Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista internacional de contaminación ambiental
versión impresa ISSN 0188-4999
Rev. Int. Contam. Ambient vol.31 no.4 Ciudad de México nov. 2015
Acute toxity and sublethal effects on macromolecules concentration, caloric content, and lipid peroxidation during exogenous-feeding of Danio rerio larvae exposed to Cu2+
Toxicidad aguda y efectos subletales en la concentración de macromoléculas, contenido calórico y lipoperoxidación en larvas de Danio rerio con alimentación exógena expuestas a Cu2+
Jesús Rodríguez-Estrada1,2,3, Alma Socorro Sobrino-Figueroa2 and Fernando Martínez-Jerónimo1*
1 Laboratorio de Hidrobiología Experimental, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Prolongación Carpio y Plan de Ayala s/n. Colonia Santo Tomás, México, D. F., México, C.P.11340*Corresponding author: fjeroni@ipn.mx; ferjeronimo@hotmail.com
2 Laboratorio Alejandro Villalobos, Departamento de Hidrobiología, Universidad Autónoma Metropolitana Iztapalapa, Avenida San Rafael Atlixco No. 186, Colonia Vicentina, México, D.F, México, C.P. 09340
3 Programa de Doctorado en Ciencias Biológicas y de la Salud, Universidad Autónoma Metropolitana, Iztapalapa, Avenida San Rafael Atlixco No. 186, Colonia Vicentina, México, D.F, México, C.P. 09340
Received January 2015;
accepted April 2015
Abstract
Copper is an essential micronutrient, involved as coenzyme in different metabolic processes. Due to its toxic effect, copper is also used as algicide and herbicide. Residual concentrations of this metal are released into aquatic environments as effluents from mining activities, industrial applications, and other sources, producing toxic effects on the aquatic biota. Although Danio rerio (zebrafish) embryos and larvae have been broadly used as surrogate species to assess toxic effects in water, scarce information exists on copper's effects on fish larvae starting to feed exogenously (after yolk sac depletion). For this reason, the toxic effect of Cu on exogenous feeding larvae was assessed. Acute toxicity (96 h) was determined in 10 and 20 day post-fertilization (dpf) larvae. Additionally, the effect of sublethal Cu concentrations on the main macromolecules concentration, caloric content, and lipid peroxidation was also determined in 10 dpf larvae, as these were the most sensitive during acute exposure. The median lethal concentration (LC50) and confidence limit values (P = 95 %) were 148.52 ± 13.5 and 274.20 ± 40.21 μg/L for 10 and 20 dpf larvae, respectively. The caloric content, glycogen, and lipid concentration were markedly reduced in the 10 dpf larvae exposed to Cu. The malondialdehyde concentration was significantly modified in D. rerio larvae exposed during 48 h to the toxicant. Cu2+ concentrations as low as 16.04 and 32.08 μg/L produced biochemical impairment in D. rerio exogenous feeding larvae. Our results warn about harmful effects on the aquatic biota exposed to Cu2+, because its concentrations in polluted waters can be higher than those assayed here.
Key words: zebrafish, toxic metals, oxidative stress, biomarkers, early life stages.
Resumen
El cobre es un micronutriente esencial involucrado en diferentes procesos metabólicos. Sin embargo, debido a que en concentraciones superiores a las requeridas tiene efecto tóxico, se usa como alguicida y herbicida, por lo que residuos de este metal son liberados en ambientes acuáticos, así como a efluentes de actividades mineras y aplicaciones industriales, causando efectos negativos en la biota acuática. Aunque los embriones y larvas de Danio rerio (pez cebra) han sido ampliamente utilizados para evaluar la toxicidad en el agua, existe poca información sobre los efectos del cobre en larvas que han iniciado su alimentación exógena (después de que las larvas han agotado el vitelo), por lo cual en el presente estudio se evaluó la toxicidad del Cu2+ en esta fase crítica del desarrollo. Se determinó la toxicidad aguda (96 h) en larvas de 10 y 20 días post fertilización (dpf) y se evaluó el efecto de concentraciones subletales sobre la concentración de macromoléculas, contenido calórico y peroxidación de lípidos en larvas de 10 dpf, debido a que fueron las más sensibles durante la exposición aguda. Los valores de CL50 e intervalos de confianza (P = 95 %) fueron 148.52 ± 13.5 y 274.29 ± 31.15 μg/L, para larvas de 10 y 20 dpf, respectivamente. El contenido calórico y la concentración de glucógeno y lípidos disminuyeron significativamente en las larvas expuestas a cobre, asimismo, se observó peroxidación de lípidos. La concentración de malondihaldeído se modificó significativamente en las larvas de D. rerio expuestas durante 48 h al tóxico. Concentraciones de Cu2+ tan bajas como 16.04 y 32.08 μg/L, produjeron efectos negativos en las larvas de D. rerio, por lo que se pueden presuponer efectos nocivos en la biota expuesta a este metal, debido a que las concentraciones en ambientes contaminados pueden ser más altas que las aquí ensayadas.
Palabras clave: pez cebra, metales tóxicos, estrés oxidativo, biomarcadores, estadios tempranos de desarrollo.
INTRODUCTION
Copper (Cu2+) is considered a persistent contaminant that enters the environment as a byproduct or residue from different anthropogenic activities such as the use of pesticides, Cu mining activities, and derived from industrial or municipal wastes (WHO 2004, Kiaune and Singhasemanon 2011). Cu is an essential micronutrient required at low concentrations by organisms to perform some vital functions (Kamunde et al. 2002). However, at levels above those required, it becomes toxic, causing alterations in membrane permeability, interfering with oxygen transport, and affecting energetic metabolism in fish (Carvalho and Fernandes 2008, Kiaune and Singhasemanon 2011). In the zebrafish (Danio rerio), Cu delays or avoids hatching of embryos and produces malformations and mortality. Besides, at low concentrations, it tends to accumulate in larvae, its excess causes oxidative stress and can damage gills, liver, intestine, and the nervous system (Craig et al. 2007, Hernández and Allende 2008). Zhang et al. (2012) assessed the effect of Cu on D. rerio embryos, reporting 9.49 % mortality at a concentration of 100 μg/L. According to Luzio et al. (2013), Cu2+ produces apoptosis in D. rerio's gill epithelium at concentrations of 12 and 100 μg/L.
Copper can produce free hydroxyl radicals (OH.) by decomposing H2O2 through the Fenton reaction (Valko et al. 2005). The OH. radical is highly reactive and attacks fatty acids of cell membranes, causing changes in their structure and functions. The damage caused by oxidant radicals on lipids under oxidative stress conditions is known as lipid peroxidation. Malondialdehyde (MDA) is one of the main released byproducts that can be determined by the thiobarbituric acid reactive substances (TBARS) method to measure the affectation lipids of cell membranes (Devasagayam et al. 2003).
Exogenous feeding in fish larvae is required to obtain energy to be allocated to vital functions, such as maintenance, growth and storage during growth. When larvae are exposed to toxic substances, energy is used to face the detoxification process, causing imbalances in homeostasis and threatening the metabolic status, growth, and reproduction (Augustine et al. 2011, Sokolova et al. 2012). Results by Jezierska et al. (2009) confirm that toxic metals affect embryonic development of fish through energetic expenditure during detoxification, leaving less energy available for growth.
The main macromolecules affected during the detoxification process are carbohydrates, lipids, and proteins, which are important reserves during long periods of energy expenditure (Sokolova et al. 2012) or fasting. In fish, glycogen is one of the main energetic reserves mobilized during detoxification (Smolders et al. 2003).
Although D. rerio is a species frequently used as test organism in aquatic toxicological studies (Gerhard 2003, Hsu et al. 2007, Ulloa et al. 2008, Belyaeva et al. 2009, He et al. 2014), information related to the biochemistry in larvae starting exogenous feeding is limited, because most of the toxicity studies with early life stages have been performed in embryos or yolk-sac larvae (Flynn et al. 2009), probably because the change to exogenous feeding is usually a period of high mortality in fish (Sloman and McNeil 2012, Viegas et al. 2012). D. rerio adults have also been used as test organisms in studies on genotoxic effects of arsenic (Baez-Ramírez and Prieto García 2005, Prieto-García et al. 2006).
The United States Environmental Protection Agency (USEPA 2007) establishes that Cu occurs naturally in surface waters at values ranging from 0.2 to 30 μg/L, but Seker and Kutlu (2014) reported 0.95 and 1.37 mg/L in two rivers that they studied. The Official Mexican Norm NOM-001-SEMAR-NAT-1996 (SEMARNAT 1996) establishes the maximal permissible limits of Cu for the protection of aquatic life at 4 mg/L (monthly average) to 6 mg/L (daily average). These values indicate its high toxic potential, yet they can be easily exceeded reaching risk levels for aquatic biota. For example, in the Sonora River in Mexico, Cu concentrations in the range of 0.21 to 50.0 mg/L have been reported, but it is argued that these values are related to the mining activities in that area (Gómez-Álvarez et al. 2004).
Cu is an essential metal for life, but it is also used in numerous industrial applications and as a biocide, reaching toxic concentrations in aquatic environments. The larval stage is considered critical in oviparous fish, mainly when larvae start their exogenous feeding (after yolk sac reserves have been consumed), and this stage is, in nature, a high mortality phase due to alimentary limitations. Based on the aforementioned, the objective of the present study was to evaluate the acute toxicity of Cu2+, as well as the effects of its sublethal concentrations on the energetic balance (macromolecules concentration and caloric content) and lipid peroxidation in exogenous-feeding D. rerio larvae.
MATERIALS AND METHODS
Production of exogenous-feeding Danio rerio larvae
D. rerio adults were obtained from the Laboratory of Experimental Hydrobiology of the Escuela Nacional de Ciencias Biológicas (National School of Biological Sciences), from Instituto Politécnico Nacional (National Polytechnic Institute). To obtain embryos, mature females and males were placed in 40 L aquaria at a 1:2 proportion. The fertilized eggs were separated and placed in reconstituted hard water (192 mg/ L NaHCO3, 120 mg/L CaSO4 2H2O, 120 mg/L MgSO4, 8 mg/L KCl) (USEPA 2002) supplemented with methylene blue to prevent infections, before being incubated in environmental chambers at 25 °C with a 16:8 (light:darkness) photoperiod. Newly hatched larvae were transferred to aquaria, and before the yolk sac was fully consumed (at around the 5th day), larvae were fed ad libitum with the rotifer Brachionus angularis. Afterwards they were fed twice daily with rotifers and a micropellet of balanced food. In this way, we obtained 10 and 20 day post fertilization (dpf) larvae that were used in all the bioassays.
Acute toxicity bioassays
A Cu2+ concentrated solution was prepared from CuSO4•5H2O (J. T. Baker, purity of 98.9 %) dissolved in deionized water, and seven Cu nominal concentrations were assayed: 80, 160, 240, 320, 400, 480, and 560 μg/L plus control. The actual Cu concentration determined in the stock solution was less than 5 %, below the nominal value (HACH Bicinchoninate method 8506). Reconstituted hard water (USEPA 2002) was used as dilution water in all tests. Each toxicant concentration was tested in triplicate, exposing five 10 or 20 dpf larvae in each replicate (15 organisms for each concentration in total) and at least three bioassays were performed. Test volume was 100 mL in 150 mL flasks. Bioassays were incubated in a bioclimatic chamber with a 16:8 (light:darkness) photoperiod and at 25 °C.
The assessed response was immobility or mortality at 96 h. Test organisms were not fed during the exposure time. Mortality data recorded at the end of the test (96 h) were used to determine the median lethal concentration (LC50) through the Probit method using the Risk Assessment version 1.0 software package.
Lipids, proteins, carbohydrates, and glycogen content
From the LC50 values obtained from the acute toxicity bioassays, three sublethal concentrations were established, corresponding to 1/25, 1/10, and 1/3 of the previously determined LC50. In this case, 10 dpf larvae were exposed for 24 h to 6.42, 16.04, and 53.46 μg Cu+2/L.
After the 24 h exposure, five larvae were placed in 1.5 mL Eppendorftubes and homogenized with 500 μL of 2 % sodium sulfate. From this homogenate, 200 μL were taken to determine lipids, carbohydrates, and glycogen, and 150 μL were used to quantify protein.
The concentrations of lipids, carbohydrates, and glycogen were determined according to Dubois, as described in Gündüz et al. (2010), with slight modifications. Briefly, to 200 μL of the homogenate, 450 μL of 2:1 (v/v) chloroform:methanol were added, vortexed, and 50 μL of deionized water were incorporated to separate the phases. The organic phase was removed to quantify lipids, whereas the aqueous phase was used to quantify total carbohydrates. The white precipitate in the tubes was used to determine the amount of glycogen. The carbohydrates fraction and the white precipitate were supplemented each with 200 μL of 5 % phenol + 500 μL of concentrated H2SO4. The amount of carbohydrates and glycogen was determined by interpolating the absorbance values (490 nm) in a dextrose curve (Arzate-Cárdenas and Martínez-Jerónimo 2012a).
The fraction with lipids was dried at 80 °C, then supplemented with 40 μL of H2SO4 and heated at 70 °C for 2 min. Afterwards, samples were cooled on ice to add 960 μL of vanillin reagent. Tubes were vortexed and absorbance was read at 525 nm, a standard cholesterol curve was used for quantification (Cheng et al. 2011).
The amount of protein was determined by the Bradford method, taking 150 μL of the homogenate + 450 μL of Bradford's reagent. Absorbance was read at 595 nm, a standard curve of bovine serum albumin was used for quantification (Arzate-Cárdenas and Martínez-Jerónimo 2012b).
Caloric content
The amounts of lipids, protein, and glycogen were used to calculate the caloric content in D. rerio larvae. The amount of macromolecules was multiplied by the following factors: 9.45 cal/mg for lipids, 5.65 cal/mg for total proteins, and 4.10 cal/mg for glycogen (Arzate-Cárdenas and Martínez-Jerónimo 2012a). Results are expressed as millicalories per larva (mcal/larva).
Lipid peroxidation
One of the main products of lipid peroxidation is the formation of malondialdehyde (MDA), which was determined by the TBARS method (Devasagayam et al. 2003). We distributed 21 larvae of 10 dpf per concentration in three replicates that were exposed for 24 and 48 h to two sublethal concentrations equivalent to 1/25 and 1/5 of the LC50 of Cu (6.42 and 32.08 μg/L). After the exposure period, seven larvae for each replicate were homogenized in 2.5 mL of potassium phosphate buffer (0.2 mM) pH = 7.2, using a tissue homogenizer. Homogenates were centrifuged at 1000 g for 15 min, at 4 °C. Two mL of the thiobarbituric acid (TBA) reagent (15 % trichloroacetic acid [w/v], 0.5 N TBA, and 0.25 N HCl) were added to 1 mL of the supernatant; samples were incubated at 70 °C during 25 min to develop a pink color. Finally, the samples were ice-cooled and absorbance (532 nm) was determined. The amount of MDA was calculated using the molar extinction coefficient, 1.56 x 105/M/cm.
Statistical analysis
Student's t-test was used to determine significant differences in the LC50 values between the two larva ages assessed (10 and 20 dpf).
Protein, lipids, carbohydrates, and glycogen content, as well as the caloric content, were evaluated with one-way analysis of variance (ANOVA) and Dunnett's tests.
MDA levels were analyzed with two-way ANOVA (taking as factors the exposure time and the toxicant's concentration), and the Fisher's Least Significant Difference (LSD) test was used to determine differences among treatments. All statistical analyses were performed with the Statistica ver. 7.0 software for Windows.
RESULTS
The average LC50 and the 95 % confidence limit values obtained in three bioassays with Cu2+ for the 10 dpf D. rerio larvae was 148.52 ± 13.5 pg/L, whereas in the five bioassays for the 20 dpf larvae was 274.29 ± 31.15 μg/L. Student t-test revealed statistically significant differences in the LC50 between both larval ages (P = 0.0011), the 10 dpf individuals corresponded to the most sensitive stage.
Table I shows that larvae exposed to Cu exhibited a significant reduction in the amount of lipids and in caloric content as compared to the control (P < 0.01). Glycogen concentration was also lower in the exposed organisms, but the differences were only significant at the 6.42 and 53.46 μg/L concentrations, because of this, it was not possible to establish a pattern between the observed effect and the toxicant's concentration. The concentration of protein and carbohydrates did not differ in any of the treatments as compared to the control.
MDA concentration was not modified in control larvae during the experiment, but at 48 h of Cu2+ exposure an increase in MDA levels was observed as compared to the control group at the two tested concentrations (LSD test, P < 0.05; Fig. 1).
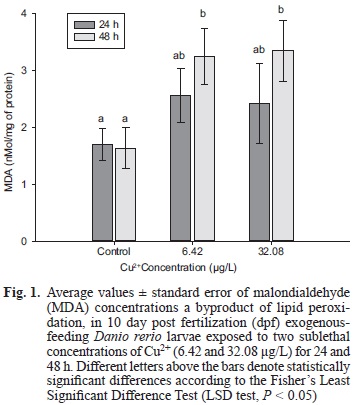
DISCUSSION
The 10 dpf larvae were more sensitive to Cu, which could be because at this age individuals are still undergoing anatomical and physiological development, and metabolic changes are fast (Parichy et al. 2009), since larvae complete their full development to juveniles in around 26 days (Augustine et al. 2011). It is also possible that the 20 dpf larvae were less sensitive to Cu2+ because they were able to retain a larger amount of energy reserves obtained from the exogenous food, which would allow them to tolerate larger concentrations of the toxicant. In addition, older larvae might have a more complete enzymatic system, including some of the enzymes required for the detoxification process.
The difference in copper sensitivity with respect to age in D. rerio was also observed by Alsop and Wood (2011), who exposed yolk-sac larvae, as well as adults, and found LC50 values of 148.4 and 212.1 μg/L, respectively. On the other hand, Hernandez et al. (2011) reported that yolk-sac larvae exposed to Cu were less sensitive than adults (LC50 = 878.20 μg/L and 120.74 μg/L, respectively), but Freiry et al. (2014) observed that 10 day D. rerio larvae were more sensitive to Cu than 60 day adults. Results obtained herein indicate that, in individuals that have started their exogenous feeding, the younger larvae (10 dpf) are more sensitive to Cu than the older individuals (20 dpf). It can be hypothesized that the 20 dpf larvae developed metabolic mechanisms of physiological tolerance related to a better use of nutrients obtained from food than the 10 dpf larvae, which have an incipient enzymatic system, with oral and feeding structures still under development.
Johnson et al. (2007) found, in D. rerio embryos, that Cu at 93 to 464 μg/L concentrations causes a decrease in larval length, and the yolk sac increases in size, which could hypothetically mean an increase in energetic reserves, but this is an anomalous condition with probable negative effects on development.
According to Campagna et al. (2008), exposure to toxic copper concentrations affects survival and growth, and produces damage in the gills of D. rerio larvae. In this respect, Kamunde et al. (2002) mention that the juvenile stages of fish are probably more susceptible to suffer the effects of intoxication because they have an immature excretion system and high energetic demands directed to growth.
Oliveira-Filho et al. (2004) assessed in D. rerio adults the effect of different pesticides containing Cu and reported LC50 values of 95, 75, and 38 μg/L, which are lower than those obtained in our study for larval stages. These differences in sensitivity could be because they used soft water (hardness of 40 to 48 mg/L as CaCO3), whereas we used reconstituted hard water as dilution medium (hardness of 160 to 180 mg/L as CaCO3), it has been documented that Ca and Mg ions reduce metal toxicity (Ebrahimpour et al. 2010). According to the aforementioned, only exceptionally, results in fish could indicate a higher sensitivity in adults with respect to their early developmental stages.
In D. rerio adults exposed to HgCl2 (0.07 mg/L), a decrease in the amount of glycogen and protein has been recorded in gills, muscle, and viscera (Vutukuru and Basani 2012). In our study, glycogen content was also significantly reduced with the highest and lowest Cu concentrations. Sokolova et al. (2012) determined that under normal conditions, organisms allocate energy to maintenance, growth and reproduction. Part of this energy is stored but, under stress conditions the energy stored in lipids, carbohydrates, and proteins is used to maintain basal activities and survival. We observed that, compared to the control, the lipid content decreased significantly when Cu concentration increased. This result demonstrates that exogenous-feeding larvae use mainly lipids as the main energy reserve under stress conditions to cope with the negative effect of this toxic element, a similar situation occurs during starvation periods in D. rerio larvae (Flynn et al. 2009)
In copper-exposed larvae, peroxidation of lipids (measured as MDA concentrations) increased with respect to the toxicant's concentration, and also with exposure time, being significantly higher at 48 h. Similar results have been found in other fish, but at higher copper concentrations than those tested herein. In this regard, Trivedi et al. (2012) observed increased MDA levels in the liver of Carassius auratus exposed to Cu2+ (0.1 to 1.5 mg/L), while Kong et al. (2013) observed, in embryos and larvae of this same species, an increase in MDA levels at Cu concentrations of 0.4 to 1 mg/L. These results confirm the cell membrane damage produced by Cu exposure, revealing the negative physiological effects exerted by this metal.
CONCLUSIONS
In conclusion, the main energetic reserves mobilized during the intoxication process in larvae exposed to Cu were lipids and glycogen. Cu effects were observed starting at the 16 μg/L concentration, which is a value below the one reported as safety limit for the aquatic environment. In Mexico, the maximum allowable limit to protect aquatic life is 6 mg/L of Cu2+ (daily average) (SEMARNAT 1996), which is much higher than the values that produce lethal and sublethal effects in exogenous-feeding D. rerio larvae, as observed herein. According to the latter, negative effects could be expected on aquatic biota, which indicates the need to review and correct the safety Cu levels to allow for the protection of freshwater environments.
ACKNOWLEDGMENTS
J. Rodríguez-Estrada is a student of the PhD program "Doctorado en Ciencias Biológicas y de la Salud" at the Universidad Autónoma Metropolitana (UAM), sponsored by a fellowship granted from the Consejo Nacional de Ciencia y Tecnología (CONACYT, No. 227293). A. S. Sobrino-Figueroa thanks the C. B. S. Division of UAM-Iztapalapa for financing the project entitled: Evaluación del estado de salud de organismos de importancia ecológica y/o económica presentes en sistemas acuáticos. F. Martínez-Jerónimo thanks to the Instituto Politécnico Nacional (IPN)-Secretaría de Investigación y Posgrado, and COFAA and EDI (IPN) for their support to this project. Finally, we thank the two anonymous reviewers whose comments and critical evaluation helped to improve this paper.
REFERENCES
Alsop D. and Wood C.M. (2011). Metal uptake and acute toxicity in zebrafish: common mechanisms across multiple metals. Aquat. Toxicol. 105, 385-393. [ Links ]
Arzate-Cárdenas M.A. and Martínez-Jerónimo F. (2012a). Energy reserve modification in different age groups of Daphnia schoedleri (Anomopoda: Daphniidae) exposed to hexavalent chromium. Environ. Toxicol. Parmacol. 34, 106-116. [ Links ]
Arzate-Cárdenas M.A. and Martínez-Jerónimo F. (2012b). Energy resource reallocation in Daphnia schodleri (Anomopoda: Daphniide) reproduction induced by exposure to hexavalent chromium. Chemosphere 87, 326-332. [ Links ]
Augustine S., Gagnaire B., Adam-Guillermin C. and Kooijman S.A.L.M. (2011). Developmental energetics of zebrafish, Danio rerio. Comp. Biochem. Physiol. Part A 159, 275-283. [ Links ]
Báez-Ramírez O.A. and Prieto-García F. (2005). Genotoxic damage in zebra fish (Danio rerio) by arsenic in waters from Zimapán, Hidalgo, Mexico. Mutagenesis 20, 291-295. [ Links ]
Belyaeva N.F., Kashirtseva V.N., Medvedeva N.V., Khudoklinova Y.Y., Ipatova O.M. and Archakov A.I. (2009). Zebrafish as model system for biomedical studies. Biochemistry (Moscov) suppl. series B: Biomed. Chem. 3, 343-350. [ Links ]
Campagna A.F., Fracácio R., Rodrigues B.K., Eler M.N., Fenerich-Verani N. and Espíndola E.L.G. (2008). Effects of the copper in the survival, growth and gill morphology of Danio rerio (Cypriniformes, Cyprini-dae). Acta Limnol. Bras. 20, 253-259. [ Links ]
Carvalho C.S. and Fernandes M.N. (2008). Effects of copper on liver key enzymes of anaerobic glucose metabolism from freshwater tropical fish Prochilodus lineatus. Comp. Biochem. Physiol. A. 151, 437-442. [ Links ]
Cheng Y. S., Zheng Y., Jean S. and Gheynst. (2011). Rapid quantitative analysis of lipids using a colorimetric method in a microplate format. Lipids 46, 95-103. [ Links ]
Craig P.M., Wood C.M. and McClelland G.B. (2007). Oxidative stress response and gene expression with acute copper exposure in zebrafish (Danio rerio). Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1882-R1892. [ Links ]
Devasagayam T.P.A., Boloor K.K. and Ramasarma T. (2003). Methods for estimating lipid peroxidation: an analysis of merits and demerits. Indian J. Biochem. Biophys. 40, 300-308. [ Links ]
Ebrahimpour M., Alipour H. and Rakhshah S. (2010). Influence of water hardness on acute toxicity of copper and zinc on fish. Toxicol. Ind. Health 26, 361-365. [ Links ]
Flynn III E.J., Trent C.M. and Rawls J.F. (2009). Ontogeny and nutritional control of adipogenesis in zebrafish (Danio rerio). J. Lipid Res. 50, 1641-1652. [ Links ]
Freiry R., Stelzer J.A.A., Maltchik L. and Arenzon A. (2014). Sensitivity of Danio rerio (Teleostei, Cyprinidae) during two stages of development based on acute toxicity test. Bull. Environ. Contam. Toxicol. 93, 442-445. [ Links ]
Gerhard G.S. (2003). Comparative aspects of zebrafish (Danio rerio) as a model for aging research. Exp. Geront. 38, 1333-1341. [ Links ]
Gómez-Álvarez A., Villalba-Atondo A., Acosta-Ruíz., Castañeda-Olivares G. and Kamp D. (2004). Metales pesados en el agua superficial del río San Pedro durante los años 1997 y 1999. Rev. Int. Contam. Ambie. 20, 1-8. [ Links ]
Gündüz E.A., Gülel A., Isitan V.Ö., Boz A. and Cesur Ö. (2010). Effect of sugar feeding on lipid, glycogen, and total sugar levels of a female parasitoid, Bracon hebetor (Say) (Hymenoptera: Braconidae). Turk. J. Agric. For. 34, 343-347. [ Links ]
He J.H., Gao J.M., Huang C.J. and Li C.Q. (2014). Zebrafish models for development and reproductive toxicity. Neurotoxicol. Teratol. 42, 35-42. [ Links ]
Hernandez P., Undurraga C., Gallardo V.E., Mackerzie N., Allende M.L. and Reyes A.E. (2011). Sublethal concentrations of waterborne copper induce cellular stress and cell death in zebrafish embryos and larvae. Biol. Res. 44, 7-15. [ Links ]
Hernández P.P. and Allende M.L. (2008). Zebrafish (Danio rerio) as a model for studying the genetic basis of copper toxicity, deficiency, and metabolism. Am. J. Clin. Nutr. 88, 835S-839S. [ Links ]
Hsu C.H., Wen Z.H., Lin C.S. and Chakraborty C. (2007). The zebrafish model: use in studying cellular mechanisms for a spectrum of clinical disease entities. Curr. Neurovas. Res. 4, 111-120. [ Links ]
Jezierska B., Lugowska K. and Witeska M. (2009). The effects of heavy metals on embryonic development of fish (a review). Fish Physiol. Biochem. 35, 625-640. [ Links ]
Johnson A., Carew E. and Sloman K.A. (2007). The effects of copper on the morphological and functional development of zebrafish embryos. Aquat. Toxicol. 84, 431-438. [ Links ]
Kamunde C., Grosell M., Higgs D. and Wood C.M. (2002). Copper metabolism in actively growing rainbow trout (Oncorhynchus mykiss): interactions between dietary and waterborne copper uptake. J. Exp. Biol. 205, 279-290. [ Links ]
Kiaune L. and Singhasemanon N. (2011). Pesticidal copper (I) oxide: environmental fate and aquatic toxicity. Rev. Environ. Contam. Toxicol. 213, 1-26. [ Links ]
Kong X., Jiang H., Wang S., Wu X., Fei W., Li L., Nie G. and Li X. (2013). Effects of copper exposure on the hatching status and antioxidant defense at different developmental stages of embryos and larvae of goldfish Carassius auratus. Chemosphere 92, 1458-1464. [ Links ]
Luzio A., Monteiro S.M., Fontaínhas-Fernandes A.A., Pinto-Carnide O., Matos M. and Coimbra A.M. (2013). Copper induced upregulation of apoptosis related genes in zebrafish (Danio rerio) gill. Aquatic. Toxicol. 128129, 183-189. [ Links ]
Oliveira-Filho E.C., Lopes R.M. and Paumgartten R.J.F. (2004). Comparative study on the susceptibility of freshwater species to copper-based pesticides. Chemosphere 56, 369-374. [ Links ]
Parichy D.M., Elizondo M.R., Mills M.G., Gordon T.N. and Engeszer R.E. (2009) Normal table of post-embryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev. Dynam. 238, 2975-3015. [ Links ]
Prieto-García F., Báez-Ramírez O.A., Scott W., Gaytán-Oyarzún J.C. and Zúñiga-Estrada A. (2006). Acumulación, toxicidad y teratogénesis provocada por presencia de arsénico en aguas en el pez cebra (Danio rerio). AquaTIC 24, 72-85. [ Links ]
Seker S. and Kutlu B. (2014). Determination of copper (Cu) levels for rivers in Tunceli, Turkey. World Environment 4, 168-171. [ Links ]
SEMARNAT (1996). Norma Oficial Mexicana NOM-001-SEMARNAT-1996 que establece los límites máximos permisibles de contaminantes en las descargas de aguas residuales en aguas y bienes nacionales. Secretaría de Medio Ambiente, Recursos Naturales y Pesca. Diario Oficial de la Federación. 6 de enero de 1997. México. 29 pp. [ Links ]
Sloman K.A. and MacNeil P.L. (2012). Using physiology and behaviour to understand the responses of fish early life stages to toxicants. J. Fish Biol. 81, 2175-2198. [ Links ]
Smolders R., De Boeck G. and Blust R. (2003) Changes in cellular energy budget as a measure of whole effluent toxicity in zebrafish (Danio rerio). Environ. Toxicol. Chem. 22, 890-899. [ Links ]
Sokolova I.M., Frederich M., Bagwe R., Lannig G. and Sukhotin A.A. (2012). Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 79, 1-15. [ Links ]
Trivedi M.H., Sangai N.P. and Renuka A. (2012). Assessment of toxicity of copper sulphate pentahydrate on oxidative stress indicators on liver of gold fish (Carassius auratus). Bull. Environ. Pharmacol. Life Sci. 1, 52-57. [ Links ]
Ulloa P.E., Iturra P., Neira R. and Araneda C. (2011) Zebrafish as a model organism for nutrition and growth: toward comparative studies of nutritional genomics applied to aquacultured fishes. Rev. Fish Biol. Fisher. 21, 649-666. [ Links ]
USEPA (2002). Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms, 5th. ed. United States Environmental Protection Agency, Office of Water (4303T). NW. Washington, D.C., USA, 20640. EPA-821-R-02-012, 266 p. [ Links ]
USEPA (2007). Aquatic life ambient freshwater quality criteria copper. U. S. Environmental Protection Agency, Office Water (4304T), Office of Science and Technology. Washington, D.C., USA, EPA-822-R-07-001, 204 p. [ Links ]
Valko M., Morris H. and Cronin M.T.D. (2005). Metals, toxicity and oxidative stress. Curr. Med. Chem. 12, 1161-1208. [ Links ]
Viegas I., Carvahlo R.A., Pardal M.A. and Jones J.G. (2012) Advances and application of tracer measurements of carbohydrates metabolism in fish [online]. http://www.intechopen.com/books/new-advances-and-contributions-to-fish-biology/advances-and-applications-of-tracer-measurements-of-carbohydrate-metabolism-in-fish [ Links ]
Vutukuru S.S. and Bassani K. (2012). Acute effects of mercuric chloride on glycogen and protein content of zebrafish, Danio rerio. J. Environ. Biol. 34, 277-281. [ Links ]
WHO (2004). Cooper in drinking-water. Background document for development of WHO guidelines for drinking-water quality. World Health Organization, Geneva, Switzerland, 23 pp. [online]http://www.who.int/water_sanitation_health/dwq/chemicals/copper.pdf. [ Links ]
Zhang W., Sun X., Chen L., Lin K.F., Dong Q.X., Huang C.J., Fu R.B. and Zhu J. (2012). Toxicological effect of joint cadmium selenium quantum dots and copper ion exposure on zebrafish. Environ. Toxicol. Chem. 31, 2117-2123. [ Links ]














