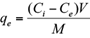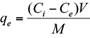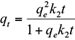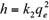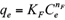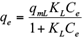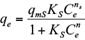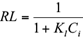INTRODUCTION
Toxic heavy metals can enter the environment from different natural and anthropogenic sources (Salazar-Camacho and Villalobos-Peñalosa 2017). The presence of toxic heavy metals in aqueous environments can be harmful to humans and living organisms even at low levels (Sparks 2003). Thus, it is essential to remove heavy metals from contaminated waters for protection of the aquatic ecosystem’s health.
Various physical and chemical methods are available for removal of heavy metals from wastewaters, including chemical precipitation (Kurniawan et al. 2006), coagulation-flocculation (López-Maldonado et al. 2014), electrochemical treatments (Mohammadi et al. 2005), membrane filtration (Chen et al. 2006), ion exchange (Inglezakis and Poulopoulos 2006, Inglezakis and Loizidou 2007), and biosorption (Milojković et al. 2016). Among these methods, biosorption is an eco-friendly and cost-effective technology for removal of metals contained in effluents (Milojković et al. 2016).
In recent years, different organic materials have been chosen for biosorption of heavy metals from contaminated water (Fomina and Gadd 2014). Among organic materials that exhibit high affinity for metal cations, activated carbon appears to be the most promising, but it is very expensive (Moussavi and Barikbin 2010).
Removal of heavy metals from water and wastewater by raw agricultural waste is a low-cost treatment method (Šćiban et al. 2008). Various raw agricultural waste materials have been used to eliminate toxic heavy metals from contaminated water, such as cork waste (López-Mesas et al. 2011), pistachio by-products (Moussavi and Barikbin 2010), sugarcane bagasse waste (Ullah et al. 2013), tea waste (Albadarin et al. 2013), tree bark powder (Munagapati et al. 2010), and pine cone shell (Martín-Lara et al. 2016). To utilize the high efficacy and simplicity of biosorption for the treatment of metal contaminated water, further investigation on low-cost biosorbents is essential.
Pistachio (Pistacia vera L.) is an important tree nut crop cultivated in arid and semi-arid regions of the world. Four major pistachio producers in 2014 were Iran (415 531 t), the United States (23 3 146 t), Turkey (80 000 t) and China (76 943 t) (FAO 2017).
Every year, a large amount of pistachio hull is generated in large quantities as waste, creating environmental problems. For example, approximately 250 000 t of pistachio by-products are produced each year in Iran (Moussavi and Barikbin 2010).
Although a large number of studies on the removal of heavy metal ions from water and wastewater using different raw agricultural wastes exist in the literature, every special material requires individual research. Pistachio hull has been evaluated for the removal of dye (Deniz and Kepeki 2016) and chromium (Moussavi and Barikbin 2010) from wastewater. In the present study, an attempt was made to use pistachio hull powder as an inexpensive adsorbent for the removal of Cd(II) and Pb(II) ions from aqueous solutions.
Biosorption is a complex process involving different mechanisms and controlled by diverse factors that can interact. Factors such as the solution pH, the nature of adsorbent materials, metal ion concentrations, metal ion properties and ionic strength can affect the adsorption of metal ions by adsorbents (Deniz and Kepeki 2016). A better understanding of the kinetics and effective parameters on biosorption of metals by adsorbents will provide engineers and scientists information to implement better strategies for dealing with contaminated soil and water (Hamidpour et al. 2011).
The ability of pistachio hull powder to remove Cd(II) and Pb(II) was investigated as a function of the initial metal ions concentration, the contact time and pH. The equilibrium and kinetic data of biosorption were tested using three isotherm models (Freundlich, Langmuir and Sips) and two kinetic models (pseudo-first-order and pseudo-second-order). The Fourier transform-infrared (FTIR) spectroscopy analysis before and after adsorption of Cd(II) and Pb(II) onto pistachio hull was also performed to explain the mechanism of biosorption.
MATERIALS AND METHODS
Reagents and adsorbent
All reagents used were extra pure analytical grades: Pb(NO3)2, Cd(NO3)2 and Ca(NO3)2. 4H2O (Merck, Germany). Pistachio hull was obtained from a local pistachio processing factory (Rafsanjan, Iran). The collected sample was washed with deionized water and dried in an oven at 50 °C for 24 h, grinded, and sieved through a 270 mesh sieve. The cation exchange capacity (CEC) was measured using the Na-acetate method (Rhoads 1986). The CEC of the pistachio hull powder was 77 cmol(+)/kg.
Infrared spectrums of untreated and Cd- and Pb-loaded adsorbent were obtained using a FTIR spectrometer (Perkin-Elmer Spectrum 100 FTIR-ATR Spectrometer) in the range of 450-4000 cm−1.
Adsorption procedure
The adsorption experiments of Cd and Pb were separately conducted in batch experiments using 0.1 g of adsorbent in a background electrolyte and at room temperature (23 ± 2 °C). The background electrolyte (0.01 M Ca(NO3)2) was used to fix the ionic strength of solutions. All samples were prepared in duplicate. Blank samples were prepared without adsorbent for all experiments.
The effects of contact time (0-24 h), initial pH values (pHi 3-10), and initial metal concentration (50-200 mg/L for CdENT#091;IIENT#093; and 100-1000 mg/L for PbENT#091;IIENT#093;) on the adsorption process were investigated. The pH values of the suspensions were adjusted to desired values by adding predetermined volumes of 0.03 M HNO3 or NaOH solutions. The suspension pH was not adjusted during the experiment and the final pHs of suspensions were measured. Suspensions were shaken for 24 h with a rotary shaker at a speed of 150 rpm. After each adsorption experiment, the suspensions were centrifuged at 5000 g for 10 min, the supernatant filtered and Cd(II) and Pb(II) concentrations were determined by an AAnalyst Perkin-Elmer 200 Atomic Absorption Spectrophotometer (AAS). The operating conditions of the adsorption experiments are summarized in table I. The metal adsorption capacity of pistachio hull was calculated by the mass balance equation:
TABLE I PARAMETERS AND OPERATING CONDITIONS IN SORPTION EXPERIMENTS
| Sorption experiments | Initial metal concentration (mg/L) | pH of suspension | Shaking time (h) | Adsorbent dose (g/L) | |||
| Cd(II) | Pb(II) | Cd(II) | Pb(II) | ||||
| Time-dependent | 5-48٭ | 88.7-883٭ | 5.5 | 5.5 | 0.5-24 | 10 | |
| pH-dependent | 8 | 100 | 3-8 | 4-10 | 24 | 10 | |
| Isotherm | 50-200 | 100-1000 | 5.5 and 6.5 | 5.5 and 6.5 | 24 | 10 | |
٭Approximately, on the basis of 10 and 100 % of the maximum sorption capacity (SCmax) of the biosorbent. The SCmax of the pistachio hull waste was determined in a preliminary study
where q e is the adsorbed amounts of Cd(II) or Pb(II) (mg/g), C i the initial metal concentration in the solution (mg/L), C e the equilibrium metal concentration (mg/L), M the mass of the adsorbent used (g), and V the volume of the solute solution (L).
Theoretical basis
The adsorption kinetic data were tested with the pseudo-first-order and pseudo-second-order models. Eqs. (2) and (3) represent non-linear forms of pseudo-first-order and pseudo-second-order kinetic models, respectively (Ho and McKay 1999).
where q e and q t are the amount of the metal ions adsorbed (mg/g) at equilibrium and at time t (h), respectively, and k 1 (1/h) and k 2 (g/mg/h) are the pseudo-first-order and pseudo-second-order equilibrium rate constants, respectively.
On the basis of pseudo-second-order models, the initial adsorption rate h (mg/g/h) at t = 0, can be defined as:
The Freundlich, Langmuir and Sips isotherms were used to fit the equilibrium data of Cd(II) and Pb(II) onto pistachio hull. Eqs. 5-7 represent non-linear form of Freundlich, Langmuir and Sips isotherms, respectively (Ho et al. 2002).
where q e and C e are the amount of adsorbed metal per unit mass of adsorbent (mg/g) and equilibrium liquid phase concentration (mg/L), respectively, and K F (L/g) and n F (-) are the Freundlich constants characteristics. K F and n F are indicators of adsorption capacity and adsorption intensity, respectively (Hamidpour et al. 2011); q mL (mg/g) and K L (L/mg) are the Langmuir constant related to the maximum adsorption and energy of adsorption, respectively; and q ms is the monolayer adsorption capacity (mg/g), and K s (L/mg) is the Sips constant related to energy of adsorption.
The parameters of adsorption kinetics and isotherms were determined by the non-linear curve fitting analysis method using the DataFit statistical software.
Chemical species of Cd(II) and Pb(II) in the solutions were also predicted using Visual MINTEQ, a computer code developed to simulate equilibrium processes in aqueous systems (Visual MINTEQ 2013).
RESULTS AND DISCUSSION
FTIR spectra
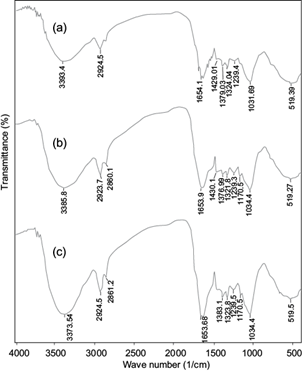
The cell wall of pistachio hull consists of lignin, hemicelluloses and cellulose that have different functional groups in their structure (Deniz and Kepekci 2016). The FTIR spectra of pistachio hull powder before and after Cd(II) and Pb(II) adsorption are presented in figure 1. The broad band around 3393 cm-1 in pure pistachio hull powder is attributed to stretching vibrations of hydroxyl (-OH) and amine (-NH2) groups (Moussavi and Barikbin 2010). This peak shifted from 3393 to 3385 cm-1 for Cd(II) and to 3373 cm-1 for Pb(II) and these bands became more intense after Cd(II) and Pb(II) adsorption onto pistachio hull waste. The peaks around 2920 and 2860 cm-1 may be assigned to C-H deforming vibration, and the sharp band centered around 1654 cm-1 can be related to the aromatic C=O ring stretching or C=C stretching vibration of aromatic groups (Moussavi and Barikbin 2010). These peaks were greatly intensified after adsorption of Cd(II) and Pb(II). The bands in 1300-1100 cm-1 can be attributed to stretching vibrations of C-O and C-H groups (Deniz and Kepekci 2016). After adsorption, a shift in the positions of the C-O and C-H group peaks occurred. This shift indicated that the Cd(II) and Pb(II) forms chemical bonds with these functional groups of the biosorbent. The sharp and intense peak at 1030 cm−1 can be assigned to the C-O-C, P-O or P-OH stretching (Deniz and Kepekci 2016) which shifted to 1034 cm−1 after adsorption of Cd(II) and Pb(II). The FTIR results show that the pistachio hull waste has a variety of functional groups, such as carboxyl, hydroxyl and amine, and these groups are likely involved in the biosorption of Cd(II) and Pb(II). Generally, biosorption of heavy metal ions onto biosorbents involves complex mechanisms of ion exchange, chelation (surface complexation), and adsorption by physical forces (Park et al. 2010, Fomina and Gadd 2014). Similar findings have been reported for sorption of Cd(II), Cu(II) and Pb(II) to Acacia leucocephala bark powder (Munagapati et al. 2010) and sorption of Cr(VI) to pistachio hull waste biomass (Moussavi and Barikbin 2010). Also, the FTIR spectrum of Pb-loaded pistachio hull waste was similar to that observed for Cd(II) (Fig. 1b, c).
Kinetics
The kinetics of adsorption describe the rate of metal removal from a solution and the time required to reach equilibrium, which is important for the understanding of the biosorption process and adsorbent performance. The biosorption capacity of pistachio hull as a function of time and initial concentration of Cd(II) and Pb(II) is shown in figure 2. For Cd(II), the equilibrium was achieved in 30 min for both initial metal concentrations of 5 and 48 mg/L, where 87 and 90 % of Cd(II) were adsorbed, respectively. For Pb(II), the equilibrium was achieved in 120 min for both initial metal concentrations, where 90 % of Pb(II) was adsorbed. This may be due to the different initial concentrations of Cd(II) and Pb(II) in suspensions. Solutions with lower metal concentration are likely to reach equilibrium in a shorter time period (Hamidpour et al. 2011).

Fig. 2 Effect of initial metal concentration on kinetics of: (a) Cd(II), and (b) Pb(II) adsorption onto pistachio hull (pH = 5.5, adsorbent dose = 10 g/L, temp = 23 ± 2 °C)
The biosorption kinetics of Cd(II) and Pb(II) consisted of two steps (Fig. 2): An initial rapid step where adsorption was very fast, which may be due to an increased number of vacant sites available at the initial stage of the biosorption process, resulting in an increased concentration gradient between the liquid bulk and the adsorbent surfaces (Hamidpour et al. 2011). The second phase is the gradual biosorption stage before the Cd(II) and Pb(II) uptake reach equilibrium. The concentration gradient is reduced with increasing contact time because of the accumulation of Cd(II) and Pb(II) on the vacant adsorption sites, which leads to a decreased adsorption rate of metals at the later stages of adsorption (Singh and Balomajumder 2016).
An increase in initial concentration of Cd(II) and Pb(II) leads to an increase in the adsorption capacity of pistachio hull biomass. As the initial metal concentration increased from 5 mg/L to 48 mg/L for Cd(II) and from 88.7 mg/L to 883 mg/L for Pb(II), the experimentally observed adsorption capacity (q exp ) increased from 0.4 to 4.5 mg/g and from 8.1 to 82.2 mg/g for Cd(II) and Pb(II), respectively. Increasing initial metal concentration accelerates the diffusion of Cd(II) and Pb(II) from the solution onto the surface of the adsorbent due to the increasing driving force at higher metal concentrations (Hamidpour et al. 2011).
Table IІ shows the kinetic parameters of the pseudo-first-order and pseudo-second-order models obtained using the non-linear least-square analysis method. The results show that both models satisfactorily described the time-dependent Cd(II) and Pb(II) adsorption onto pistachio hull (R2 ≥ 0.99) and the experimental adsorption capacity (q exp ) were close to that calculated from the these models (Table IІ). The fitness of experimental data to both the pseudo-first-order and pseudo-second-order kinetic models indicates that both physical and chemical interactions between the metals ions and pistachio hull biomass are necessary for the adsorption process. Good correlations of experimental data with the pseudo-first-order and pseudo-second-order kinetic models have been reported for the biosorption of Pb(II) and Cd(II) on Acacia leucocephala bark powder (Munagapati et al. 2010) and cork waste biomass (López-Mesas et al. 2011).
TABLE ІІ KINETIC PARAMETERS OBTAINED USING PSEUDO-FIRST-ORDER AND PSEUDO-SECOND-ORDER KINETIC MODELS
| Models constant | Cd(II) | Pb(II) | ||||
| 5 mg/L | 48 mg/L | 88.7 mg/L | 883 mg/L | |||
| q exp (mg/g) | 0.46 | 4.5 | 8.2 | 89.0 | ||
| Pseudo-first-order | ||||||
| q e (mg/g) | 0.46 | 4.4 | 8.2 | 88.2 | ||
| k 1 (1/h) | 3.4 | 3.65 | 5.1 | 5.9 | ||
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | ||
| SSE | 0.03 | 0.02 | 0.06 | 0.68 | ||
| Pseudo-second-order | ||||||
| q e (mg/g) | 0.46 | 4.45 | 8.2 | 81.65 | ||
| k 2 (g/mg/h) | 25.7 | 33.1 | 2.6 | 0.39 | ||
| h (mg/g/h) | 5.43 | 565 | 174.0 | 2603 | ||
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | ||
| SEE | 0.03 | 0.02 | 0.02 | 0.37 | ||
q exp : experimentally observed adsorption capacity, q e : solid phase metal ion concentration at equilibrium, k 1 : pseudo-first-order rate constant, k 2 : pseudo-second-order rate constant, h: initial adsorption rate, R2: correlation coefficient, SEE: standard error of the estimate
As shown in table І, the values of the initial biosorption rate (h) and the pseudo-second-order rate constant (k 2 ) decreased with the increase in initial Cd(II) and Pb(II) concentrations, indicating that the solution with lower metal concentration is likely to reach equilibrium in a shorter time period. Hamidpour et al. (2011) reported similar findings for Cd(II) and Pb(II) adsorption from aqueous solutions onto zeolite and bentonite.
Effects of the equilibrium pH of the solution
The pH value of a liquid solution is the most important factor in the biosorption of metal ions (Ullah et al. 2013). The effect of pH on the amount of metal removal efficiency depended on the kind of metal. The biosorption capacity of Cd(II) increased with an increase in the pH of the aqueous solution (Fig. 3a). The biosorption efficiency increased from 61.2 % at pH 3.2 to 95.0 % at pH 8.0 and maximum removal of Cd(II) by the adsorbent was found at pH range of 7.0-8.0.

Fig. 3 Effect of final pH of suspension on percent removal of: (a) Cd(II), and (b) Pb(II) by pistachio hull (contact time = 24 h, adsorbent dose = 10 g/L, temp = 23 ± 2 °C, Cd(II) concentration = 8 mg/L, Pb(II) concentration = 100 mg/L)
For Pb(II), two separate sections could be observed (Fig. 3b). Between pH 3.0 and pH 8.0, adsorption efficiency of pistachio hull slightly increased. In the second section (pH > 8) the adsorption efficiency decreased from 97 to 87 %.
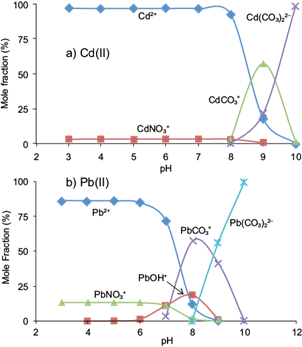
In order to interpret the effect of pH on the biosorption behavior of Cd(II) and Pb(II) ions onto pistachio hull, knowledge of the metal speciation is essential. The results of Visual MINTEQ indicated that Pb2+ and Cd2+ were the dominant species present in solutions in pH ≤ 7.0 and pH ≤ 8.5, respectively (Fig. 4a, b). Therefore, the lower adsorption of Cd(II) and Pb(II) ions at acidic pHs is probably due to the presence of excess H+ ions competing with the Cd2+ and Pb2+ species for the adsorption sites (López-Mesas et al. 2011). With the increase of the pH of the solution, the functional groups on surfaces of the adsorbent become negatively charged favoring Cd(II) and Pb(II) species adsorption (Sparks 2003). For Pb(II), the results of speciation showed that soluble hydroxylated species such as Pb(OH)+, Pb(OH)2 ° and Pb3(OH)4 2+ were the major species at pHs ≥ 8.0. Lower adsorption of Pb(II) at pHs ≥ 8.0 may be attributed to the formation of these soluble species. Munagapati et al. (2010) reported similar findings for Pb(II) biosorption from aqueous solutions onto Acacia leucocephala bark powder. According to Visual MINTEQ, none of the suspensions were super-saturated with regard to solid phases of Cd(II) or Pb(II). However, the under saturation of the systems does not exclude the possibility of metal surface precipitation or co-precipitation processes (McBride, 2000). The formation of solid phases of Cd(OH)2, CdCO3, Pb(OH)2, PbCO3, and Pb3(OH)2(CO3)2 is probable. Therefore, processes such as electrostatic attraction, the binding of Cd(II) and Pb(II) to functional groups of the adsorbent and precipitation may be responsible for the removal of 95 % of Cd(II) and Pb(II) from solutions for pHs in the range of 7.0-8.0.
Adsorption isotherms
The analysis of the equilibrium adsorption data is important to develop a model which correctly describes the results and which could be used for design purposes. In this study, adsorption isotherms were fitted to the Freundlich, Langmuir and Sips equations using non-linear least-square analysis. Fitted models parameters, coefficients of determination (R2) and standard errors of estimate (SEE) for adsorption of Cd(II) and Pb(II) are shown in table IІІ. On comparison of SEEs and correlation coefficient values (R2), the Sips model correctly simulates the adsorption behavior of Cd(II) and Pb(II) onto pistachio hull (Figs. 5 and 6). This indicates that the adsorption process of Cd(II) and Pb(II) onto the adsorbent followed a combined Freundlich and Langmuir isotherm. The maximum adsorption capacities for the Sips isotherm increased from 84.6 to 92.4 mg/g and from 7.2 to 9.4 mg/g with an increase in the pH of the suspension from 5.5 to 6.5 for Pb(II) and Cd(II), respectively. The values of maximum adsorption capacities obtained using the Sips isotherm were very close to the experimental values.
TABLE ІІІ ADSORPTION ISOTHERMS CONSTANTS AND STATISTICAL COMPARISON VALUES FOR DIFFERENT MODELS
| Models | Constants | Pb(II) | Cd(II) | |||
| pH = 5.5 | pH = 6.5 | pH = 5.5 | pH = 6.5 | |||
| Freundlich | K F (L/g) | 3.6 | 4.77 | 0.77 | 0.99 | |
| n F (-) | 0.94 | 0.83 | 0.71 | 0.92 | ||
| R2 | 0.81 | 0.89 | 0.93 | 0.96 | ||
| SEE | 12.4 | 9.5 | 0.80 | 0.50 | ||
| Langmuir | qmL (mg/g) | 142.0 | 127.9 | 14.9 | 20.3 | |
| K L (L/mg) | 0.038 | 0.044 | 0.03 | 0.06 | ||
| R2 | 0.91 | 0.95 | 0.95 | 0.94 | ||
| SEE | 9.2 | 6.4 | 0.6 | 0.69 | ||
| Sips | q s (mg/g) | 84.6 | 92.49 | 7.9 | 9.4 | |
| K s (L/mg) | 0.004 | 0.019 | 0.00004 | 0.03 | ||
| n S (-) | 2.3 | 1.59 | 4.03 | 2.3 | ||
| R2 | 0.98 | 0.97 | 0.97 | 0.99 | ||
| SEE | 5.02 | 5.2 | 0.7 | 0.36 | ||
K F : Freundlich isotherm constant related to adsorption capacity, n F : Freundlich isotherm exponent, q mL : Langmuir monolayer adsorption capacity, K L : Langmuir isotherm constant, qs: Sips monolayer adsorption capacity, K s : Sips isotherm constant, ns: Sips isotherm exponent, R2: correlation coefficient, SEE: standard error of the estimate

Fig. 5 Sips isotherm for biosorption of Cd(II) onto pistachio hull at: (a) pH 5.5, and (b) pH 6.5 (contact time = 24 h, adsorbent dose = 10 g/L, temp = 23 ± 2 °C)
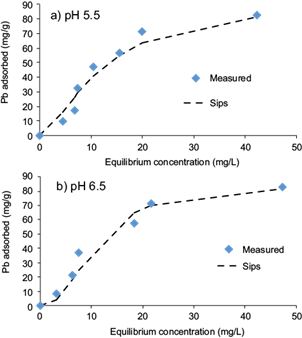
Fig. 6 Sips isotherm for biosorption of Pb(II) onto pistachio hull at a) pH 5.5 and b) pH 6.5 (contact time = 24 h, adsorbent dose = 10 g/L, temperature = 23 ± 2 °C)
The Langmuir and Freundlich isotherms fitted the equilibrium adsorption data well, confirming heterogeneous biosorption mechanisms (Hamidpour et al. 2011). The “n” values of the Freundlich isotherm were between 0 and 1, which demonstrated the suitability of pistachio hull as a biosorbent for removal of Cd(II) and Pb(II) from polluted water (Hamidpour et al. 2011). Also, the suitability of pistachio hull as a biosorbent for Cd(II) and Pb(II) was assessed using a dimensionless separation factor (RL), which was derived from the Langmuir model by the following relationship (Hamidpour et al. 2011):
The RL values for Cd(II) and Pb(II) adsorption onto pistachio hull were in the range of 0-1, which indicates that the biosorption process is favorable at studied conditions.
The values of the Freundlich adsorption capacity (K F ) increased from 3.6 to 4.7 L/g for Pb(II) and from 0.77 to 0.99 L/g for Cd(II), with an increase in the pH of the solution from 5.5 to 6.5 (Table ІIІ). The same trend is observed for the values of Langmuir adsorption energy (K L ) for both Cd(II) and Pb(II). As the pH of the suspension increases, the number of negatively charged sites on the adsorbent increase. This favors the adsorption of Cd(II) and Pb(II) ions through the electrostatic force of attraction and chelating of metals with the surface functional groups of the adsorbent. Also, surface precipitation of Cd(II) and specially Pb(II) ions should be considered as another mechanism at higher pHs in the solution.
Several researchers have studied the adsorption capacity of biosorbents. However, the comparison of the pistachio hull biosorption capacity with other biosorbents reported in the literature is difficult because of the different experimental conditions (e.g., initial metal concentration, pH of the solution, temperature) applied in those studies. The adsorption capacity of pistachio hull for the removal of Cd(II) and Pb(II) has been compared with those of other biosorbents reported in literature (Table IV). The values were reported in the form of monolayer adsorption capacity. The experimental data of the present study show that the pistachio hull used in this study exhibited a reasonable adsorption capacity for Cd(II) and Pb(II), therefore, may be useful for removal of these metals from polluted environmental sites. Furthermore, the pistachio hull used in this study is a raw material (without modification) that can be easily obtained from pistachio processing industries in large amounts at no cost.
TABLE IV COMPARISON OF LANGMUIR BIOSORPTION CAPACITIES OF SOME BIOSORBENTS FOR Cd(II) AND Pb(II)
| Adsorbent | q mL (mg/g) | pH | References | |
| Pine cone shell | Pb(II) | 25.4 | 5.0 | (Martín-Lara et al. 2016) |
| Cork waste | Pb(II) | 13.57 | 5.0 | (Lopez-Mesas et al. 2011) |
| Cork waste | Cd(II) | 2.39 | 5.0 | (Lopez-Mesas et al. 2011) |
| Pine cone shell | Pb(II) | 25.24 | 5.0 | (Martín-Lara et al. 2016) |
| Wheat straw | Cd(II) | 14.6 | 6.0 | (Dang et al. 2009) |
| Pistachio hull | Cd(II) | 14.9 | 5.5 | This study |
| Pistachio hull | Pb(II) | 142.0 | 5.5 | This study |
q mL : Langmuir monolayer adsorption capacity
CONCLUSIONS
Pistachio hull powder showed a high biosorption behavior for removing Cd(II) and Pb(II) from aqueous solutions without chemical modification. Biosorption of Cd(II) and Pb(II) is pH-dependent showing a maximum value at pH 8.0. Kinetic and equilibrium studies reveal that the pseudo-first-order and pseudo-second-order kinetic models gave the best fit, and the Sips adsorption model described the interaction between Cd(II) and Pb(II) and the adsorbent better than the Freundlich and Langmuir models. The FTIR results confirmed the interactions between metals and functional groups present on the surface of the pistachio hull. These findings showed that the pistachio hull biomass can be used as a cheap and eco-friendly biosorbent for efficient removal of Cd(II) and Pb(II) from water. Although, pistachio hull powder showed high sorption capacity for Cd(II) and Pb(II), more studies should be carried out for recovery of metals and regeneration and reuse of the material. Moreover, further experiments should be performed at the pilot scale to examine the feasibility of pistachio hull waste for industrial applications.











 nueva página del texto (beta)
nueva página del texto (beta)