INTRODUCTION
Metals which enter the environment may generate several problems that demand practical solutions. Among these, conventional methods of remediation such as filtration, precipitation or ion exchange, are costly and at times ineffective (Panchanadikar 1994, Cañizares-Villanueva 2000, Vieira and Volesky 2000), while biological systems or biosorbents, including bacteria, fungi or algae are cheaper and frequently more effective, especially when metal concentrations are low (Rodríguez et al. 2006).
Among different groups of microorganisms, bacteria may play an important role in bioremediation because most of them may adapt easily to unfavorable conditions. Some strains of Bacillus, Pseudomonas and Streptomyces have successfully been used as biosorbents (Yilmaz 2003, Gabr et al. 2008, Vijayaraghavan and Yun 2008). Although most of these microorganisms studied for metal bioremediation were isolated in metal-polluted areas, especially industrial wastewaters and mine tailings (Panchanadikar 1994, Samuel et al. 2012). Therefore, their metal resistance might be due to their ability to prevent metal absorption and/or accumulation through some metal-excluding mechanism, or because of genetically determined detoxification processes (Saier 2003, Marrero-Coto et al. 2010). For this reason, they might be less suitable for bioremediation than those able to absorb and accumulate metals in their biomass (Romero et al. 2006, Monge-Amaya et al. 2008).
The Mexican Pacific coast receives contaminants from natural and anthropic sources. Among these, the Pb and Cd contents in fish and seafood indicate the extent of their environmental impact, which in some cases may reach the level of concern for human health (Frías-Espericueta et al. 2010). In this study two bacterial species were adapted to high external concentrations of Cd and Pb, in order to evaluate their ability to remove one or both of these metals by biosorption.
MATERIAL AND METHODS
Isolation and adaptation to metals
Samples of urban wastewater were plated onto trypticase soy agar medium (TSA), and incubated at 30 ºC for 24 h to allow bacterial growth. Colonies with different morphologies were purified with standard procedures (APHA 1992) and maintained on TSA. The pure strains were adapted to Cd and Pb with the adaptive pressure selection technique (Monge-Amaya et al. 2008), using progressively increasing concentrations up to values higher than those reported for mining effluents.
For this, the strains were grown in liquid trypticase soy (TS) medium spiked with concentrations of Cd increasing from 0.25 to 2.83 mg/L and from 2.5 to 17.4 mg/L of Pb. The lowest concentrations selected correspond to the respective chronic concentration criterion (CCC; USEPA 2009). The highest concentrations were higher than those found in mining effluents (0.309 mg/L for Cd and 6 mg/L for Pb) by Ruíz-López et al. (2010) and Lavado et al. (2010).
Adaptation was evaluated comparing the growth rate and the bacterial concentration determined as optical density (OD), absorbance at 680 nm of the initial, non-adapted strain, to that of the strain grown in metal-added TS medium. The OD readings were taken after 12 and 24 h, since preliminary experiments with adapted and non-adapted strains showed that in both cases, these periods of time marked the end of exponential growth and the onset of the stationary phase of growth, respectively. Adaptation was assumed when growth rates and OD values of the adapted strains were significantly higher than those determined in the non-adapted strain (Samuel et al. 2013).
Identification of adapted strains
The strains adapted to Cd and Pb, were used for total genomic DNA extraction with a Cyclo-PrepTM Genomic DNA isolation kit (Amaresco®). The 16S ARNr gene amplification with PCR was performed using 50 ng/µL of the DNA of each strain and the universal primers: Forward 27f.1 (AGR GTT TGA TCM TGG CTC AG) and Reverse 1492R2 (GGT TAC CTT GTT ACG ACT T). The amplification program was: 16S: 94 ºC/2' → 35 cycles (94 ºC/1' → 56 ºC/1' → 72 ºC/1') → 72 ºC/5' → 4 ºC/∝. For species identification, the sequences obtained were compared to the public database EzTaxon available online with its analytical functions at http://www.ezbiocloud.net/eztaxon (Kim et al. 2012).
Metal removal and analysis
The potential of Cd and Pb removal by the two adapted strains were determined in two separate experiments. In each experiment both strains were grown in metal-free TS medium for 24 h, centrifuged (3000 rpm, room temperature) and used to start new triplicate cultures in 16 mL test tubes with 10 mL of TS medium, added with 2.83 mg/L of Cd and 17.4 mg/L of Pb. Triplicate cultures in metal-free medium served as control group, all cultures were maintained in a thermoregulated (37 ºC, 180 rpm, continuous operation) Thermo Scientific MaxQ 4000 orbital shaker.
After 24 h all cultures were centrifuged at 5000 rpm in a refrigerated centrifuge (Sorvall Legend T, 4 ºC) for 30 min. The supernatant was decanted and stored for metal analysis, the pellet was re-suspended in 10 mL of 10 mM EDTA, and vortexed for 15 min to separate the metal adsorbed on the bacterial cell surface (Bashkar and Bhosle 2006). The re-suspended bacteria were centrifuged again at 5000 rpm at 4 ºC for 20 min. The resulting pellet was digested in 3 mL of concentrated nitric acid (Fluka®) in a Teflon vessel at 120 ºC for 4 h. No solid residues were left after this digestion.
Samples of the medium of the adsorbed metal-containing EDTA and of the digested bacterial biomass diluted with 7 mL of Milli-Q water, were transferred to polypropylene vials, which were stored and refrigerated until analysis (Frías-Espericueta et al. 2009) by atomic absorption spectrophotometry (AAS; Varian SpectrAA-220).
Blanks were used after every 25 samples and the accuracy of the analytical method was evaluated using certified reference materials IAEA-392 (algae) and IAEA-331 (spinach) (IAEA 2009). The recoveries for Cd were 107.02 and 97.31 %, in algae and spinach, respectively. While for Pb was 90.42 % in algae.
Statistical analysis
The mean OD values, determined after 12 and 24 hof growth of the initial and adapted strains of each species, were compared using Student´s t-test or the equivalent Mann-Whitney test when data were not normal or homoscedastic (Kolmogorov-Smirnov and Fishers' F test, respectively). The same tests were used to compare the amounts of Cd and Pb in the medium, those adhered to the cell walls and that contained in the biomass of each adapted strain. All tests were performed with ∝ = 0.05.
RESULTS
The strains were identified as Pseudomonas aeruginosa JCM 5962 (T) and Enterobacter cloacae dissolvens LMG 5962 (T), with similarity percentages of 99.8 and 99.5 %, respectively. There were significant differences between the OD readings determined after 12 and 24 h of growth for the adapted and not adapted strains in TS medium spiked with 2.83 of Cd or 17.4 mg/L of Pb. This difference was especially evident with both metals in the case of E. cloacae (Table I).
Table I Mean absorbance values (± standard deviation) at 680 nm (optical densities, OD) of adapted (A) and not adapted (NA) strains of Enterobacter cloacae (Ec) and Pseudomonas aeruginosa (Pa) after 12 and 24 hours with 2.83 mg/L of Cd or 17.4 mg/L of Pb
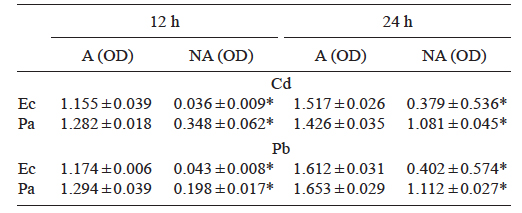
* indicates significant differences between mean values of adapted and non-adapted strains (t-test).
In the biosorption experiments, both strains retained Cd with equally low efficiency: after 24 h of exposure the amount adsorbed on cell walls was approximately 7 % of the Cd added to the growth medium, and the amount in the endocellular fraction was slightly higher, ranging from 7.4 to 9.6 % in E. cloacae and P. aeruginosa cultures, respectively. There were no significant differences (P > 0.05) between the total Cd removed by either strain (Table II).
Table II Mean concentrations of Cd (mg/L) in culture medium (Me), cell wall (M) and bacterial biomass (BM) of Enterobacter cloacae (Ec) and Pseudomonas aeruginosa (Pa) culture in TS medium added with 2.83 mg/L of Cd. The percentages of the initial concentration are in parenthesis
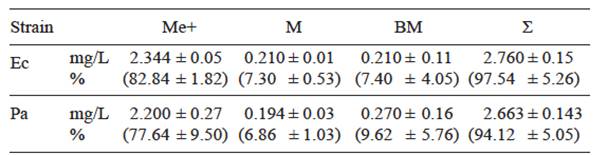
+ = Non parametric (Mann-Whitney) test, Ʃ = Total concentration (sum of Me+M+BM)
Removal efficiency was far higher in the case of Pb. The mean amount contained in the spent growth medium of the E. cloacae cultures was significantly higher than that determined in the medium of P. aeruginosa (2.13 and 1.27 mg/L, equivalent to 12.5 and 7.3 % of the initial values, respectively). The amount retained by the biomass of P. aeruginosa was significantly higher than that of E. cloacae. Cell walls adsorbed approximately similar amounts, close or higher to 15 % of the total Pb added to the growth medium (Table III).
Table III Mean concentrations of Pb (mg/L) in culture medium (Me), cell wall (M) and bacterial biomass (BM) of Enterobacter cloacae (Ec) and Pseudomonas aeruginosa (Pa) cultures in TS medium added with 17.4 mg/L of Pb. The percentages of the initial concentration are in parenthesis
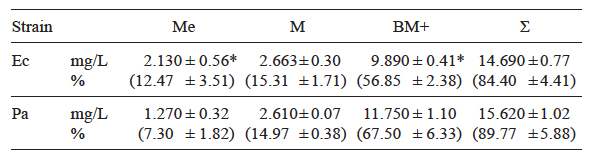
+ = Non parametric (Mann-Whitney) test, * = significant difference between strains (t-test), Ʃ= Total concentration (sum of Me+M+BM)
DISCUSSION
Most studies on bacteria-mediated remediation of metal-contaminated wastewaters, used bacterial strains isolated from industrial effluents or mining sites. In these cases, bacteria were used after selective pressure, which served to adapt the strains to concentrations of the pollutant higher than those of their original site (Romero et al. 2006, Monge-Amaya et al. 2008, Samuel et al. 2012, 2013). This work provides evidence that the same technique can be used for adaptation to high metal concentrations of bacterial strains isolated from wastewater of different origin. Additionally, these adapted strains are good candidates for effluent bioremediation, at least in the case of Pb.
Pb removal by both strains would have occurred initially by adsorption to the bacterial cell wall, followed by active transmembrane transport, chelation or sequestration of metal ions and accumulation within the cell (King et al. 2007). In the case of Cd, the lack of accumulation in the cell biomass may be explained by metal exclusion, or by detoxification mechanisms similar to those described for antibiotic resistance, which represent the main defense of bacteria in the presence of external toxicants (Saier 2003, Pana 2012).
A similar resistance to several metals, including Cd, was observed in Alcaligenes eutrophus by Nies and Silver (1988). There are numerous additional examples such as the ability to withstand high arsenic concentrations described for several aquatic bacterial strains by Takeuchi et al. (2007). This ability may be due to the activity of members of the cation diffusion facilitators (CDFs) family, which favors the efflux of divalent cations, thus preserving metal homeostasis (Zeytuni et al. 2014), or to one or more of the several, sometimes overlapping, metal resistance systems summarized by Nies (2003) and more recently by Marrero-Coto et al. (2010).
CONCLUSION
Both strains could be adapted to high external concentrations of Cd and Pb, which was shown by the poor growth rates of the original (not adapted) strains when cultured in Cd and Pb-spiked media. However, the differences in Cd and Pb retention shown by both strains indicate different resistance mechanisms, which in one case (Pb) allow bacteria to accumulate the metal within their cell biomass. Although, in the second (Cd) bacteria might prevent metal influx, or could maintain internal concentrations of the metal within safe levels through the balance of metal influx and efflux.
For this reason, neither strain is a good candidate for bioremediation of Cd, while both may be used for the treatment of Pb-contaminated wastewater, since Pb is retained by both strains, in part through adsorption to the cell walls, but mostly through its retention by absorption within the bacterial biomass.











 text new page (beta)
text new page (beta)


