INTRODUCTION
Organophosphorus (OP) and carbamate (CA) insecticides cause a practically irreversible inhibition of acetylcholinesterase (AChE) and other cholinesterases (ChE) activities and a continuous stimulation of the nervous synapses and physiological and behavioral changes. This leads to death of target and of non-target organisms when these compounds reach the aquatic environment. For this reason, ChE activity has been suggested as a biomarker of exposure to these pollutants (Fulton and Key 2001, Mwila et al. 2013), although several studies suggest that this response may be caused also by exposure to other stressors (Dias et al. 2006, Oliva et al. 2012).
Agriculture is an important activity for most coastal states of the Mexican NW. In the state of Nayarit, the area dedicated to irrigated and dryland (rain-dependent) agriculture is 6024 km2, and chemical insecticides are applied in 29.6 % (178 310.4 ha) of this area (INEGI 2012). Considering an average use similar to that of the neighboring state of Sinaloa, of between 3 and 3.5 kg of insecticide/ha/year (Karam 2002), the amount of insecticides used in Nayarit would be in the order of 535 to 623 t/year and, since 62 % of the insecticides used in Nayarit are OP and CA (González et al. 2010), the amount of ChE-inhibiting OP and CA would be between 332 and 386 t/year.
The hydrological regions of Nayarit, Presidio-San Pedro (RH11), Lerma-Santiago (RH12) and Huicicila-San Blas (RH13) to the north, and Ameca (RH14) to the southare named from the main rivers that flow across the state eventually reaching the Pacific Ocean coastline. Together with their tributaries and lesser streams, these rivers drain the most productive agricultural areas of the state carrying pesticides to the coastal zone in amounts and concentrations which depend on their seasonal use and on the varying volumes of agricultural runoff during the dry and the rainy seasons.
Oysters are sessile filter-feeders, and are among the most common organisms used for pollution monitoring programs (De Freitas et al. 2003, Luna et al. 2010). Therefore, as indicated by Zha et al. (2013), the ChE activity of the tissues of local oysters should indicate the degree and the variability of pesticide-related stress of the coastal environment.
Since the type and amount of pesticides may vary geographically as well as seasonally, depending on local conditions and planned crops (González et al. 2010), the mean ChE activity was determined in oysters obtained in different coastal zones of the state of Nayarit during the rainy and dry seasons.
MATERIALS AND METHODS
The sampling points Los Murillo (LM), La Boca de Camichín (BC), El Conchal (EC), Miramar (MR), and Platanitos (PL) are located in the Pacific coastal plain, under the influence of the state´s main rivers Acaponeta (LM), San Pedro Mezquital and Grande de Santiago (BC), as well as Huicicila and El Naranjo (EC, MR and PL) which drain areas of intensive agriculture. La Peñita de Jaltemba (LP) lies in the same Sierra de la Costa de Jalisco physiographic province as the two southernmost sampling stations La Cruz de Huanacaxtle (LC) and Bucerias (BS). However, LC and BS are located along the Bahía de Banderas coastline (Fig. 1), where tourism is an increasingly important economic activity, although maize, sorghum, tobacco and other main crops are produced through intensive, mainly irrigated agriculture (INEGI 2012).
Fig. 1 Sampling points: Los Murillo (LM), La Boca de Camichín (BC), El Conchal (EC), Miramar (MR), Platanitos (PL), La Peñita de Jaltemba (LP), La Cruz de Huanacaxtle (LC), Bucerias (BS)
Oysters tend to adhere to hard substrates and to each other forming clumps of several individuals. Samples (2.5 to 5.5 cm Crassostrea sp. from four different clumps) were hand-collected at each sampling station at the beginning of September 2008 (rainy season) and end of February 2009 (dry season), and transported to the laboratory in separate plastic bags contained in an ice box. The selected tissues of each oyster were adductor muscles, gills and labial palps (Bocquené et al. 1997, Nardi et al. 2008). One g of each tissue of each oyster was homogenized separately in a 1300-D Polytron at 12 000 rpm for one minute in phosphate buffer in a 1:5 ratio (w/v) (García et al. 2006).
The resulting suspensions were centrifuged at 10 000 rpm and 4 ºC for 30 minutes (Beckman GS-15R centrifuge). Four 50 µL subsamples of the supernatant were mixed with 250 µL of a composite reaction solution of acetylthiocholine 0.075 mM and 10 mM of 5,5 dithiobis-2-nitrobenzoic acid (DTNB), and the ChE activity of each of these mixtures was calculated using two absorbance readings at 414 nm after 10 and 15 minutes with a Bio-Rad Model 550 plate reader as described by Guilhermino et al. (1996). Proteins were quantified with the Bradford (1976) method, using bovine serum albumin as standard. Bio-Rad reactive was used as a reaction solution in a proportion of 1:4 (v/v) with double distilled water and absorbance readings were made at 595 nm.
Since data were not normal or homoscedastic (Kolmogorov-Smirnov and Bartlett’s tests), ChE activities obtained in each station for each tissue were compared with Kruskall-Wallis and Dunn’s tests, performed separately for the dry and the rainy season. However, data were normal when the values of all stations were pooled for separate tissue-to-tissue comparison in each season: their mean values were compared with separate one way blocked analysis of variance (ANOVA) tests, and differences were assessed with Tukey’s tests. The level of significance was p < 0.05 for all tests.
RESULTS
The general trend of ChE activity was gills>palps> muscle. With few exceptions, low activities and thus higher inhibitions were determined in both seasons in the northernmost station LM and the southern stations LC and BS. There were significant differences in the mean activities determined at different stations in the three tissues. In gills, high values were found in both sampling seasons in stations BC, EC and MR. The lowest were in LC and BS, which were significantly different from the value of BC.
In muscle, EC and LP had high activities in the rainy season and the lowest was in LC, while in the dry season BC and PL had high values and the lowest was again that of LC. As to palps, the highest values of the rainy season were in LM, BC and MR. In this case, LP had the lowest value. In the dry season the high values were in BC and PL and the lowest was that of BS (Table I).
TABLE I MEAN VALUES (± S.E.) OF ChE ACTIVITY (nmol/min/mg OF PROTEIN) OF GILLS, PALPS AND MUSCLE OF OYSTERS COLLECTED IN DIFFERENT COASTAL AREAS OF NAYARIT STATE IN THE RAINY AND DRY SEASONS (n = 8)
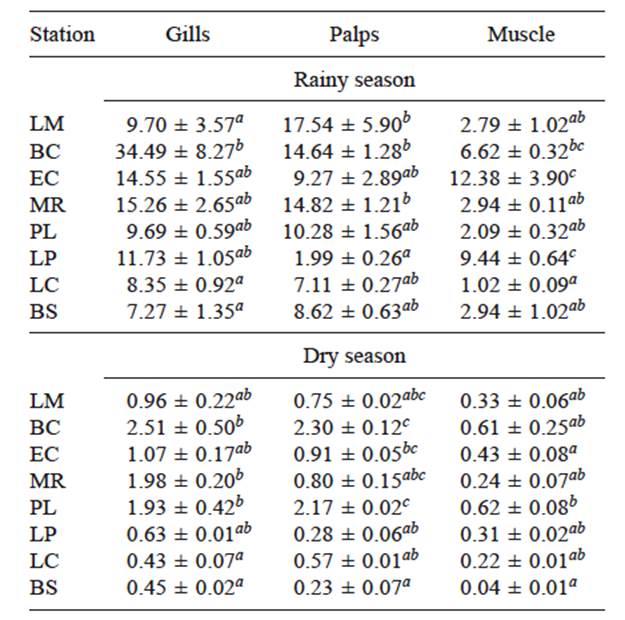
Different letters indicate significant differences between stations sampled in the same season (Kruskal-Wallis and Dunn´s tests performed separately with the data collected in the rainy and in the dry season, p < 0.05, a≤ab≤abc and a<b<c). (LM) Los Murillo, (BC) La Boca de Camichín, (EC) El Conchal, (MR) Miramar, (PL) Platanitos, (LP) La Peñita de Jaltemba, (LC) La Cruz de Huanacaxtle and (BS) Bucerias
DISCUSSION
The ChE activities were one order of magnitude higher in the rainy than in the dry season, indicating lower inhibition in summer. This might be due to the prevalent use of insecticides in winter while that of herbicides is more common during spring-summer in the northern stations. However, insecticides are the main type of pesticide used in both planting seasons in the southern part of the state (González et al. 2010). For this reason, although at least in part this difference could be due to fluctuations in enzyme levels and activity in oyster tissues linked to seasonal factors, the most probable explanation for this common seasonal tendency is the dilution effect caused by the high runoff volumes during summer months, as was observed for other contaminants in the San Pedro River by Guzmán et al. (2011).
In spite of the traditional use for fishery, tourism and aquaculture of Nayarit coastal water bodies, information on the presence and levels of contaminants is limited. Páez et al. (2002) and Osuna et al. (2009) found measurable levels of Pb and Cd and of some organochlorine pesticides in oysters and mussels of two lagoons of northern Nayarit, and Bernal et al. (2010) determined a low AChE activity in the oysters of one of the stations used in this work (BC). This is the main oyster-producing area of Nayarit State (INEGI 2011), with an estimated 2011 production of 1076 t. This station is located in the Marismas Nacionales estuarine system, generated by the confluence of several continental streams which drain some of the state’s most productive agricultural areas.
Our data and those of Bernal et al. (2010) coincide in indicating a higher impact of pesticides during the dry than in the rainy season. Additionally, the comparatively high ChE activity of BC oysters both in the rainy and in the dry seasons seem to indicate a low local level of impact by agricultural activities in comparison with the rest of the coastal stations selected for this study.
Possibly because of their respiratory function, which causes continuous exposure to water-borne contaminants, it has been suggested that gills may be the appropriate tissue to evaluate the toxic effects of pollutants in laboratory experiments (Bernal et al. 2010, Zha et al. 2013), followed by labial palps and adductor muscle (Bocquené et al. 1997). Although the tendency to higher ChE values in gills than in muscle corresponds to that reported in other bivalve species, it corresponds only in part with the data of this study, since gills and labial palps had similar activities in either season (Table II).
TABLE II MEAN VALUES (± S.D.) OF ChE ACTIVITY (nmol/min/mg OF PROTEIN) IN DIFFERENT TISSUES OF MARINE BIVALVES OF CONTAMINATED SITES OR AFTER EXPOSURE TO ORGANOPHOSPHORUS PESTICIDES
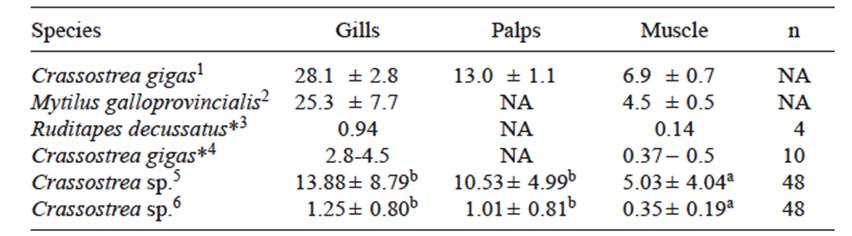
1Bocquené et al. (1997), 2Mora et al. (1999), 3Nadji et al. (2010), 4Ochoa et al. (2013), 5This study (rainy season), 6This study (dry season). *calculated from bar diagrams. n= number of specimens analyzed. NA= data not available. Different letters indicate significant differences among ChE activities determined in the rainy and dry seasons in this study (One-way block ANOVA and Tukey’s tests, p < 0.05)
Bivalves such as clams, oysters and mussels are the most common indicators of aquatic pollution (Boening 1997), and their cholinesterase activity has been suggested as biomarker of pesticide pollution (Cajaraville et al. 2000, Damiens et al. 2004). This seems to agree with the values reported in other studies such as Bocquené et al. (1997) and Mora et al. (1999) who reported high activities in oysters and mussels collected in low polluted areas. These results seem to indicate a higher degree of pollution in Nayarit coastal waters during the dry season, while the values determined in the rainy season are close to those reported by Nadji et al. (2010) and Ochoa et al. (2013) in clams and oysters challenged with OP pesticides or transplanted to heavily polluted areas.
However, cholinesterases show a considerable variety of biochemical characteristics, properties and sensitivities to different organic pollutants (Bocquené et al. 1990, 1997). Additionally, they may be affected also by metals, although the effect may be either metal- or species-dependent since, while no effect was observed in mussels exposed to Cu (Regoli and Principato 1995), Machreki-Ajmi et al. (2008) and Bonacci et al. (2008) observed cholinesterase inhibition in clams when exposed to Cd.











 nova página do texto(beta)
nova página do texto(beta)


