended with 100 (○), 200 (∆) or 500 mg toluene/kg dry soil (□)
Tsao et al. (1998) found that nearly 56 % of 14C-labelled toluene added to a sandy loam soil at 3 µl/g was mineralized within 4 weeks. Mineralization of toluene, considering no priming effect was faster in this study. Differences in mineralization rates between soils depend on soil characteristics, especially clay and silt content (Chung and Alexander 2002).
An increase in emission of CO2, which is the result of mineralization of the contaminant, was only apparent after 2 days. Consequently, there was a lag between the break-up of the aromatic ring and its mineralization. Zhang and Bouwer (1997) reported that a pre-equilibrated soil-water slurry amended with 200 mg C7H8/L showed a lag phase of 3 days. Similarly, Davis and Madsen (1996) mentioned a lag phase in the degradation of toluene applied at a concentration of 50 mg/kg in soil with pH of 5.4, 7.8 and 7.9. It can be hypothesized that the lag phase is a result of the absorption of toluene within the cells. Once in the cells, toluene can be metabolized along eight different metabolic pathways (Jindrová et al. 2002). None of the pathways reported included extracellular decarboxylation reactions so the toluene must first be transported into the cell before being metabolized explaining the lag.
CONCLUSIONS
No abiotic process affected the concentration of toluene in soil. In the unsterilized soil, toluene was quickly removed independent from the amount applied. Within two days 73 %, 59 % and 69 % was dissipated from the arable soil and 88 %, 93 % and 96 % when 100, 200 or 500 mg C7H8/kg was applied, respectively. The removal of toluene was faster from the alkaline than from the arable soil. The mineralization of toluene as evidenced by an increase in emissions of CO2 showed a lag phase of two days compared to the decrease in the concentration of toluene. Considering no priming effect, then approximately 81 % of the toluene added to the Texcoco soil and 62 % in the Otumba soil was mineralized. Consequently, most of the toluene was removed from the soil and its effect on the environment will thus be limited.











 text new page (beta)
text new page (beta)


