INTRODUCTION
Oil exploration, exploitation, and storage are key economic activities in Patagonia, which have occasionally produced oil pollution events (SAyDS 2000, Barquín et al. 2011). The advance of these activities can endanger Patagonian aquatic ecosystems, which are very well preserved until now. However, in the early 20th century, the black gold fever had already impacted some Patagonian environments in several ways, such as the footprint of old unproductive wellbores. One of these old wellbores is located near to Bariloche city, North West Patagonia, and was under production from 1900 up to the 1940s, when it was dismantled leaving a permanent heavy oil spill (Mancini and Serna 1989). This spill runs into La Mina stream, a low order stream located in a steppe-forest transition area in the sub-Andean Mountains within the Ñirihuau river system that drains into the Nahuel Huapi Lake (Mancini and Serna 1989, Cazau et al. 2005), a glacial lake, with great touristic and conservation value (Fig. 1). Considering the public concern about adverse health effects of persistent oil pollution on highly valued aquatic ecosystems, particularly on water and edible fish, it is necessary to develop tools to assess the impact of this oil spill.

Fig. 1 Location of the study site in La Mina stream, a tributary of Ñirihuau river, 30 km from Bariloche City. (A) Oil remains afloat on the surface of stream water. (B) Oil spill sources running towards the stream. (C) Oil extraction well next to the stream in the 30s (photograph by Pedro Penna, Parsons 2002)
Fish have proved to be useful models when it comes to evaluating the health of aquatic ecosystems. Rainbow trout Oncorhynchus mykiss is a useful model for toxicology studies because of its sensitivity to chemicals (Bailey et al. 1996,Buhler and Wang-Buhler 1998) and because it has been introduced in water courses around the world and is particularly abundant in Patagonia (Quirós 1990, Pascual et al. 2002), including La Mina stream. Since direct measurement of tissue hydrocarbon concentrations generally does not provide a useful indicator of petroleum exposure in fish and could be expensive (Collier et al. 1996), we focused on the transcription of genes possibly involved in aromatic hydrocarbons metabolism, in order to find sensitive biomarkers of oil exposure. The phase I xenobiotic metabolizing enzymes are represented mainly by cytochrome P450 (CYP) multi-enzymatic complex, that catalyzes hydrolysis, oxidation and reduction reactions, by adding or exposing functional groups (Danielson 2002). Seven CYP families have been reported for the rainbow trout (Uno et al. 2012).
CYP1A mRNA expression is induced by minuscule amounts of diverse aromatic compounds through the aryl hydrocarbon receptor (AhR) signaling pathway, being a sensitive biomarker of exposure to aromatic hydrocarbon contaminants (Uno et al. 2012). Hence, CYP1A transcript expression serves as reference in biomarkers of crude oil pollution. The CYP2 family of enzymes is represented by CYP2K1 and CYP2M1 in rainbow trout (Buhler et al. 1994, Yang et al. 1998). CYP2K1 is the most abundant constitutively expressed hepatic cytochrome P450 found in sexually immature rainbow trout and it shows an unusually high sensitivity to carcinogens (Bailey et al. 1996). CYP2K1 has benzphetamine N-demethylase and steroid hydroxylase activities, and both CYP2K1 and CYP2M1 are involved in lauric acid metabolism and some longer fatty acids in rainbow trout (Yang et al. 1998, 2000) and both are involved in the metabolism of estrogenic hormones (Buhler and Wang-Buhler 1998, Buhler et al. 2000). A tropical reef fish exposed to oily water showed an induction of CYP2K1 and CYP2M1 proteins (Zhu et al. 2008). Thus, anomalous expression of both CYP2 could impact on steroid and fatty acid homeostasis in rainbow trout (e.g. Buhler et al. 2000). The CYP3 family of enzymes is represented by CYP3A27 and CYP3A45 in rainbow trout, being both involved in steroid hormone metabolism (Lee et al. 2001, Lee and Buhler 2003). The mRNA of CYP3A27 is 55 % identical to human CYP3A4, and their encoded proteins (LMC5-like) have structural and functional similarities (Miranda et al. 1991). Through pregnane X receptor (PXR) signaling pathway, some polycyclic aromatic hydrocarbons induce human CYP3A4 (Luckert et al. 2013). Similarly, aromatic hydrocarbons could act as endocrine-disrupting chemicals in O. mykiss by inducing CYP3A27 mRNA expression, possibly affecting fish reproduction. The CYP3A27 induction could provide a useful biomarker for the environmental monitoring. Consequently, since several CYPs could be influenced by exposure to polycyclic aromatic hydrocarbons, oil contamination could affect biosynthesis and metabolism of many important endogenous constituents of rainbow trout.
In this study we analyzed the suitability of different possible CYPs molecular biomarkers in O. mykiss for detection of oil contamination. We focused on gill and liver tissues because the gills are the primary route of hydrocarbon uptake from water, and the liver is the main organ for metabolism of toxic oil constituents (Thomas and Rice 1981). In these organs, we assessed the effects of petroleum on the transcriptional expression of CYP1A, CYP2K1, CYP2M1 and CYP3A27. Our aim was to assess the use of mRNAs levels, by semiquantitative reverse transcription polymerase chain reaction (RT-PCR), of these CYP forms in rainbow trout gill and liver. First, we explored the heavy crude oil effects on these possible biomarkers in wild juvenile rainbow trout that were caught in the La Mina stream, up and downstream of the oil spill. Second, we investigated the effects of the water-accommodated fractions (WAF) from La Mina’s oil, through laboratory experiments, on these possible biomarkers in juvenile rainbow trout.
MATERIALS AND METHODS
Study area
There are four oil spills located at 5-20 m from the water’s edge, covering an area of 400 m2. The spills converge into a unique source point of pollution, with 2-4 liters of crude oil per day entering the stream (in February 2015). The spills are the relict of a wellbore, an attempt to have oil production during the early 20th century and then abandoned (Mancini and Serna 1989). The oil sampled from surface seepage was characterized as immature heavy crude petroleum (Ro = 0.44-0.53 %, American Petroleum Institute degree (API) = 18º and sulfur = 0.45 %; Cazau et al. 2005), composed by 33.7 % saturated, 17.8 % aromatics, 5.9 % asphaltenes and 42.6 % NSOs (compounds with nitrogen, sulfur, oxygen and heavy metals; data provided by YPF S.A. Argentina).
The study was conducted in two 300-m-long reaches: upstream-control (41º17’34’’ S - 71º11’14’’ W, 1011 masl) and downstream-impacted (41º17’21’’ S - 71º10’58’’ W, 1001 masl) of the oil spill during summer (dry season), 1-2 February 2015. The riparian vegetation of the reaches is well preserved, with dominance of shrubby plants and the herbaceous Festuca gracillima. The upstream reach is up of a 1.4 m high waterfall, 50 m upstream from the spill. This impairs juvenile fish migrate from the downstream to the upstream reach, but it might not necessarily prevent migration from the upstream to the downstream reach. The stream bottom is composed of boulder cobble substrates and the stream channel has alternated riffle-pool habitats. The climate is cold-temperate, with a mean annual temperature of 8.7 ºC and annual precipitations of 850 mm. During the study, the stream reaches were characterized by mean water velocity of 0.31 ± 0.11 m/s, discharge of 78 ± 12 L/s, mean width of 0.75 ± 0.48 m, mean depth of 0.35 ± 0.31 m, water temperature 16-19 ºC, conductivity 80 ± 27 μS/cm and pH 6.8 ± 0.2. Fish are represented by a single species, the exotic rainbow trout O. mykiss.
Total petroleum hydrocarbons (TPH)
Triplicate samples were collected at the upstream reach (-200, -250 and -350 m), at the oil spill (zero meters), at the downstream reach (50, 100 and 200 m) and further downstream (400, 800 and 1600 m). Water samples were collected in polyethylene bottles and filtered through glass-fiber filters: pore size = 0.7 μm; Whatman GF/F, Maidstone, UK. Sediment samples were collected with a core sampler (4 cm diameter, 3 cm depth), from stream pools and stored in polyethylene flasks at 4 ºC in the dark. The TPH were estimated in the WAF solution, and in stream water and sediment samples. The WAF was prepared according to Singer et al. (2000) using 4.75 g of crude petroleum from the spill per L of stream water. Each sample was acidified with HCl to a pH <2. The TPH was extracted from each with carbon tetrachloride and was estimated by the analysis of the triple peak method (EPA 1978) at three spectral regions: 2930 cm-1 (-CH2), 2960 cm-1 (-CH3) and 3030 cm-1 (Ar-CH), using a Fourier transform infrared (FT-IR) spectrometer (Thermo Scientific™; detection limit = 0.04 mg/L). The procedure was replicated twice for each sample, with an intra-deviation < 10 %.
Fish samples and treatments
Wild, juvenile rainbow trout (10.7 ± 6.1 g, 101 ± 23 mm, n = 24, 12 individuals per reach) of both sexes were caught by net casting. Fish were killed by a blow to the head. Liver and gills from four fish were aseptically dissected out and pooled into one sample of each organ, obtaining a total of three pooled samples per organ and per reach. For the experiments, juvenile rainbow trout (3.9 ± 0.8 g, 75 ± 5 mm) were obtained from the Centro de Ecología Aplicada de Neuquén (CEAN) hatchery (Neuquén, Argentina) and acclimatized for 2 days before exposure. Fish were kept in 1.5 L glass fishbowls (4 fish per fishbowl, ~10 g fish/L) with continuously aerated fresh water from Chimehuin river, at a temperature of 16-18 ºC and pH 7.4-7.6 (12 h light-12 h dark photoperiod). Treatments consisted in 24 h and 96 h exposure to 1 % and 5 % WAF, by sextuplicate, with the respective controls. Fish were killed and dissected as described above for field samples. Field and laboratory samples for mRNA expression analysis were stored in RNA-later® (Life technologies) at -20 ºC.
CYPs transcriptional expression
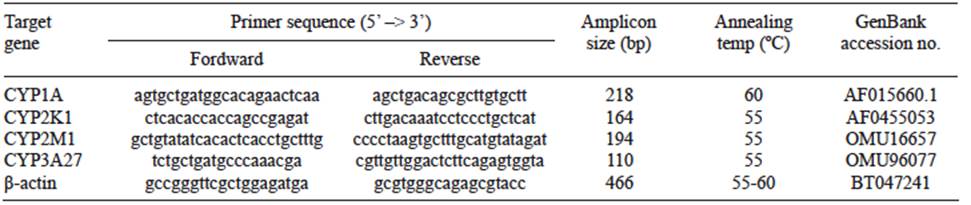
Total RNA was extracted from 80 - 120 mg of each sample using TRIzol® reagent according to manufacturer’s instructions. RNA concentration was measured at 260 nm and quality was examined by 260/230 and 260/280 ratios. Samples were diluted with ultra-pure water to adjust to a concentration of 2.0 μg/μL and stored at -80 ºC. The synthesis of cDNA was performed with Moloney Murine Leukemia Virus (M-MLV) Reverse Transcriptase kit (Invitrogen). Duplex PCR reactions were performed with the GoTaq® Green Master Mix kit (Promega), in a Swift Maxi Gradient Thermal Cycler, as follows: 94 ºC for 3 min, 27 cycles at 94 ºC for 30 s (denaturation), 55-64 ºC for 30 s (annealing) and 72 ºC for 30 s (DNA chain extension) with a final extension at 72 ºC for 10 min. The number of cycles of denaturation phase was selected to catch the exponential amplification, by previous calibration of each mRNA. The annealing temperatures and primer pairs used during the duplex PCR for each gene are shown in Table I. The amplified products were separated by 2 % agarose gel electrophoresis for 50 min at 90 V in TAE 1x (0.04 M Tris, 0.0001 M EDTA, pH 8.0) buffer, visualized using SYBR® Safe DNA gel stain (Invitrogen), and photographed under UV light. Bands were analyzed densitometrically using Image J software. A 100 bp ladder (Winkler) was used as the molecular mass marker. The presence of a single product of the appropriate size, identical to the reference experiments, was considered as a positive result. β-actin was used as housekeeping gene and loading control. The relative expression of each gene was calculated as the ratio gene/β-actin.
Data analysis
All statistical analyzes were performed using SPSS 11.5 (Inc., Chicago, Illinois, USA), under the license from Universidad Nacional de Luján, Argentina. All data are expressed as the mean ± standard deviation (n). The mRNA expression (gene/β-actin) data from field samples were analyzed by Student’s T-test, and data from the laboratory experiment were analyzed by two-way ANOVA. The explanatory variables in the two-way ANOVA were exposure time (24 and 96 h) and WAF concentration (0, 1 and 5 %). We performed post hoc comparisons between control and treatments, for both 24 and 96 h, of all pairs of groups using two-sided significance levels with a Bonferroni adjustment.
RESULTS
Total petroleum hydrocarbons
The TPH was 5.2 ± 0.7 mg/L in the stock solution of WAF, 74.5 ± 18.1 μg/L in the WAF 1 % solution and 284.4 ± 75.2 μg/L in the WAF 5 % solution. The TPH concentrations in the stream at the oil spill discharge (zero meters) were 23.2 ± 8.7 μg/L in water and 1.9 ± 0.3 mg/g in sediments of La Mina stream. TPH concentration in water was 4.5 ± 15.0 μg/L before the oil spill (upstream, -200 to -350 m), 106.4 ± 51.1 μg/L after the oil spill (downstream; 5 to 200 m) and 89.3 ± 78.2 μg/L further downstream (400 to 1600 m) of the oil spill; with an increase of 23-fold from up to downstream reach. TPH concentration in sediments was 0.17 ± 0.20 mg/g in upstream reach, 3.16 ± 1.55 mg/g in downstream reach and 1.26 ± 0.92 mg/g in further downstream reach.
CYPs transcriptional expression in wild rainbow trout
The expression levels of CYP1A mRNA were significantly higher in liver and gill (1.34 and 1.24-fold, respectively) of trout from the impacted reach relative to the control trout (from upstream of the spill). The levels of CYP2K1 mRNA were significantly lower in liver and gill (1.54 and 1.46-fold, respectively) in trout from the impacted reach relative to control. In liver, the CYP2M1 mRNA levels were lower (1.47-fold) in trout from the impacted reach relative to control; while CYP3A27 mRNA levels were higher (1.58-fold; Fig. 2).

Fig. 2 mRNA expression levels (relative to β-actin) of CYP327, CYP2M1, CYP2K1 and CYP1A genes in liver and gill of wild rainbow trout Oncorhynchus mykiss from upstream (control) and downstream of the oil spill (impacted) in La Mina stream. Each value is the mean ± SD (n = 3). Asterisks indicate differences between upstream and downstream fish (*p < 0.05 and **p < 0.005, Student’s T-test)
CYPs transcriptional expression in rainbow trout with WAF treatment
The transcript levels of CYP1A were induced by 24 h exposure to 1 % and 5 % WAF in liver and gill (1.2 and 1.4-fold, respectively), and by 96 h exposure to 5 % WAF in liver (1.4-fold), meaning that gill CYP1A levels returned to control values after 96 h. The transcript levels of CYP2K1 were induced by 24 h exposure to 5 % WAF in liver and gill (2.0 and 1.8-fold, respectively), but they were down-regulated by 96 h exposure to 5 % WAF in liver and gill (1.2 and 2.1-fold, respectively). The transcript levels of CYP2M1 declined 1.8-fold after 96 h exposure to 5 % WAF in liver, and the CYP3A27 transcript levels did not show changes after treatments (Fig. 3, Table II). Significant interactions denote different responses to WAF at 24 and 96 h (Table II). The controls of CYPs levels tended to increase over time, except gill CYP2M1 levels, being a significant source of variability (Fig. 3, Table II).

Fig. 3 mRNA expression levels (relative to β-actin) of CYP327, CYP2M1, CYP2M1 and CYP1A genes in liver (A) and gill (B) of rainbow trout Oncorhynchus mykiss after 24 hours (dotted line) and 96 hours (solid line) of exposure to 1 % WAF and 5 % WAF (WAF: water accommodated fraction prepared from the crude oil obtained in La Mina spill). Values are mean ± SD (n = 6). Letters denote significant differences among treatments within each time, 24 h (lowercase letters) and 96 h (capital letters) from Bonferroni test (Table II)
TABLE II RESULTS OF TWO-WAY ANOVAS FROM TROUT CYPS TRANSCRIPTS AFTER CRUDE OIL WATER ACCOMMODATED FRACTION (WAF) TREATMENT FOR LIVER AND GILL. TABLE SHOWS THE EFFECTS OF WAF CONCENTRATION, TIME OF EXPOSURE AND INTERACTION BETWEEN BOTH, AND THE PERCENTAGE OF RELATIVE CONTRIBUTION OF EACH FACTOR TO EXPLAIN EACH MODEL. ASTERISKS INDICATE *p < 0.05 AND **p < 0.005 FROM BONFERRONI TEST

DISCUSSION
The rainbow trout Oncorhynchus mykiss is widely distributed around the world and is present in most of the freshwater ecosystems of Patagonia. This, along with the fact that its metabolic pathways related to oil contaminants detoxification are relatively well known, makes this species a good bioindicator (Moore et al. 2004). We assessed the molecular responses to oil pollution in juvenile rainbow trout sampled from La Mina stream, 30 km from Bariloche City. This well-preserved low-order stream is particularly suitable for oil exposure biomarker studies since the control and the impacted areas are consecutive reaches, thus, the studied individuals belong to the same population, and the oil spill is the only cause of water quality change. The field study was combined with laboratory exposure to WAF obtained from the oil source, which affects the same stream. Gene transcript expression of several cytochrome P450 oxidases was studied by semi-quantitative PCR in both field and laboratory.
CYP1A is a hallmark of AhR signaling pathway activation in fish and is a sensitive biomarker of exposure to PAHs and other oil compounds (Varanasi 1989, Jönsson et al. 2006, Clark et al. 2010). Here, as it was to be expected, crude oil exposure induced CYP1A transcription in O. mykiss liver and gill. These clear and consistent changes reaffirm CYP1A transcript expression as a good semiquantitative biomarker for crude oil exposure. The maximum induction of CYP1A transcript expression has been reported to start eight to sixteen hours after exposure (Minura et al. 1999, Nahrgang et al. 2009, Chang et al. 2014) and remained elevated in exposed fish (Jönsson et al. 2010). This explains the similar response in liver CYP1A transcript levels at 24 and 96 h during our experiments, without dose-dependent effects. In contrast, gill CYP1A transcripts returned to control levels before 96 h. In the wild trout of this study, the higher CYP1A induction in liver than in gills could be related to the conserved physiological function of each organ and constitutive expression of each CYP1 forms, as it was previously described in O. mykiss (Jönsson et al. 2010) and other fish (Jönsson et al. 2007, Zanette et al. 2009). Also, the difference between gill and liver CYP1A levels in the experiment and stream samples could be a consequence of AhR pathway down-regulation by, for instance, the induction of the AhR repressor (AHRR), which competes with AhR for the aryl hydrocarbon nuclear translocator (ARNT) heterodimerization (Mimura et al. 1999, Meyer et al. 2003); or by proteolysis of AhR through ubiquitination (Ma and Baldwin 2002). These possible explanations exceed the scope of our research and further studies will be needed to understand gill CYP1A mRNA regulation in rainbow trout.
CYP1A are involved in the detoxification pathway of hydrocarbons, while CYP2K1, CYP2M1 and CYP3A27 are involved in other metabolisms. Hence, the response of the last ones to crude oil could be less specific but not less useful indicating some metabolic alterations. The transcription of CYP2s and CYP3 are affected by foreign compounds as well as by endogenous substrates (Uno et al. 2012), which implies that internal metabolism should change their mRNA levels over time, maybe explaining experimental-control differences over time. In this study, CYP2K1 was induced after 24 h exposure to WAF, as it was previously observed in reef fish (Zhu et al.2008). In contrast, more extended time and high PAH concentration (e.g. exposed trout to 5 % WAF, 96 h, and exposed wild trout) appears to down-regulate CYP2K1. More studies are needed to understand if down-regulation of liver CYP2K1 and CYP2M1 and gill CYP2K1 were caused by the disruption of physiologic homeostatic processes in which CYP2K1 and CYP2M1 enzymes participate (Zhu et al. 2008, Browne et al. 2010), or by alterations in the metabolism of estrogenic hormones (testosterone, progesterone, estradiol) and fatty acids, which could lead to the inhibition of CYP2K1 and CYP2M1 transcript expression, for example, by estradiol (Buhler et al. 2000,Sovadinová 2006). Although the results in the WAF experiment are not conclusive, differences among time response of CYP2 expression could be useful as complementary indicators of pollution by crude oil.
We found induced CYP3A27 transcript levels only in liver of impacted wild trout, suggesting an induction at longer time than four days. Gene expression of CYP3A27 has been reported to be regulated by pregnane X receptor (PXR) in rainbow trout hepatocytes (Wassmur et al. 2010). Since PAHs activate the PXR signaling pathway in humans (Luckert et al. 2013), it could be speculated that oil PAHs induce O. mykiss CYP3A27 expression through PXR. However, there are few studies on the nature of PXR ligands in fish (Ekins et al. 2008, Krasowski et al. 2011, Bainy et al. 2013) and their results show differences in ligand affinity between fish and mammalian PXRs, and also among fish species. Moreover, crosstalk between AhR and PXR cannot be discarded as an alternative explanation for PXR signaling activation by oil contaminants.
Thus, while CYP1A mRNA expression appears to be a more robust biomarker for detecting the effects of crude oil under different dose and exposure time, the expression of CYP2K1, CYP2M1 and CYP3 mRNAs could supply additional information such as different exposure time to oil pollution in freshwater systems. The results obtained in wild rainbow trout could be particularly useful to evaluate other freshwater pollution, since the TPH concentration varied along the stream reach and rainbow trout was not exposed to a constant concentration of the pollution for several hours, unlike the experiments. The study of mRNA expression patterns of multiple CYP genes in rainbow trout gills and liver would be extended in order to evaluate their use for monitoring programs of crude oil pollution and remediation actions on Patagonian freshwater ecosystems. As far as we know, this is the first ecotoxicological study on the oil spill in La Mina stream, and one of the few related studies in Patagonia. Since oil exploration and extraction activities are expected to be enhanced in this region in the future, further studies and monitoring programs are needed to protect the quality of valuable water ecosystems, fish production in downstream reservoirs and human health.











 nueva página del texto (beta)
nueva página del texto (beta)