INTRODUCTION
The amount of potentially available water on the planet is approximately 1386 million km3, of which 97.5 % is marine and brackish water, and only 2.5 % or 35 million km3 is surface freshwater. Of this amount, 70 % is not available for immediate human consumption because it is in the form of glaciers, snow or ice (CONAGUA 2012). Available ground water in aquifers is located at depths from few to several hundred meters, depending on the geohydrology of the water table (Shah et al. 2007). Ground water represents approximately 97 % of the available freshwater resources, excluding the water enclosed in the polar ice caps (Burchi and Mechlem 2005). Of this amount, it is estimated that 37 % is used for irrigating, approximately 89 million ha (Burke 2002). At present, agriculture, livestock and forestry production face great challenges in feeding the growing human population, which is projected to reach 9 billion people in 2050 (FAO 2014). The increase in food production using traditional techniques will demand more water to cover crop irrigation needs. It is estimated that approximately 7130 km3 of water is evapotranspirated by crops, a value that could reach 13 500 km3 by 2050 (IWMI 2007). Fulfilling these growing water demands cannot be solved by only increasing the amount of water pumped from aquifers because this action will cause additional problems, such as higher concentrations of salts, arsenic or other pollutants in the water extracted from ground water reservoirs (Karim 2000), in addition to imposing more pressure on already overexploited aquifers.
Thus, a comprehensive approach must be applied to look for alternative water sources for food production (IWMI 2007), that combines several options for an efficient extraction and use of water resources. The alternatives for procuring such water include unconventional sources of ground water, such as connate or formation waters, which are aquifers normally located at a depth more than 200 m (Ruggieri et al. 2010). There is a lack of available data on the interaction between subterranean aquifers and connate water bodies and on the global volume of connate water or the maximum depths at which they can be found. However, the total land surface of the earth is approximately 148.94 × 106 km2. Thus, the potential volume of connate water is enormous. Recently, another type of ground water reservoir has been described, and it includes water located in the earth's mantle between 410 and 660 km depth and located in hydrated minerals at up to 3 % by weight (Pearson et al. 2014, Schmandt et al. 2014).
Access to connate water deposits occurs because the geological structures that produce hydrocarbons normally contain this type of water, and it is brought to the surface along with the extraction of oil or gas (SEMARNAT 2003). The characteristics and physicochemical composition of connate water depend on the reservoir, minerals in contact with it, and geology and age of the formation in which the oil or gas is produced (Lee et al. 2002, Veil et al. 2004, Clark and Veil 2009). Among other constituents, connate waters may contain salts, oils, fats, and organic and inorganic compounds (SEMARNAT 2003, DOE 2012) at different concentrations, which promote significant variability in chemical characteristics and increases the likelihood of obtained connate water with low or high salt or organic compound contents (Veil et al. 2004, Martel-Valles et al. 2013). The composition of connate waters is modified during the industrial process of gas or oil production because of the addition of surfactants, gels and inhibitors, which is why such waters are referred to as "produced waters" (Manfra et al. 2010).
Several studies have found high variability in the salinity characteristics and content of different elements in produced water, which occurs between hydrocarbon extraction sites that are relatively close to each other (Veil et al. 2004) and in produced water from offshore platforms and inland operations (Veil et al. 2004, Manfra et al. 2010). Therefore, certain types of produced water exhibit salt contents that are acceptable for agricultural use, and the application of such water has been experimentally tested (Veil et al. 2004, DOE 2012, Martel-Valles et al. 2013, Martel-Valles et al. 2014).
However, in most of the of oil-producing countries, produced water is not reused for irrigation purposes. Instead, it is re-injected to improve the extraction of hydrocarbons (SEMARNAT 2003, Veil et al. 2004, CNH 2010) and pumped toward recipient subterranean formations (unproductive wells) or deposited into marine waters (SEMARNAT 2003, Veil et al. 2004).
The use of produced waters in agricultural, livestock or forest applications could represent a feasible option to unconventional water resources, with the potential to reduce the environmental and economic impact of produced water disposal activities within the hydrocarbon industry. Therefore, a literature review was conducted about the origin, characteristics, and applications of produced waters currently permitted by environmental regulations according to USEPA (1993) and SEMARNAT (2003). The objective of this document is to present an updated state-of-the-art review of these topics and proposals on the current and potential use of this important natural resource.
ORIGIN OF PRODUCED WATERS
Ground water reservoirs are believed to originate from meteoric or surface water that seeped or infiltrated into the subsoil through the vadose zone, faults or permeable rocks (FAO 2005). A portion of these waters remained trapped in geological formations and were absorbed into the pores formed by clay or sandy particles in subterranean sediments (Llamas 1993), which explains why they are not considered part of the hydrological cycle (Birkle et al. 2009) and are known as connate or formation waters (Llamas 1993).
The geological structures that produce hydrocarbons normally contain connate waters (SEMARNAT 2003), and the most accepted hypothesis suggests that most of the oil formations were completely saturated with water prior to invasion by oil (Veil et al. 2004).
Once the hydrocarbons become contained in the bedrock, oil fluids are continuously removed through the phenomenon of expulsion toward porous neighboring rocks (Santamaría-Orozco et al. 2009). During primary migration, lower density hydrocarbons migrate to trap sites and displace a portion of the water inside the formation (Veil et al. 2004), then this mass of oil and gas subsequently migrates upward through porous strata because of gravity or the pressure of tectonic plates (Luo et al. 2007) in what is known as secondary migration. This mass becomes a hydrocarbon reservoir that may contain petroleum, gas and water (Veil et al. 2004), which suggests that waters from these formations have been associated with petroleum or gas for millions of years and remained isolated from the atmosphere since then (Martel-Valles et al. 2014).
In the process of producing oil or gas, connate waters are extracted with hydrocarbons (Morales-Bautista et al. 2011), and when hydrocarbon production wells are perforated, the composition of the connate waters is modified through the addition of surfactants, gels and inhibitors. In consequence, this water is called "produced water" (Manfra et al. 2010). During the extraction process, produced waters are brought to the surface along with gas or petroleum and later separated through the dehydration process (Deng et al. 2005).
COMPOSITION OF PRODUCED WATERS
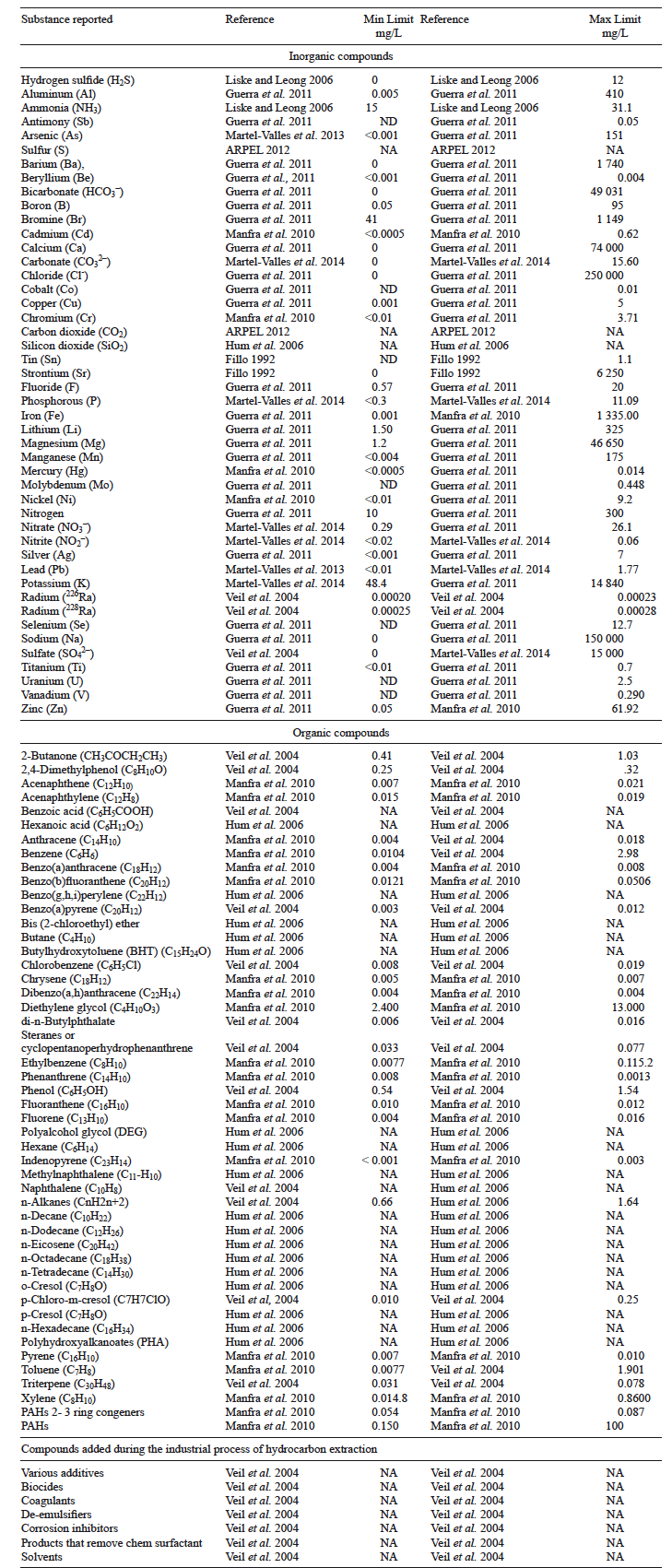
Studies on the characterization of produced waters are abundant, suggesting a wide variation in chemical composition. Results compiled from different publications include data gathered on the different elements, including natural and artificial chemical compounds found in samples of produced waters that have been analyzed and published elsewhere (Table I).
Because connate water has been trapped inside the pores of sedimentary rocks during formation (Veil et al. 2004), associated with petroleum or gas during hundreds of millions of years and because it has been isolated from the atmosphere since then (Martel-Valles et al. 2014), produced water should reflect the composition of the associated geological strata and hydrocarbons. In addition, the effects of interactions along time suggest that produced waters should contain a mixture of organic and inorganic compounds that have dissolved along time (SEMARNAT 2003, Veil et al. 2004, Fakhru'l-Razi et al. 2009).
To corroborate these hypotheses, Manfra et al. (2007) conducted a study to analyze the minerals in sediments surrounding an offshore petroleum-producing platform and correlated their findings with the minerals found in produced water. In this sense, they found that most of elements contained in the sediments corresponded to those quantified in the produced water. In addition, the components found in high concentrations in sediments coincided with those in the samples of produced waters. Similar results were obtained by Connolly et al. (1990), who conducted isotopic measurements of 87Sr/86Sr, D and 18O in produced waters and rocks underlying the Alberta watershed, in Canada, and demonstrated that the composition and variability of produced waters is a result of their origin and also of the water-rock interactions in the watershed.
Produced waters with high salinity can contain up to five or six times dissolved solids as seawater (36000 mg/L) and may reach Cl- concentrations of 150 000 to 180 000 mg/L (Veil et al. 2004). In comparison, seawater contains an average Cl- concentration of 35 000 mg/L (FAO 1994) and an average EC of 55 dS/m (SWRCB 2004). In addition, produced water can contain low molecular weight compounds (benzene, toluene, xylene, and others), organic acids, condensates, oils, fats, aromatic hydrocarbons (PAH), phenols and microorganisms (Head et al. 2003, SEMARNAT 2003). When these compounds are present in produced water, individually or collectively, they can exert negative impacts on the environment, mainly on soil, water and ecosystems, and therefore on living organisms (Veil et al. 2004, Clark and Veil 2009).
The concentration of metal ions in produced water has a complex relationship with the interactions between rocks and water (Rittenhouse et al. 1969). Such concentrations and availability differ from one site to the next because the variations in age and geology of the formations from which petroleum or gas are extracted (Veil et al. 2004). In average, water extracted from gas-producing wells contains several times the concentration of metals in ionic form relative to water from petroleum-producing wells (Jacobs et al. 1992). In addition, the natural composition of such water can be affected by chemical additives used during drilling and production operations (Clark and Veil 2009).
Moreover, produced waters may contain microorganisms (Head et al. 2003), and there is evidence of active microbial communities in petroleum reservoirs (Bailey et al. 1973), where the highest level of activity of such microorganisms is normally found at the water-hydrocarbon interface (England et al. 1987). Specifically, these bacteria are anaerobic and thermophilic and capable of living at depths where connate waters are found (Head et al. 2003) because of a variety of physiological and metabolic adaptations. Such microorganisms include several different phylogenetic affiliations, including methanogenic archaea, bacteria, sulfate-reducing archaea and firmicutes, as well as a large number of fermenting bacteria genera with mesophilic, thermophilic, hyperthermophilic and halophytic attributes. Many of these organisms use hydrocarbons as a source of energy, as they produce biogenic gas (CO2 and CH4) and reduce the viscosity of petroleum (Youssef et al. 2009). This metabolism of petroleum reduces the content of saturated and aromatic hydrocarbons (Head et al. 2003) and increases petroleum's density, sulfur content, acidity and metal content, thus altering the quality of the product (Peters and Fowler 2002). In addition, microbial activity modifies the chemical characteristics of produced water (Murali-Mohan et al. 2013).
During the exploitation period of a hydrocarbon-producing well, the concentration of organic and inorganic compounds in the produced water does not remain constant but varies over time, in consequence, it is difficult to predict it (Veil et al. 2004). Organic and inorganic components of produced water can vary in their physical states, including solution, suspension, emulsion and adsorption of particles (Tibbetts et al. 1992). Manfra et al. (2007) conducted a series of sampling runs on produced water from four platforms in gas-producing wells over three years, and they found that a single well could include variations in the concentrations of organic and inorganic components over time without a predictable trend. Results of sampling conducted by Kuipers et al. (2004) showed that the characteristics of produced water varied among different sites and even between sites that were located in close proximity. Similar results were reported by Rittenhouse et al. (1969), who analyzed 823 samples of produced water from different locations in the United States and Canada.
Variability in the composition of produced water
Because of the wide variability in the content and concentration of inorganic and organic compounds in produced waters (Veil et al. 2004, Manfra et al. 2007, Manfra et al. 2010, Martel-Valles et al. 2013), all sources must be analyzed before use in productive activities. Depending on results, such produced water must be treated using different technologies (osmosis, distillation, ion exchange, physical separation, coalescence, etc.) before use (Fakhru'l-Razi et al. 2009, NPC 2011).
If the produced water has a low percentage of total dissolved solids (TDS), it may represent a valuable resource for crop irrigation (GWPRF 2003, Veil et al. 2004). Based on an analysis of the minerals found in produced waters, Paetz and Maloney (2002) concluded that the most critical variables for determining the direct use of produced water on agricultural lands are salinity (which affects plants), measured as TDS or EC, sodicity or relative content of Na over other cations (which affects soil), and the potential toxicity, mainly determined by the presence of hydrocarbons and metals.
In a study conducted by Manfra et al. (2010), different degrees of sensitivity to produced waters were observed among different organisms. This sensitivity varied according to species and trophic level, as certain levels can transform inorganic substances into organic substances, whereas others can feed on and degrade organic matter. Because the composition and concentration of different components varies in produced water extracted from different sites (Clark and Veil 2009) and different responses to such components are observed among different organisms (Manfra et al. 2010), each source of water must be analyzed and proposed for a specific potential use. Such uses will most likely be limited on a regional basis, however, there is a significant information gap related to such uses.
To characterize produced waters, the following documents (FAO 1994, Hum et al. 2006, ARPEL 2012) have been suggested as guidelines:
Water quality guide for agricultural irrigation (FAO 1994).
Heavy metal limits in water (SEMARNAT 1996, ARPEL 2012).
Organic and inorganic compound standards for water quality in the United States of America (SEMARNAT 1996, ECFR 2015).
Criteria for drinkable and irrigation water (USEPA 2014).
Concentration of trace elements in plant tissues for solutions under normal and toxic growth conditions (ARPEL 2012).
Normal and toxic concentrations of trace elements in soils related to plant growth (Berrow and Burridge l979, Kabata-Pendias and Pendias l984).
Concentration of elements in plants related to toxicity and tolerance in animal feed (ARPEL 2012).
In a case study of the possible agricultural application of produced waters, Martel-Valles et al. (2013) carried out tests in which they used three sources of produced water that were characterized according to Mexico's NOM-143-SEMARNAT-2003, which establishes the environmental specifications for handling connate water associated with hydrocarbons, and according to FAO (1994) for irrigation water quality. These waters were diluted with irrigated water of good quality (SEMARNAT 1996) to reduce the EC and element concentration, and the mixture was used to irrigate tomato plants. Results suggested that produced waters diluted with water of good quality can be used for crop irrigation, although certain water characteristics may limit mineral absorption (except Na), which negatively affected the morphological variables and the number of harvested fruits. The highest impact was found in plants irrigated with produced water containing high concentrations of hydrocarbons, Cu+2 and Cl-1. Produced waters commonly have a cost associated with extraction, separation, storage, transportation and confinement. Such waters can be used directly or diluted, such as in the previously mentioned study in which the ions in the produced water were sufficient to achieve growth and fruit production of plants.
Therefore, the characterization of produced waters has been extensive and variable, and in most cases, has focused on measuring dissolved salts, either through the content of dissolved solids or via the EC, as well as the amount of heavy, medium and light hydrocarbon fractions carried by the water, because this information on salinity and hydrocarbons will guide the potential applications or uses of this water resource. However, a review of different publications indicates that a number of variables have been used to describe the properties of water, which are shown in table II.
GENERATED VOLUME OF PRODUCED WATERS
The quality of produced water can vary with time, and the volume of water extracted from petroleum- or gas-producing wells can also be modified over time (Veil et al. 2004). The water-petroleum ratio can change according to the well age, and wells with a short time under production generate a smaller water volume relative to the petroleum content. However, the opposite trend occurs with time, with the percentage of water increasing and the percentage of petroleum decreasing to the point where the productive activities of the well are finally suspended (Khatib and Verbeek 2003). In the case of gas, an opposite trend to that of petroleum has been found, with newer wells usually producing a large amount of water and the volume of gas increasing and the amount of water decreasing over time as gas converges into the reservoir in the space previously occupied by water (Lee et al. 2002). It is estimated that the oil and gas industry in the United States of America generates ten times more water than oil and gas on average (U.S. Bureau of Reclamation and Sandia National Laboratories 2003). In 2007, the United States of America generated approximately 3.3 × 109 m3 of produced water from nearly 1 000 000 oil and gas producing wells (Clark y Veil 2009).
The situation in Mexico appears to be different because much less water from oil industry is produced than oil and gas. In 2002, a volume of 12.09 × 106 m3 (equivalent to approximately 76.04 × 106 barrels) of produced water were obtained (SEMARNAT 2003), and in 2010, the amount was 12.04 × 106 m3 according to the social responsibility report by the company Petróleos Mexicanos (PEMEX 2010). However, even though these numbers are significantly lower than those reported by the United States of America, they still represent a significant volume of water.
CURRENT AND POTENTIAL USES OF PRODUCED WATERS
At present, 71 % of the produced water is used by the oil industry to maintain pressure in oil reservoirs and to hydraulically drive the hydrocarbon to producing wells (GWPRF 2003). In terms of volume, almost all the water extracted from oil and gas producing wells is injected into non-productive reservoirs, and the remainder is dumped into the sea after treatment (GWPRF 2003, SEMARNAT 2003).
However, available literature report that there are other urban and industrial uses for that water, such as the production of potable water using desalinization systems, recharging of shallow aquifers with water obtained after treating produced water (NETL 2014) or the direct recharging of aquifers with untreated produced water that exhibits low salt concentrations (GWPRF 2003). Industrial uses include dust control on unpaved roads, in which water does not leave the boundaries of the road or is applied near streams or buildings (Murphree 2002), and fire control in locations where fire would cause greater damage relative to the application of saline water to the soil (GWPRF 2003). Other uses include vehicles and equipment washing before transport to other fields to avoid the distribution of seeds or undesirable pathogens (Veil et al. 2004), steam generation (Brost 2002), and cooling of electricity production systems (Veil et al. 2004).
In addition, produced water has also been considered for food production use, such as the agricultural, livestock or forestry sectors.
Agricultural application of produced water
Produced waters can contain essential minerals for the nutrition of plants, such as K+, Ca+2, Mg+2, Na+, Zn+2, Cu+2, SO4 -2, CO3 -2, Cl-, NO3 -, and others (Martel-Valles et al. 2014), which availability is a function of salinity and the interaction of essential ions with other organic and inorganic components present in the produced water (Pessarakli 2011).
Studies on the application of produced waters in crops should include at least an analysis of essential minerals both in water and plants, especially those plant parts consumed by animals or humans. These analyses can be framed according to environmental guidelines and may even include a greater number of variables than those required by the guidelines to define the composition of both, water and plants (Martel-Valles et al., 2013, 2014). Such studies can be broadened to include heavy metals or toxic metalloids, organic compounds and even radioactive elements, with the objective of ensuring the safety of food and the health of ecosystems.
Jackson and Myers (2002) used produced water in a combination of hydroponics and aquaculture and found that the production of tomato and lettuce was lower when produced water was used as compared with a fertilizer solution. They mentioned that this effect was possibly caused by nutritional imbalances in the produced water, which is consistent with the results of Martel-Valles et al. (2013). However, the complete system described by Jackson and Myers (2002) was viable for the production of vegetables and fish. Contact between the produced water and soil was avoided by using a system for recycling water (Jackson and Myers 2002, Veil et al. 2004, NPC 2011). In another study, Paetz and Maloney (2002) used produced water from methane gas extraction to irrigate 100 ha of arid land to produce native forage. These authors applied careful management techniques and treated each water source as unique by constantly monitoring the volume of water and its composition, and also verifying the concentration of Na+ Ca+2, Mg+2 and HCO3 -. Similarly, soil parameters were controlled with the application of amendments as required. The productive process described by Paetz and Maloney (2002) produced a successful harvest, although an analysis of the forage similar to that of Martel-Valles et al. (2013) was not conducted to determine the effect of produced waters on the harvest.
The variability of chemical compounds in terms of their profile and concentration in produced waters will have an effect on the composition of food, but limited investigations have studied this aspect. The mentioned authors used produced waters diluted with irrigation water to irrigate tomatoes in greenhouses and evaluated their morphological response variables such as root, stem, leaf and fruit mineral composition and fruit hydrocarbon content. They concluded that the use of several sources of produced water did not cause detrimental effects in the quality and productivity of the plants.
In another study with tomato plants (Martel-Valles et al. 2014), the use of produced water led to results consistent with those of their 2013 study. However, it is difficult to extrapolate these results to other crops, and additional experiments with other plant species are required, including ornamental and medicinal species.
There is a lack of data on the impact of produced waters on soils, although certain sources of produced water are known to cause negative impacts on the environment, including soil degradation, as well as ground water and surface water pollution (Otton 2006), because they may contain high levels of salts, heavy metals and hydrocarbons (Benko et al. 2008). As a result, hydrocarbon-producing countries have published regulations on the safe limits of compounds contained in the water and authorized methods of disposing of such water (USEPA 1993, SEMARNAT 2003) because its components can have negative impacts on the environment when improperly handled (SEMARNAT 2003).
Mexican regulations state that all sources of connate water are saline or hyper saline. However, studies on the characterization of produced water in Mexico indicate that this not always occurs, and there are sources of water with EC values below the maximum value for irrigation water (Martel-Valles et al. 2014).
Livestock applications for produced water
Produced waters with low concentrations of salt and hydrocarbons have been used as a water source for animals. In the Rocky Mountains of Colorado, reservoirs were built as water sources for wildlife, fish habitat and water fowl (GWPRF 2003). However, species of fish or birds were not included, and regulations on this type of produced water were not reported, although negative impacts from the produced waters in the local wildlife were not observed. For the application of produced waters in water holes for livestock, such water must not contain more than 1000 mg/L of TDS (GWPRF 2003). Although tests have been conducted showing that animals survive after consuming those water (GWPRF 2003, Veil et al. 2004), there are also reports of produced water provoking diarrhea. In a study by Jackson and Myers (2002), produced waters were used to farm tilapia, and the fish reached greater weights compared with those in the control treatment. Nevertheless, the authors reported deaths in certain fish living in produced water tanks, although they did not specify any causes, neither the composition and salt concentration of the water.
The mentioned tests suggest that it is possible to use produced waters in livestock applications at a larger scale. However, because of the great variability evidenced by such waters, it is necessary to chemically characterize and monitor the water sources to avoid toxic effects. Similarly, constant monitoring of weight, size and mineral absorption in animals is essential to prevent illness.
Forestry application of produced water
In California, USA, Brost (2002) described a system used by Chevron Texaco for the treatment of produced water that provides approximately 76 314 m3 to the Kern River field in central California. After treatment and filtering the water, it is pumped to the Cawelo water district to be used for irrigation of orchard and other crops, and it is also used to recharge shallow aquifers. Although, this study does not mention the effects on soil, trees, fruit or their development, and also it does not mention the kind of analyses conducted on the fruits.
CONCLUSIONS
Produced waters are used by the hydrocarbon-producing industry to increase pressure within oil-producing wells, which is often the only productive use. National and international regulations (USEPA 1993, SEMARNAT 2003) consider such waters toxic, which is why only the maximum allowable limits are established for their discharge into receiving bodies and confinement to non-producing wells is recommended.
According to their characteristics, produced waters have the potential to be used in domestic, industrial and agricultural sectors. However, it is essential to characterize such waters according to the applicable rules for specific sites and situations before incorporating them into productive activities. Although produced waters have been used to irrigate crops, limited information is available on their effects on plants, animals, microorganisms and in the whole ecosystem. Therefore, it is suggested to conduct experiments to analyze the response of organisms exposed to such waters.
In most the reviewed references, an insufficient number of variables were used to characterize the produced water quality and organisms in contact with it. The available information suggests that produced water has potential to be used in agriculture, livestock and forestry, although its possible environmental impact is unknown.











 nova página do texto(beta)
nova página do texto(beta)