Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista internacional de contaminación ambiental
versión impresa ISSN 0188-4999
Rev. Int. Contam. Ambient vol.31 no.2 Ciudad de México ago. 2015
Synthesis of zeolitic materials from volcanic ash in presence and absence of cetyltrimethylammonium bromide
Síntesis de materiales zeolíticos a partir de ceniza volcánica en presencia y ausencia de bromuro de cetiltrimetilamonio
Vilma Mayerling Sanhueza Núñez* and Leonardo Daniel Bennun Torres
Universidad de Concepción. Edmundo Larenas s/n, Concepción 3, Chile *Autor de correspondencia: vsanhuez@udec.cl
Recibido marzo 2014;
aceptado enero 2015
ABSTRACT
Zeolitic materials as Na-phillipsite, Na-K-phillipsite-like zeolites and the mixtures of zeolites (phillipsite+analcime and phillipsite+chabazite+analcime) were synthesized from volcanic ash, either in presence and absence of cetiltrimetilamonium bromide (CTAB). The ash sample used in the laboratory experiments contains 75.36 % SiO2 and 14.11 % Al2O3, abundances. The reaction time as well as the influence of CTAB were studied in the zeolitic materials crystallization. The experiments were carried out under hydrothermal conditions, autogenic pressure and temperature of 150 °C, as well as reaction time from 8 to 116 h. Products from this hydrotermal treatment were identified by X-ray diffraction (XRD) and characterized by scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDS). Of the zeolitic materials obtained the Na-K-phillipsite-like zeolite was found to be the most effective for the retention of cations Pb2+, Zn2+, and Ba2+.
Key words: zeolites, phillipsite, chabazite, analcime, ash, synthesis.
RESUMEN
Materiales zeolíticos tipos Na-phillipsita, Na-K-phillipsita y mezclas de zeolitas (phillipsita+analcima y phillipsita+chabazita+analcima) fueron sintetizados a partir de ceniza volcánica, en presencia y ausencia de bromuro de cetiltrimetilamonio (BCTA). La muestra de ceniza usada en los experimentos de laboratorio posee un 75.36 % de SiO2 y 14.11 % de Al2O3. El tiempo de reacción y la influencia del BCTA fueron los parámetros estudiados en la cristalización de los materiales zeolíticos. Los experimentos se llevaron a cabo bajo condiciones hidrotermales, a presión autógena y una temperatura de 150 °C, así como tiempos de reacción de 8 a 116 h. Los productos de este tratamiento hidrotermal fueron identificados por difracción de rayos-X (DRX) y caracterizados por microscopía electrónica de barrido con espectrometría dispersiva de rayos-X (MEB-EDRX). La zeolita Na-K-phillipsita resultó ser la más efectiva para la retención de los cationes Pb2+, Zn2+ y Ba2+.
Palabras clave: zeolitas, phillipsita, chabazita, analcima, ceniza, síntesis.
INTRODUCTION
Zeolites are microporous aluminosilicate crystalline materials with well-defined pore structures and compositions. Because of their properties such as thermal stability, adsorption, catalytic activity, acidity, cation exchange and molecular sieves, zeolites have important applications in refining processes at the petrochemical industry, as well as gas separation, water purification at mining industry and environmental catalysis (Zhang et al. 2011, Izidoro et al. 2012).
Most zeolites are synthesized from commercial materials (Martucci et al. 2009, Tanaka et al. 2009, Trejda et al. 2010, Morales-Pacheco 2011, Xue et al. 2012). Many of them have been synthesized, at a given temperature and crystallization time, from gels containing Na2O-Al2O3-SiO2-H2O. Some sources of silicon are: Na-silicate HS-40 (Sig and Seung 2004, Chen et al. 2009), Na-silicate, Cab-O-Sil M-5 fused silica (Rivallan et al. 2010), fumed silica (Xu et al. 2010), UltraSil silica TMA-SiO2 and Cab-O-Sil TMA-SiO2 (Shvets et al. 2008). On the other hand, some of the sources of aluminum that have been employed are: Na-aluminate (Anuwattana and Khummongkol 2009, Gupta et al. 2009) and Alisopropylate (Tosheva et al. 2005). Surfactants of different chain lengths have been used as templates. Among them, the following can be mentioned: C16H33(CH3)3N-OH/Cl, C12H25(CH3)3 N-OH/Cl, C14H29(CH3)3N-Br, C16TMA-OH and C8TMA-Br (Han et al. 2009, Sakthivel et al. 2009).
Ashes generated by thermoelectric plants are another starting material that has been widely used to synthesize zeolites. In fact different zeolites such as NaA, NaP1, analcime, gmelite and phillipsite, have been synthesized from fly ash (Moriyama et al. 2005, Terzano et al. 2005, Tanaka et al. 2008, Walek et al. 2008, Font et al. 2009, Kumar et al. 2009, Ríos et al. 2009, Goni et al. 2010), lignite and rice husk ash (Ahmaruzzaman 2010).
On the contrary experiments oriented to synthesize zeolites by means of natural products as starting materials are relatively scarce. The chemical synthesis of zeolites is subject to disturbance caused by the impurities present in these materials. Some natural raw materials that have been used are kaolin (San-hueza et al. 1999, Mignoni et al. 2008, Miao et al. 2009), bentonite (Boukadir et al. 2002, Hongchao et al. 2010), pumice (Sanhueza et al. 2006), diatomite (Sanhueza et al. 2003, 2004, 2006, 2011 and Chilean patents 2004, 2006, 2009, 2010), and perlite glass (Christidis and Papantoni 2008).
Other raw materials for obtaining zeolites are natural zeolitic rocks hydrotermally treated (Wata-nabe et al. 2005). Phillipsite and chabazite were obtained from trachytic glass by hydrothermal conversion at 200 °C (De Gennaro et al. 1999). Wilkin and Barnes (2000) used Na-clinoptilolite zeolite as a starting material to synthesize anal-cime zeolite. Phillipsite and merlinoite zeolites have been synthesized by chemical reaction between an obsidian and NaOH or KOH solutions at hydrothermal conditions, autogenic pressure and temperatures between 150 °C and 200 °C (Kawano and Tomita 1997).
The products from Chaitén volcano (eruption occurred on May 2, 2008) including its ash are vitro-phyric and mostly rhyolitic in composition, constituting a natural low cost source of silicon and aluminium (Lara 2009). The Chaiten volcano is located in the southern area of the Southern Volcanic Zone of the Andes-Chile at 42°50'S.
Fortunately in this kind of events, not all is disaster and always there is something positive that can be rescued from. This fact motivated us to evaluate the possibility of investigating the reactivity of ash as a raw material thrown by the Chaiten volcano eruption in the formation of zeolites and/ or zeolitic materials.
MATERIALS
The volcanic ash comes from the Chaitén (2008) volcanic eruption. The chemical composition of the ash was determined by X-ray fluorescence (XRF; Table I), and it was used to carry out the experiments. The volcanic ash (molar ratio SiO2/Al2O3 = 9.1) was collected at the bottom of the Chaiten volcano. Minerals found correspond mainly to anorthite and quartz, followed by minor amounts of rutile, anatase, amphibole, chlorite and ilmenite, which probably come from the volcanic ash. With the aim of converting the volcanic ash into zeolite or zeolitic materials, several experiments were done.
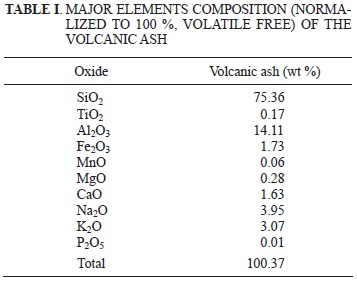
Zeolites preparation
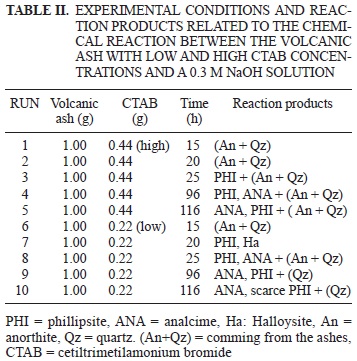
The volcanic ash sample was dried at room temperature, ground and the grain size employed in the synthesis was less than 200 mesh. The experiments (Tables II and III) were carried out in Parr steel autoclave reactor (150 cc of capacity) in static conditions under hydrothermal conditions, autogene pressure, temperature of 150 °C, with high and low concentrations of cetiltrimetilamonium bromide (Table II). The runs 1, 2, 3, 4 and 5 were prepared with high CTAB concentration. The aim of runs 6, 7, 8, 9 and 10 was to investigate whether phillipsite-like zeolite and zeolitic materials would be formed by introducing half CTAB (low concentration of cetiltrimetilamonium bromide). The runs in Table III were prepared without CTAB.
Once the run was completed and the system cooled down, the products were washed off with abundant distilled water (Milli-Q water 18.2 Mil/ cm resistivity), filtered using Advantec 5C filter paper and dried at 120 °C for 15 h. The calcination of samples prepared with template agent were carried out in air at 600 °C for 6 h. The heating rate of the furnace was 1.5°/min.
The retention capacity of the zeolitic materials was determined through aqueous solutions of Pb (NO3)2, ZnCl2 and BaCl2. 100 mL were taken of each solution containing 400 ppm of the cation to be analyzed. 0.1 g of zeolitic material was added in polyvinylchloride bottles at room temperature under continuous stirring until equilibrium. Then the samples were filtered and the solutions were stored at 4 °C for later analysis.
Characterization
The major elemental components of volcanic ash were determined using a Rigaku X-ray fluorescence spectrometer. Mineralogy was determined by a Qem-scan with Tescan System Vega LSH. The zeolites were identified through a D4 Endeavor X-ray Diffraction equipment, Cu-a1 radiation (X = 1.5406 A) at 30 kV and 15 mA was employed. The morphology of the zeolites was determined by means of a JEOL JMS 6380 LV with EDS. Before mounting, the samples were ultrasonically dispersed in a 40 % solution of water/ethanol. Metal concentrations were analyzed using a Hitachi Z-8100 Polarized Zeeman Atomic Absorption Spectrophotometer.
RESULTS AND DISCUSSION
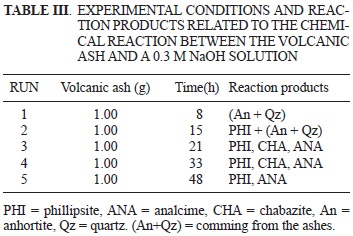
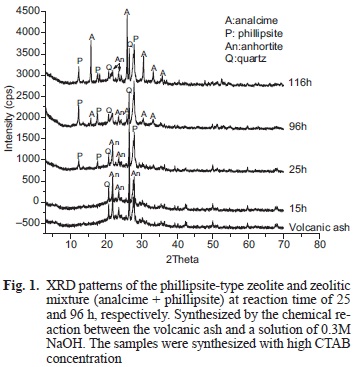
Fifteen experiments were carried out (Tables II and III). In both cases products are the result of a chemical reaction between the volcanic ash and a NaOH 0.3M solution at 150 °C temperature reaction whether in presence or in absence of CTAB, respectively. Phillipsite-like zeolite crystallized at 25 h of reaction time (Fig. 1). If the concentration of CTAB halved the crystallization time for phillipsite-like zeolite decreases to 20 h of reaction (Fig. 2).
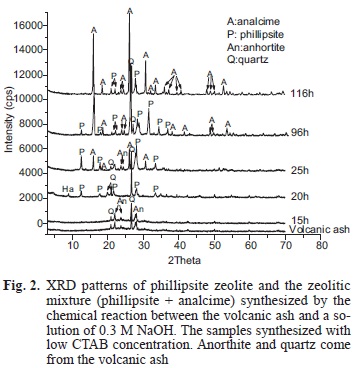
Within a 25 h time of reaction (Fig. 2) and a low concentration of CTAB, two phases crystallize (PHI, ANA), whereas if the CTAB concentration increases, then a 96 h time of reaction would be needed to reach the same phases (PHI, ANA; Fig. 1)
The XRD pattern of the sample synthesized without CTAB shows high intensity peaks that correspond to the phillipsite-like zeolite (Fig. 3, 15 h). On the other hand, the patterns of the samples obtained at the same reaction time (15 h) with low and high CTAB concentrations (Figs. 1 and 2) show only quartz and anorthite coming from the volcanic ash. An extra peak at 20 h appeared in the pattern (Fig. 2) indicating that another phase, most probably halloysite, was forming together the phillipsite-like zeolite.
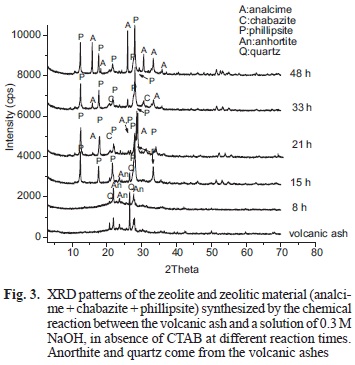
In figure 3 no crystallization is observed during the first 8 h of reaction. Two new zeolite-like are added to the reaction (21 and 33 h) chabazite and analcime-like phases. Similar mixtures of natural zeolites phillipsite + chabazite and phillipsite + chabazite + analcime have been reported by Dwairi et al. (2009) in Tall Amir and Tall Juhira, two important volcanoes located in southern Jordan.
The study ended after 48 h of reaction where the mixtures of zeolites phillipsite + analcime prevail.
The results of the kinetics experiments to measure the retention of metal ions from aqueous solutions as a function of time are shown in figure 4a, b, c and d. Differences in the sequences of selectivity of cations by the zeolitic materials are expected due to differences in composition. In figure 4a selectivity sequence is Ba2+ > Zn2+ > Pb2+, in about an hour and then reaches equilibrium. In figure 4b the sequence selectivity is Pb2+ > Zn2+> Ba2+, it takes about 2 h to reach equilibrium. Sequence selectivity in figure 4c is Zn2+ > Pb2+ > Ba2+, with a period of approximately 2 h to reach equilibrium. And finally figure 4d shows the preference of cations in descending order: Pb, Ba and Zn. The Na-K-phillipsite-like zeolite was the most effective with a 60 % retention for Pb2+, 56 % for Zn2+ and 43 % for Ba2+. The retention of cations by the ash is very low. 3.6 % for Zn2+, 3.9 % for Ba2+and 4.6 % for Pb+2.
Under SEM examination, the volcanic ash shows evidence of angular glassy shards (Fig. 5). In figure 6a, it is possible to observe Na-phillipsite-like zeolite (Si/Al = 1.6). In figure 6b, trapezohedron crystals of analcime and groups of prismatic crystals of phillipsite are observed. In figure 6c, analcime appears as the most abundant zeolite. All the above experiments were performed with high concentrations of cetyl-trimethylammonium bromide. When the previous experiments were carried out with low cetyltrimethyl-ammonium bromide, concentration clearly decreases the crystallization time of analcime. The transformation of a zeolite phase into another by dissolu-tion-recrystallization is frequently observed during hydrothermal syntheses, this transformation is known as metastability. In this case it shows the formation of analcime at expense of phillipsite-like zeolite (Fig. 6d) at a reaction time of 25 h. Photographic evidence not previously reported. Presumably temperature of 150 °C is lost structural water phillipsite-like zeolite lattice destabilizing its evolving into the more stable cubic phase thermodynamically. In presence or in absence of CTAB the morphology of the phillipsite is the same. Phillipsite exhibits a well defined morphology called sword blade (Figs. 6a and 7a). In figure 6f analcime crystals finalizing the process of crystallization can be observed.
Highly crystalline Na-K-PHI-like zeolite with a Si/ Al = 3.1 ratio crystallizes at 15 h of reaction (Fig. 7a). After 21 h a mixture of phillipsite and chabazite is obtained (Fig. 7b). Increasing the reaction time to 33 h, analcime is added to the phillipsite and chabazite (Fig. 7c). This suggests that the crystallization sequence is phillipsite — chabazite — analcime. De Gennaro et al. (1999) obtained similar results from synthetic monocationic glasses. However, they obtained the chabazite — phillipsite — analcime sequence at 200 °C and not at 150 °C, like in our experiments. Probably, the difference in the reaction temperature caused the change ofthe crystallization sequence. Following the methodology of De Gennaro et al. (1999) a temperature of 200 °C and 48 h of reaction time are required to crystallize the phillipsite. In our case, the phillipsite crystallization took place at 150 °C and in just 15 h of reaction time.
Höller and Wirsching (1988) reported the synthesis of chabazite with different morphologies using volcanic glasses as starting materials. Among the products they obtained at 200 °C and 20 days of reaction time, there was a mixture of chabazite + phillipsite + analcime. However they did not specify the crystallization sequence of the members of this mixture.
On the other hand, Ibrahim (2004) reports the following paragenetic sequence: smectite®phillipsi te®chabazite®natrolite®analcime®calcite. The zeolites were synthesized using volcanic glass granules transformed into palagonite as starting material by the action of percolating waters in a closed hydrothermal system. The paragenetic sequence is similar to the one we obtained in the sense that the chabazite crystallization took place after the crystallization of phillipsite.
CONCLUSIONS
It is possible to synthesize a single phillipsite-like zeolite and zeolitic mixtures (phillipsite + analcime and phillipsite + chabazite + analcime) by chemical reactions between the Chaitén volcanic ash and a NaOH 0.3 M solution at 150 °C, under hydrothermal conditions, autogenic pressure and either in presence or absence of the template agent CTAB. The Na-K-phillipsite-like zeolite was the most effective to retain cations with about a 60 % retention for Pb2+, 56 % for Zn2+ and 43 % for Ba2+.
Obtaining phillipsite-like zeolite at 150 °C and 15 h of reaction time would represent an economical advantage if the process could be carried out at industrial level. This would contribute to mitigate the socioeconomic damages produced by the Chaitén (2008) volcanic ash. In the light of the results obtained it is possibly to postulate that zeolites could be also synthesized from others SiO2, Al2O3 and K2O rich volcanic products like rhyolitic domes and silicic pyroclastic deposits.
ACKNOWLEDGMENTS
This paper was possible thanks to the collaboration of the Instituto GEA. We also give our thanks to the Vicerrectoría de Investigación y Desarrollo of the Universidad de Concepción (Chile). The authors are grateful to Dr. Andrés Tassara and Priscilla Riveros who donated the Chaitén volcano ash samples analyzed, also to Mónica Uribe for her help in the identification of the synthesized minerals by XRD and to Julio Pugin and Hugo Pacheco for their assistance in the SEM characterization of the synthesized zeolites.
REFERENCES
Ahmaruzzaman M. (2010). A review on the utilization of fly ash. Prog. Energ. Combust. 36, 327-363. [ Links ]
Anuwattana R. and Khummongkol P. (2009). Conventional hydrothermal synthesis of Na-A zeolite from cupola slag and aluminum sludge. J. Hazard Mater. 166, 227-232. [ Links ]
Boukadir D., Bettahar N. and Derriche Z. (2002). Synthesis of zeolites 4A and HS from natural materials. Ann. Chim-Sci. Mat. 27, 1-13. [ Links ]
Chen Y., Zhu G., Peng Y., Bi H., Feng J. and Qiu S. (2009). Synthesis and characterization of pure-silica-zeolite Beta low-k thin films. Micropor. Mesopor. Mat. 123, 45-49. [ Links ]
Christidis G. and Papantoni H. (2008). Synthesis of FAU Type Zeolite Y from Natural Raw Materials: Hydrothermal SiO2-Sinter and Perlite Glass. Open Mineral. J. 2, 1-5. [ Links ]
Corma A. (2003). State of the art and future challenges of zeolites as catalysts. J. Catal. 216, 298-312. [ Links ]
De Gennaro M., Langella A., Cappelletti P. and Colella C. (1999). Hidrotermal conversión of trachytic glass to zeolite 3. Monocationic model glasses. Clay Clay Miner. 47, 348-357. [ Links ]
Dwairi R., Khoury H. and Ibrahim K. (2009). Mineralogy and Authigenesis of Zeolitic Tuff from Tall-Juhira and Tall Amir, South Jordan. JJEES. 2, 72- 83. [ Links ]
Font O., Moreno N., Díez S., Querol X., López-Soler A., Coca P. and García Peña F. (2009). Differential behavior of combustion and gasification fly ash from Puertollano Power Plants (Spain) for the synthesis of zeolites and silica extraction. J. Hazard. Mater. 166, 94-102. [ Links ]
Goni S., Pena R. and Guerrero A. (2010). Hydrothermal synthesis of zeolite from coal class F fly ash. Mater. Construcc. 60, 51-60. [ Links ]
Gupta S., Agarwal D.D. and Banerjee S. (2009). Thermal Stabilization of Poly(vinyl chloride) by Hydrotalcites, Zeolites, and Conventional Stabilizers. J. Vinyl Addit. Techn. 15, 164-170. [ Links ]
Han S., Bun M., Min H. and Bong S. (2009). Zeolite synthesis in the tetraethylammonium-tetramethylam-monium mixed-organic additive system. Micropor. Mesopor. Mat. 123, 160-168. [ Links ]
Höller H. and Wirrsching U. (1988). Experiments on the formation conditions and morphology of chabazite from volcanic glasses. In: Ocurrence and properties of natural zeolites. (D. Kalló, H.S. Sherry, Eds.). Akademiai Kiadó, Budapest, Hungría, pp. 171- 191. [ Links ]
Hongchao M., Quantong Y., Yinghuan F, Chun M. and Xiaoli D. (2010). Synthesis of Zeolite of Type A from Bentonite by Alkali Fusion Activation Using Na2CO3. I and EC Research. 4, 454-458. [ Links ]
Ibrahim K. (2004). Mineralogy and chemistry of natrolite from Jordan. Clay Miner. 39, 47-55. [ Links ]
Izidoro J.C., Fungaro D.A., Dos Santos F.S. and Wang S. (2012). Characteristics of Brazilian coal fly ashes and their synthesized zeolites. Fuel Process. Technol. 97, 38-44. [ Links ]
Kawano M. and Tomita K. (1997). Experimental study on the formation of zeolites from obsidian by interaction with NaOH and KOH solutions at 150 and 200 °C. Clay Clay Miner. 45, 365-377. [ Links ]
Kumar V., Nagae M., Matsuda M. and Miyake M. (2009). Zeolite formation from coal fly ash and heavy metal ion removal characteristics of thus-obtained Zeolite X in multi-metal systems. J. Environ. Manage. 90, 2507-2514. [ Links ]
Lara L. (2009). The 2008 eruption of the Chaitén volcano, Chile: a preliminary report. Andean Geol. 36, 125-129. [ Links ]
Martucci A., Parodi I., Armbruster T. and Alberti A. (2009). Mobility of acidic protons in zeolites: A neutron diffraction study of D-heulandite. Micropor. Mesopor. Mat. 123, 15-20. [ Links ]
Miao Q., Zhou Z., Yang J., Lu J., Yan S. and Wang J. (2009). Synthesis of NaA zeolite from kaolin source. Front. Chem. Eng. Chin. 3, 8-11. [ Links ]
Mignoni M., Petkowicz D., Fernandes Machado N. and Pergher S. (2008). Synthesis of mordenite using kaolin as Si and Al source. Appl.Clay. Sci. 41, 99-104. [ Links ]
Morales-Pacheco P., Domínguez J.M., Bucio L., Ál-varez F., Sedran U. and Falco M. (2011). Synthesis of FAU(Y) y MFI(ZSM5)-nanosized crystallites for catalytic cracking of 1,3,5-triisopropylbenzene. Catal. Today. 166, 25-38. [ Links ]
Moriyama R., Takeda S., Onozaki M., Katayama Y., Shiota K., Fukuda T., Sugihara H. and Tani Y. (2005). Large-scale synthesis of artifitial zeolite from coal fly ash with a small charge of alkaline solution. Fuel 84, 1455-1461. [ Links ]
Ríos C., Williams C. and Roberts C. (2009). A comparative study of two methods for the synthesis of fly ash-based sodium and potassium type zeolites. Fuel 88, 1403-1416. [ Links ]
Rivallan M., Yordanov I., Thomas S., Lancelot C., Mintova S. and Thibault-Starzy F. (2010). Plasma Synthesis of Highly Dispersed Metal Clusters Confined in Nanosized Zeolite. Chem. Cat. Chem. Catalysis. 2, 1074-1078. [ Links ]
Sakthivel A., Lida A., Komura K., Sugi Y. and Chary K. (2009). Nanosized P-zeolites with tunable particle sizes: Synthesis by the dry gel conversion (DGC) method in the presence of surfactants, characterization and catalytic properties. Micropor. Mesopor. Mat. 119, 322-330. [ Links ]
Sanhueza V and Cid R. 2010. Proceso para obtención de la zeolita NaA de bordes biselados a partir de diatomita en presencia de NaOH, aluminato de sodio y agua. Chile, patent No. 46732 (C01B39/16). 05 Aug 2010. Solicitude 200102836, 21 nov 2001. 7 pp. [ Links ]
Sanhueza V. Kelm U. and Cid R. 2004. Síntesis de zeolitas a partir de diatomita o kieselguhr. Chile, patent No. 42218. (CO1B39/26; CO1B39/36), 30 jul 2004. Solicitude 199901604, 31 Mar 2000. 7 pp. [ Links ]
Sanhueza V. Kelm U. and Cid R. 2006. Proceso para la obtención del material mesoporoso MCM-41 a partir de diatomita como fuente de sílice. Chile, patent No. 43035. (C01B33/2; C01B33/26; C01B39/02), 26 sep 2006. Solicitude 200301678, 20 Aug 2003. 8 pp. [ Links ]
Sanhueza V. U Kelm. and Cid R. 2009. Proceso para obtener zeolita microporosa ZSM-5 de la familia pentasil. Chile, patent No. 44641 (C01B39/26; C01B39/36), 09 Abr 2009. Solicitude 200003595, 26 dec 2000. 7 pp. [ Links ]
Sanhueza V., Kelm U. and Alfaro G. (2011). Synthesis of zeolites nap-gis, with different morphologies, from two diatomites. Rev. Mex. Ing. Chim.10, 117-123. [ Links ]
Sanhueza V., Kelm U. and Cid R. (1999). Synthesis of molecular sieves from Chilean kaolinites: 1. Synthesis of NaA type zeolites. J. Chem. Technol. Biotechnol. 74, 358-363. [ Links ]
Sanhueza V., Kelm U. and Cid R. (2003). Synthesis of mordenite from diatomite: a case of zeolite synthesis from natural material. J. Chem. Technol. Biotechnol. 78, 485-488. [ Links ]
Sanhueza V., Kelm U., Cid R. and López-Escobar, L (2004). Synthesis of ZSM-5 from diatomite: A case of zeolite synthesis from natural material. J. Chem. Technol. Biotechnol.79, 686-690. [ Links ]
Sanhueza V., López-Escobar L., Kelm U. and Cid R. (2006). Synthesis of a mesoporous material from two natural sources. J. Chem. Technol. Biotechnol. 81, 614-617. [ Links ]
Shvets O.V., Zukal A., Kasian N., Zilková N. and Cejka J. (2008). The role of crystallization parameters for the synthesis of germanosilicate with UTL topology. Chemistry- A European Journal 14, 10134-10140. [ Links ]
Sig Y. and Seung W. (2004). Crystallization of zeolite L from Na2 O-K2 O-Al2 O3-Si2- H2O system. Powder Technol. 145, 10-19. [ Links ]
Snyder M.A. and Tsapatsis M. (2007). Hierarchical Nano-manufacturing: From Shaped Zeolite Nanoparticles to High-Performance Separation Membranes: Angew. Chem. Int. Ed. 46, 7560-7573. [ Links ]
Tanaka H., Fujii A., Fujimoto S. and Tanaka Y. (2008). Microwave-Assisted Two-Step Process for the Synthesis of a Single-Phase Na-A Zeolite from Coal Fly Ash. Adv. Powder Technol. 19, 83-94. [ Links ]
Tanaka S., Okada H., Nakatani N., Maruo T., Nishiyama N. and Miyake Y. (2009). Mesoporous aluminosilicates assembled from dissolved LTA zeolite and triblock copolymer in the presence of tetramethylammonium hydroxide. J. Colloid Interface Sci. 333, 491-496. [ Links ]
Tao Y., Kanoh H., Abrams L. and Kaneko K. (2006). Mesopore-Modified Zeolites: Preparation, Characterization, and Applications. Chem. Rev. 106, 896-910. [ Links ]
Terzano R., Spagnuolo M., Medicib L., Tateoc F. and Ruggiero P. (2005). Zeolite synthesis from pre-treated coal fly ash in presence of soil as a tool for soil remediation. Appl. Clay Sci. 29, 99-110. [ Links ]
Tosheva L., Holzl M., Metzger T.H., Valtchev V, Mintova S. and Bein T. (2005). Zeolite beta films synthesized from basic and near-neutral precursor solutions and gels. Mater. Sci. Eng., C. 25, 570-576. [ Links ]
Trejda M., Wojtaszek A., Floch A., Wojcieszak R., Gaigneaux E.M. and Ziolek M. (2010). New Nb and Ta-FAU zeolites—Direct synthesis, characterization and surface properties. Catal. Today. 158, 170-177. [ Links ]
Walek T., Saito F. and Zhang Q. (2008). The effect of low solid/liquid ratio on hydrothermal synthesis of zeolites from fly ash. Fuel 87, 3194-3199. [ Links ]
Watanabe Y., Yamada H., Tanaka J. and Moriyoshi Y. (2005). Hydrothermal modification of natural zeolites to improve uptake of ammonium ions. J. Chem. Technol. Biotechnol. 80, 376-380. [ Links ]
Wilkin R.T. and Barnes H.L. (2000). Nucleation and growth kinetics of analcime from precursor Naclinoptilolite. Am. Mineral. 85, 1329-1341. [ Links ]
Xu G., Zhu X., Li X., Xie S., Liu S. and Xu L. (2010). Synthesis of pure silica ITQ-13 zeolite using fumed silica as silica source. Micropor. Mesopor. Mat. 129, 278-284. [ Links ]
Xue T., Meng-Wang Y. and He MY. (2012). Synthesis of ultra-high-silica ZSM-5 zeolites with tunable crystal sizes. Solid State Sci.14, 409-418. [ Links ]
Zhang M., Zhang H., Xua D., Hanb L., Niuc D., Tiand B., Zhang J., Zhang L. and Wua W. (2011). Removal of ammonium from aqueous solutions using zeolite synthesized from fly ash by a fusion method. Desalination 271, 111-121. [ Links ]














