Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista internacional de contaminación ambiental
versão impressa ISSN 0188-4999
Rev. Int. Contam. Ambient vol.31 no.2 Ciudad de México Ago. 2015
Evaluation of the oxidative potential of urban PM and its relation to in vitro induced DNA damage: a spatial and temporal comparison
Evaluación del potencial oxidativo de partículas atmosféricas (PM) y su relación con el daño inducido in vitro en el ADN: una comparación espacial y temporal
Raúl Ornar Quintana-Belmares1,2*, Ernesto Alfaro-Moreno1, Claudia María García-Cuéllar1, Virginia Gómez-Vidales3, Inés Vázquez-López1, Manuel de Jesús Salmón-Salazar3, Irma Rosas-Pérez2 and Álvaro Román Osornio-Vargas4
1 Laboratorio de Toxicología Ambiental, Subdirección de Investigación Básica, Instituto Nacional de Cancerologia. Av. San Fernando 22, Col. Sección XVI, Del. Tlalpan, C.P. 14080, México D.F., México *Autor para correspondencia: qbro@hotmail.com
2 Centro de Ciencias de la Atmósfera, Universidad Nacional Autónoma de México. Circuito de la Investigación s/n, Ciudad Universitaria, Coyoacán, C.P. 04510, México D.F., México
3 Instituto de Química, Universidad Nacional Autónoma de México. Circuito de Exterior s/n, Ciudad Universitaria, Coyoacán, C.P. 04510, México D.F., México
4 Department of Paediatrics, University of Alberta, Edmonton, AB Canada. 3-591 Edmonton Clinic Health Academy, 11405 87th Avenue. Edmonton T6G 1C9, Canada
Recibido abril 2014;
aceptado noviembre 2014
ABSTRACT
Some toxic effects of particulate matter (PM) are related to the oxidative potential (OP) of the particles. The electron paramagnetic resonance (EPR) technique was used to evaluate the intensity of paramagnetic species (PS) and EPR plus spin trapping, to evaluate the OP of PM. We evaluated, in parallel, the DNA degradation potential of PM10 and PM25 collected from three regions of Mexico City in 1991 and 2003. Each region had different sources of pollution: industrial, commercial or residential. Both techniques evaluated Fenton-type reactions in the presence and absence of de-feroxamine (DFO). PM10 samples from the industrial region presented similar high OP, independently of sampling year. PM10 and PM25 collected in the commercial and residential regions in 2003 had similarly low OP. The OP induced by PM10 from the industrial region was completely inhibited by DFO, and DFO partially inhibited the OP induced by PM10 from other regions. PM25 OP was not inhibited by DFO. PM from the industrial region was the most potent inductor of DNA degradation, while PM from residential region was the least potent, correlating with the OP. DFO inhibited the degradation of DNA induced by PM. The OP of PM collected in the industrial and residential region correlated with the DNA degradation. The region, size and year of PM collection are linked to observed OP variations and DNA degradation induced by PM.
Key words: PM10, PM2.5, electron paramagnetic resonance, oxidative potential, DNA damage.
RESUMEN
Algunos de los efectos tóxicos atribuibles a las partículas atmosféricas (PM) están relacionados con su potencial oxidante (OP). La resonancia paramagnética electrónica (EPR) es una técnica que se utilizó para evaluar la intensidad de las especies paramagnéticas (PS), y la EPR más un atrapador de espín, para evaluar el OP de las PM. Se evaluó en paralelo el potencial de degradación de ADN por PM10 y PM25 muestreadas en tres regiones de la Ciudad de México en 1991 y 2003. Cada región tenía diferentes fuentes de contaminación: industrial, comercial o residencial. Ambas técnicas fueron evaluadas en reacciones de tipo Fenton en presencia y ausencia de deferoxamina (DFO). Las PM10 de la región industrial presentan alto OP, independiente del año de muestreo. Las PM10 y PM2.5 muestreadas en las regiones comercial y residencial durante 2003 tuvieron bajo OP. El OP inducido por las PM10 de la región industrial fue completamente inhibido por la DFO, el OP inducido por las PM10 procedentes de las otras dos regiones fue inhibido parcialmente. El OP de las PM2.5 no fue inhibido por la DFO. Las PM10 de la región industrial fueron el inductor más potente de la degradación del ADN, mientras que las PM10 de la región residencial fueron las menos potentes, lo que se correlaciona con el OP. La DFO inhibe la degradación del ADN inducida por las PM. El OP de las PM de la región industrial y residencial se correlaciona con la degradación del ADN. La región, el tamaño y el año de muestreo parecen estar vinculados a las variaciones del OP y a la degradación del ADN inducido por las PM.
Palabras clave: PM10, PM2.5, resonancia paramagnética electrónica, potencial oxidativo, daño ADN.
INTRODUCTION
It has been widely demonstrated that particulate matter (PM) is linked to biological effects such as lung inflammation, blood clotting, and various cardiovascular effects (Alfaro-Moreno et al. 2007). Some of these effects (such as endothelial dysfunction) have been related to the ability of the particles to induce oxidative stress (Montiel-Dávalos et al. 2010). Several studies indicate that different physical and chemical properties of the particles are related to the intensity of the biological effects (Veranth et al. 2006, Rosas-Pérez et al. 2007, Mugica et al. 2009). Differences in composition seem to be critical, it has also been shown that PM collected in different cities and even in different locations within the same city, show different toxic potentials (Alfaro-Moreno et al. 2002, Gerlofs-Nijland et al. 2009, Osornio-Vargas et al. 2011).
The toxicological evaluation of PM is a time-consuming effort. Considering the wide range of PM composition and size to which humans can be exposed, there is a need to predict potential toxic effects of PM. For this purpose, electron paramagnetic resonance (EPR) has been used to evaluate the oxidative potential (OP) of PM (Valavanidis et al. 2005b). Source-related variations in chemical components and composition may lead to different oxidative characteristics (Briede et al. 2005). Therefore, PM generated by different sources may have a different pattern of oxidative potential. The relationship between the oxidative potential's ability to induce an effect at a biological level and the oxidation of biomolecules can help to predict toxicity. EPR can be employed as an alternative method of predicting the toxic potential of PM in a quick, sensitive and reliable way.
We have already mentioned that PM represents a complex mixture and some components, such as metals, can cause inflammatory processes and increased reactive oxygen species (ROS), related to Fenton reactions (Osornio-Vargas et al. 2011).
It has been shown that different biological processes induced by the particles of Mexico City are associated with PM chemical composition (Alfaro-Moreno et al. 2002). One of these effects is DNA damage and is suggested that DNA degradation occurs as a result of metal content, and the generation of ROS involving the Fenton reaction (Lloyd 1997, Lloyd and Phillips 1999).
Naked DNA and the chelating Deferoxamine (DFO) have been used as a simplified method for evaluating the participation of metals present in PM and the induction of DNA damage (Garcia-Cuellar et al. 2002).
Oxidative stress has been identified as a key factor in causing biological effects induced by PM. Although, it is known that cells exposed to PM show a dramatic increase in oxidative activity (Soukup et al. 2000, Becker et al. 2005), the intrinsic oxidative potential of PM is not well understood. Furthermore, it has been shown that PM with a high oxidative potential is more toxic (Wessels et al. 2010). In the present study, we evaluated the signal intensity of paramagnetic species and the oxidative potential of urban PM from Mexico City collected in different regions and years using EPR.
MATERIALS AND METHODS
PM10 and PM2.5 sampling
Particulate matter with an aerodynamic diameter <10 um (PM10) was obtained from industrial (IR), commercial (CR), and residential (RR) regions of Mexico City using a high-volume sampler (GMW model 1200 VFC HV PM10; Sierra Andersen, Smyrna, GA, USA) for particles with an aerodynamic diameter < 10 um (PM10). In 1991, 24 h PM10 samples were collected using fiberglass filters (type A/E glass 61638; Gelman Sciences, Ann Arbor, MI, USA; 1.13 m3/min), three days per week during each week of the year. PM was recovered from the membranes after dry sonication for 45 min and subsequently smoothly swept with a brush into an endotoxin-free flask. All PM samples were pooled by region and stored dry in endotoxin-free glass vials, which were kept in a dryer at 4 °C until use (Alfaro-Moreno et al. 2002). In 2003, PM10 and particulate matter with an aerodynamic diameter g < 2.5 um (PM25) were collected using the same instrument, but we introduced a modification involving the use of cellulose nitrate membranes with a nominal pore size of 3 um. Membranes were cut from rolls (11302-131, Sartorius, Goettingen, Germany) and modified to preserve the airflow rate and particle size sampling performance, as previously described (Alfaro-Moreno et al. 2009).
Determination of the relative intensity of paramagnetic species in the PM
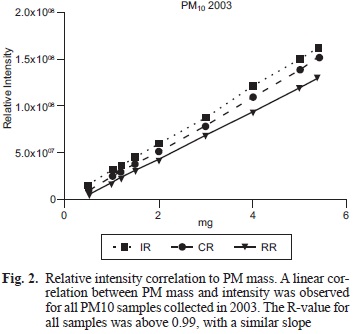
To evaluate the linearity of the method, we evaluated the relative intensity of the paramagnetic species using PM10 (1, 2, 3, 4 and 5 mg) samples from 2003 with an EPR spectrometer (Jeol JES TE-300, Tokyo, Japan) under the following experimental conditions: center field 335 mT; microwave frequency 9.4 GHz; microwave power 1 mW; sweep width +/- 4 mT; sweep time 2 min; modulation width 0.16 mT; amplitude 63; time constant 0.3 s and accumulation 1 (Fig. 1a).
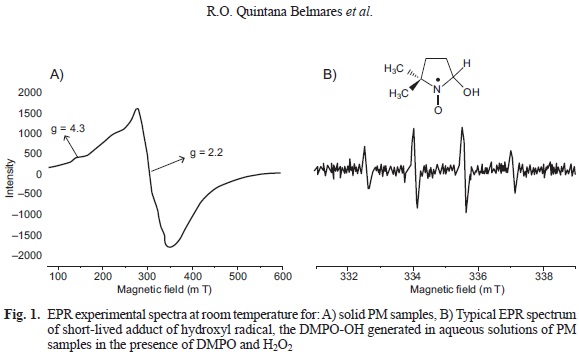
The relative intensity was obtained in relation to a standard curve of Tempol by double integration of each spectrum (Shinji et al. 2004, Dos Santos et al. 2009), using Esprit 382 series V 1.916 Jeol software. The results show the signal intensity and are expressed in arbitrary units.
Relative intensity in the solid PM
To determine the relative intensity of paramagnetic species in the solid PM samples, 3 mg of each sample was weighted and evaluated under the conditions previously described. Two independent measurements were taken for each PM sample. The final intensity was calculated based on the standard curve described above.
Evaluation of the oxidative potential of PM
The production of the hydroxyl radical (•OH) by PM10 and PM25 was evaluated in the presence of H2O2. For this purpose, a suspension of PM was prepared (3 mg/mL). A total of l00 μL of the suspension was mixed with 200 uL of 5,5-Dimethyl-l-Pyr-roline N-oxide (DMPO) (final concentration 0.1 M, Dojindo, Rockville, MD), for use as a spin trap, and 100 uL of H2O2 (final concentration 0.125 M, Fluka-Aldrich, St. Louis, MO). The mixture was incubated for 15 min at 37 °C with continuous shaking. The sample was filtered (0.2 um; Ministar-RC Syringe, Sartorius, Goettingen Germany) and transferred to a quartz flat cell for the EPR measurements. The formation of hydroxyl radical (•OH) was detected as a well-characterized 1:2:2:1 pattern of DMPO-OH adduct (Fig. 1b; Rinalducci et al. 2004) with aN=aH= 1.49 mT, g = 2.0056 under the following experimental parameters: center field = 335 mT, microwave frequency = 9.4 GHz, microwave power = 1 mW, sweep width +/- 5 mT, sweep time 0.5 min, modulation width 0.04 mT, amplitude 100, time constant 0.1 s and accumulation 3. A mixture of DMPO (0.1 M in water), phosphate buffered saline (PBS) and H2O2 (0.125 M in PBS) was used as a blank (Knaapen et al. 2000, Shi et al. 2003).
Inhibition of the oxidative potential of PM
Under the same experimental conditions described above we added DFO to the samples as an iron-copper chelating agent. A total of 100 uL of the PM suspension was mixed with 100 uL of DFO (final concentration 2.5 mM in water). The samples were incubated for 3 h with continuous shaking.
Evaluation of DNA degradation
The degradation of "naked" DNA was evaluated in DNA isolated from Balb/c 3T3 cells with a commercial kit (DNA isolation kit for cells and tissues; Boehringer, Mannheim, Germany) as previously described (Garcia-Cuellar et al. 2002). A total of 400 ng of isolated DNA was exposed to 40, 80 or 160 μg/mL of PM10 in the presence of 1 mM H2O2 (Fenton-type reaction). To inhibit the DNA degradation related to transition metals 1 mM of DFO was added. DNA exposed to CuSO4 as well as H2O2 was used as a positive control for a Fenton-type reaction. DNA was exposed to the particles for 24 h with constant shaking. The samples were evaluated by electrophoretic mobility (H5 Horizontal Gel Electrophoresis Apparatus; GIBCO-BRL Gaithersburg, MD) in 1.5 % agarose gels at 100 V for 3 hours, then stained with ethidium bromide (1.2 mg/mL) and photographed under UV light using a Kodak Gel logic 200 imaging system (New Haven, CT). All gels included DNA size markers and the following DNA degradation positive and negative controls: 1) l/Hind III (1 ug), 2) 400 ng of DNA alone, 3) 400 ng of DNA with 1 mM H2O2, 4) a "Fenton-type reaction" control using 400 ng of DNA plus 5 mM CuSO4 with 1 mM H2O2, and 5) 400 ng of DNA plus 1 mM of DFO and 1 mM H2O2.
Statistical analysis
A linear regression analysis was performed for the spin intensity vs. PM mass. The slope and Rvalue were calculated for each curve. These curves were used to calculate the intensity of spin in all evaluated samples. The data obtained for the different regions, years and sizes of PM were analyzed using an ANOVA followed by a Bonferroni test (Stata 7.0, Windows XP, College Station, TX). Differences were considered significant when p < 0.05.
RESULTS
Determination of the intensity of paramagnetic species (spin) in PM
Dry PM samples showed an pseudoisotropic broad signal (g= 2.2 and AH= 68 mT) indicating a high concentration ofparamagnetic species (PS), potentially explained by the presence of previously identified levels ofFe+3, Cu+2 and VO2+ in the PM samples (Fig. 1a). These metals are found in all three regions in different proportions, though the IR was found to have the highest concentrations of metals' related signal. The intensity evaluation of paramagnetic species in PMi0 from 2003 showed a linear correlation between PM mass and intensity under the conditions used for the measurement. When testing the PM from different regions the correlations yielded similar slopes and R-values above 0.99 for all samples (Fig. 2).
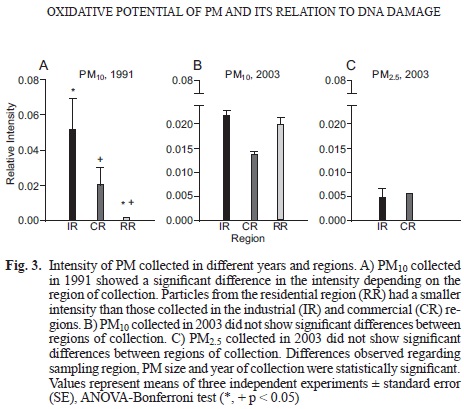
PS intensity of PM from different years and regions
PM-related PS intensity varied depending on the sampling region, the PM size and the year of collection (p < 0.05). PM10 collected in the IR during 1991 presented the highest intensity, followed by samples from the CR (p > 0.05) and the RR (p < 0.05; Fig. 3a). PM10 collected during 2003 did not yield significant differences among the sampling sites (Fig. 3b). Nevertheless, a reduction in spin intensity was observed in the IR upon comparison of samples from 1991 and 2003 (p = 0.07). In the IR and the CR, PM25 had lower PS intensities than PM10 samples, ~ 20 % of the PM10 value (Fig. 3c).
Oxidative potential of PM by EPR
PM suspensions in the presence of H2O2 produced a characteristic 1:2:2:1 DMPO-OH adduct pattern (hfcc aN=aH =1.49 mT, g=2.0056; Fig. 1b), indicative of hydroxyl radical (•OH) generation (Rinalducci et al. 2004) and indicative of OP. The OP of PM10 collected during 1991 was similar for the IR and the CR and slightly lower for the RR (Fig. 4a; p > 0.05). In contrast, the PM10 collected in the IR during 2003 demonstrated a higher oxidative potential than those collected in the CR and RR (p < 0.05; Fig. 4b). The OP of the PM10 from the IR was significantly higher in the samples collected in 2003 compared to the samples collected in 1991 (p = 0.012). PM10 and PM2.5 from 2003 did not yield significant differences in the OP (p > 0.05; Fig. 4).
Inhibition of PM oxidative potential by DFO
The OP induced by PM10 from the IR in 1991 and 2003 was strongly inhibited by the presence of DFO (p < 0.05; Fig. 4a and b). In the case of the PM10 collected in the CR and RR, no significant reductions in the OP were observed. The OP of PM2.5 was not inhibited by DFO (Fig. 4c).
DNA degradation
All samples of PM were capable of inducing DNA degradation under the experimental conditions (Fig. 5). In the case of the PM10 collected in 1991, the particles from the industrial and the commercial regions were stronger inducers of DNA degradation than those collected in the residential region (Fig. 5a). PM10 samples from 2003 presented a different pattern; those collected in the CR seemed to induce weaker responses than those from the IR and RR (Fig. 5b). In the case of PM2.5, similar effects were observed independent of the region (Fig. 5c). In all cases, DNA degradation was completely inhibited by the presence of DFO.
DISCUSSION
In the present study, we observed that the intensity of the paramagnetic species and oxidative potential of urban PM collected in Mexico City varied by year and site of collection. These parameters correlate with the ability of the PM to induce DNA degradation in vitro.
The broad signal induced by the PM dry samples is the result of the dipolar coupling of a high concentration of paramagnetic species, including metals such as Fe+3, Cu+2 and VO2+ (Valavanidis et al. 2005a). In the center field, we observed an isotropic fine signal with ΔH = 0.4 mT and g = 2.0026 that was attributable to stable organic species that could be semiquinones (data not shown). Regarding the intensity of the paramagnetic species, we observed that the PM from the IR of Mexico City collected in 1991 had a higher content of this intensity than that observed by PM from the RR. In contrast, the PM collected during 2003 did not differ depending on the sampling region. When comparing PM from both years (1991 vs. 2003) a significant reduction in the paramagnetic species content was observed for the PM from 2003 of the IR (p < 0.05), whereas the PM from the RR showed an increase (p < 0.05). The paramagnetic species in PM from the CR remained similar in both years of sampling. It has been reported that the concentration of different components in PM from Mexico City varied depending on the year of collection (Vega et al. 2004, Bae et al. 2010). The rapid growth of both, population and vehicle fleet (about 2 million units) during the period 1991-2003 has increased the production of organic compounds, PM10 and PM2.5 in Mexico City. Besides the growth of the industry, because about 70 % of non-metallic mineral industries, primary metals industry, food and beverage production, are important sources of PM (SMA-GDF 2008). For example, the content of total carbon and elemental carbon increased from 1997 to 2002, and these changes were related to increases in traffic, as well as industrial and commercial activities (Vega et al. 2011).
Previous evaluation of metal content by inductively coupled plasma-atomic emission (ICP-AES) and carbon content (TOR) in the PM10 from 1991 and 2003 revealed a higher concentration of metals and carbon in samples collected in 2003 (Alfaro-Moreno et al. 2009). Particles from 1991 had a gradient of metal content in which IR>CR>RR, while samples from 2003 had the pattern IR>RR>CR. In 1991, we observed that the carbon content had a pattern of IR=CR>RR, while in 2003, the pattern observed was IR>CR>RR (Vega et al. 2011).
When the oxidative potential of PM was evaluated, we observed that the samples collected in 1991 did not show significant differences among regions, while the PM collected in 2003 demonstrated that the PM from the IR region had a larger OP than that of the other two regions. If we compare the samples from 1991 and 2003, it is notable that the PM10 from the IR showed a significant increase in oxidative potential in 2003, while the PM from the other two regions did not significantly vary between the different years. These differences in oxidative potential could be related to the two following phenomena: 1) changes in the content of metals, mainly Fe and Cu, or 2) the oxidation of the sample due to storage time. This oxidation could increase the signal intensity in dry samples but still contribute to the OP, as with the less oxidized species. The second hypothesis could be supported by the observation that the amount of paramagnetic species is larger in the 1991 samples than in the 2003 samples. It makes sense that iron would be a significant contributor to the intensity of paramagnetic species, considering that Fe2+ is an inducer of the Fenton reaction (Granados-Oliveros et al. 2013), but Fe3+ could also participate. The nature of the reactions for the generation of hydroxyl radicals by ferric and ferrous salts in the presence of hydrogen peroxide has been studied (Yamazaki and Piette 1990), and it is accepted that the «OH free radical could be generated from ferric salt according to the mechanism proposed by Croft et al. (1992).
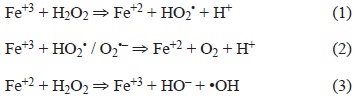
Fe2+ is not a paramagnetic species, in contrast to Fe3+ (Valavanidis et al. 2009, Gilch et al. 2010). Given this, it is likely that the formation of the hydroxyl radicals detected comes from both oxidation states of Fe. In addition to Fe, Cu+2 is a good promoter of Fenton-like reactions (Valenzuela et al. 2008) and has shown an even higher rate of oxidizing ability than Fe (Strlic et al. 2003).
The roles of Fe2+ and Fe3+ were highlighted by the suppression of the oxidative potential when DFO was added to the PM-DMPO suspension, considering that DFO is primarily an iron chelator (Valgimigli et al. 2001, Karlsson et al. 2005, Shi et al. 2006).
Various authors have shown the relationship between the oxidative potential of PM and biological effects by measuring lipid peroxidation (Shi et al. 2006), DNA damage (García-Cuéllar et al. 2002, Sánchez-Pérez et al. 2009, Wei et al. 2009) and cellular death (Chirino et al. 2010). In the present study, we observed that the intensity of DNA degradation by the Fenton-type reaction correlated with the oxidative potential. Interestingly, we observed that the addition of DFO to the mixture of DNA and PM abolished the degradation of DNA, while the oxidative potential was still measured with EPR. However, metal synergisms exist and the participation of organics has been described as key issues in PM induced biological effects (Cooper et al. 2009). Due to the design of this study, no full effect of the organic fraction can be explained, as is in the case of PM25. Thus, this result could be explained by differences in resolution between the two methods used (i.e., DNA electrophoresis did not detect minor DNA alterations; Sánchez-Pérez et al. 2009).
It has been shown that the OP of PM, as measured by EPR analysis (•OH radicals), correlates with DNA damage (Shi et al. 2006). The present study supports this previous observation. The reason for this correlation appears to be the high presence of metals. Considering that Fe3+ and Cu2+ are capable of inducing a Fenton-type reaction (Veranth et al. 2006, Gilch et al. 2010), it seems logical to conclude that the oxidative potential of PM, which can be inhibited by DFO, is mainly related to the predominant transition metals. Wessels et al. (2010) have suggested that increases in PS could be related to larger OP. As has been previously discussed, this correlation depends on the chemical species.
CONCLUSION
In conclusion, the evaluation of paramagnetic species and oxidative potential by means of EPR provides information supporting the understanding of the damage induced by the oxidative potential of PM. In some cases, the evaluation of the oxidative potential of PM could help to predict potential toxicity of these materials. However, PM is a complex mixture composed of different transition metals, organic and biological compounds that interact and can produce additive or synergistic effects. But for this study, metals are the most important sources of OP in PM samples. The EPR spin trapping technique allows us to directly monitor the formation of the 'OH free radical and to conduct DNA degradation analysis. Together, these approaches can be used as primary methods for evaluating the toxicity potential of PM.
ACKNOWLEDGMENTS
This study was partially supported by the CONACyT (project 106057). We would like to thank MSc. Yazmín Segura for her technical support during the 2003 PM sampling, Dr. Yesennia Sánchez-Pérez, MSc. Eva Salinas Cortés and MSc. Leticia Martínez Romero for technical advice in standardizing the DNA degradation assay.
REFERENCES
Alfaro-Moreno E., Martínez L., García-Cuéllar C., Bonner J.C., Murray J.C., Rosas I., Ponce de León-Rosales S. and Osornio-Vargas A.R. (2002). Biologic effects induced in vitro by PM10 from three different zones of Mexico City. Environ. Health. Perspect. 110, 715-720. [ Links ]
Alfaro-Moreno E., Ponce-de-León S., Osornio-Vargas A.R., García-Cuéllar C., Martínez L. and Rosas I. (2007). Potential toxic effects associated to metals and endotoxin present in PM10: an ancillary study using multivariate analysis. Inhal. Toxicol. 19, 49-53. [ Links ]
Alfaro-Moreno E., Torres V., Miranda J., Martínez L., García-Cuéllar C., Nawrot T.S., Vanaudenaerde B., Hoet P., Ramírez-López P., Rosas I., Nemery B. and Osornio-Vargas A.R. (2009). Induction of IL-6 and inhibition of IL-8 secretion in the human airway cell line Calu-3 by urban PM collected with a modified method of PM sampling. Environ. Res. 109, 528-535. [ Links ]
Bae S., Pan X.C., Kim S.Y., Park K., Kim Y.H., Kim H. and Hong Y.C. (2010). Exposures to PM and polycyclic aromatic hydrocarbons and oxidative stress in schoolchildren. Environ. Health Perspect. 118, 579-583. [ Links ]
Becker S., Dailey L.A., Soukup J.M., Grambow S.C., Devlin R.B. and Huang Y.C. (2005). Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress. Environ. Health Perspect. 113, 1032-1038. [ Links ]
Briede J.J., De Kok T.M., Hogervorst J.G., Moonen E.J., Op Den Camp C.L. and Kleinjanst J.C. (2005). Development and application of an electron spin resonance spectrometry method for the determination of oxygen free radical formation by PM. Environ. Sci. Technol. 39, 8420-8426. [ Links ]
Chirino Y.I., Sánchez-Pérez Y., Osornio-Vargas A.R., Morales-Bárcenas R., Gutiérrez-Ruíz M.C., Segura-García Y., Rosas I., Pedraza-Chaverri J. and García-Cuéllar C.M. (2010). PM10 impairs the antioxidant defense system and exacerbates oxidative stress driven cell death. Toxicol. Lett. 193, 209-216. [ Links ]
Cooper N.L., Bidwell J.R. and Kumar A. (2009). Toxicity of copper, lead, and zinc mixtures to Ceriodaphnia dubia and Daphnia carinata. Ecotoxicol. Environ. Saf. 72, 1523-1528. [ Links ]
Croft S., Gilbert B.C., Lindsay-Smith J.R. and Whitwood A.C. (1992). An E.S.R. Investigation of the reactive intermediate generated in the reaction between Fe+2 and H2O2 in aqueous solution. Direct evidence for the formation of the hydroxyl radical. Free Radic Res. Commun. 17, 21-39. [ Links ]
Dos Santos A.B., Siqueira S., Da Silva B., Ávila Santos L., Schmidtc M. and Baffad O. (2009). An-tioxidant properties of plant extracts: an EPR and DFT comparative study of the reaction with DPPH, TEMPOL and spin trap DMPO. J. Braz. Chem. Soc. 20, 1483-1492. [ Links ]
García-Cuéllar C., Alfaro-Moreno E., Martínez-Romero F., Ponce-de-León Rosales S., Rosas I., Pérez-Cárdenas E. and Osornio-Vargas A.R. (2002). DNA damage induced by PM10 from different zones of Mexico City. Ann. Occup. Hyg. 46, 425-428. [ Links ]
Gerlofs-Nijland M.E., Rummelhard M., Boere A.J., Lese-man D.L., Duffin R., Schins R.P., Borm P.J., Sillanpaa M., Salonen R.O. and Cassee F.R. (2009). Particle induced toxicity in relation to transition metal and polycyclic aromatic hydrocarbon contents. Environ. Sci. Technol. 43, 4729-4736. [ Links ]
Gilch S., Meyer O. and Schmidt I. (2010). Electron paramagnetic studies of the copper and iron containing soluble ammonia monooxygenase from Nitrosomonas europaea. Biometals 23, 613-622. [ Links ]
Granados-Oliveros G., Gómez-Vidales V., Nieto-Camacho A., Morales-Serna J.A., Cárdenas J. and Salmón M. (2013). Photoproduction of H2O2 and hydroxyl radicals catalysed by natural and super acid-modified montmo-rillonite and its oxidative role in the peroxidation of lipids. RSC Adv. 3, 937-944. [ Links ]
Karlsson H.L., Nilsson L. and Moller L. (2005). Subway particles are more genotoxic than street particles and induce oxidative stress in cultured human lung cells. Chem. Res. Toxicol. 18, 19-23. [ Links ]
Knaapen A.M., Schins R.P., Steinfartz Y., Ho D., Dunemann L. and Borm P.J. (2000). Ambient PM induces oxidative DNA damage in lung epithelial cells. Inhal. Toxicol. 12, 125-131. [ Links ]
Lloyd D.R., Phillips D.H. and Carmichael P.L. (1997). Generation of putative intrastrand cross-links and strand breaks in DNA by transition metal Ion-mediated oxygen radical attack. Chem. Res. Toxicol. 10, 393-400. [ Links ]
Lloyd D.R. and Phillips D.H. (1999). Oxidative DNA damage mediated by copper(II), iron(II) and nickel(II) Fen-ton reactions: evidence for site-specific mechanisms in the formation of double-strand breaks, 8-hydroxy-deoxyguanosine and putative intrastrand cross-links. Mutat. Res. 424, 23-36 [ Links ]
Montiel-Dávalos A., Ibarra-Sánchez M.J., Ventura-Gallegos J.L., Alfaro-Moreno E. and López-Marure R. (2010). Oxidative stress and apoptosis are induced in human endothelial cells exposed to urban PM. Toxicol. In Vitro. 24, 135-141. [ Links ]
Mugica V, Ortíz E., Molina L., De Vizcaya-Ruíz A., Nebot A., Quintana R., Aguilar J., and Alcántara E. (2009). PM composition and source reconciliation in Mexico City. Atmos. Environ. 43, 5068-5074. [ Links ]
Osornio-Vargas A.R., Serrano J., Rojas-Bracho L., Miranda J., García-Cuéllar C., Reyna M.A., Flores G., Zuk M., Quintero M., Vázquez I., Sánchez-Pérez Y., López T. and Rosas I. (2011). In vitro biological effects of airborne PM2.5 and PM10 from a semi-desert city on the Mexico-US border. Chemosphere 83, 618-626. [ Links ]
Rinalducci S., Pedersen J.Z. and Zolla L. (2004). Formation of radicals from singlet oxygen produced during photoinhibition of isolated light-harvesting proteins of photosystem II. Biochim. Biophys. Acta. 1608, 63-73. [ Links ]
Rosas-Pérez I., Serrano J., Alfaro-Moreno E., Baumgardner D., García-Cuéllar C., Miranda Martín Del Campo J., Raga G.B., Castillejos M., Colín R.D. and Osornio Vargas A.R. (2007). Relations between PM10 composition and cell toxicity: a multivariate and graphical approach. Chemosphere 67, 1218-1228. [ Links ]
Sánchez-Pérez Y., Chirino Y.I., Osornio-Vargas A.R., Morales-Barcenas R., Gutiérrez-Ruíz C., Vázquez-López I. and García-Cuéllar C.M. (2009). DNA damage response of A549 cells treated with PM (PM10) of urban air pollutants. Cancer Lett. 278, 192-200. [ Links ]
Shi T., Duffin R., Borm P.J., Li H., Weishaupt C. and Schins R.P. (2006). Hydroxyl-radical-dependent DNA damage by ambient PM from contrasting sampling locations. Environ. Res. 101, 18-24. [ Links ]
Shi T., Knaapen A.M., Begerow J., Birmili W., Borm P.J. and Schins R.P. (2003). Temporal variation of hydroxyl radical generation and 8-hydroxy-2'-deoxyguanosine formation by coarse and fine PM. Occup. Environ. Med. 60, 315-321. [ Links ]
Shinji A., Kazuyoshi K., Koichiro T., Masumi O., Toyoshi H., Hitoshi H. and Masanori Y. (2004). The reaction rate of edaravone (3-Methyl-1-Phenyl-2-Pirazolin-5-one-(MCI-186) with hydroxyl radical. Chem. Pharm. Bull. 52, 186-191. [ Links ]
SMA-GDF (2008). Inventario de emisiones ZMVM 2006. Secretaría del Medio Ambiente del Distrito Federal. Inventario. México D.F. 158 pp. [ Links ]
Soukup J.M., Ghio A.J. and Becker S. (2000). Soluble components of Utah Valley particulate pollution alter alveolar macrophage function in vivo and in vitro. Inhal. Toxicol. 12, 401-414. [ Links ]
Strlic M., Kolar J., Selih VS., Kocar D. and Pihlar D. (2003). A comparative study of several transition metals in Fenton-like reaction systems at Circum-neutral pH. Acta Chim. Slov. 50, 619-632. [ Links ]
Valavanidis A., Fiotakis T. K., Bakeas E., and Vlahogianni T. (2005a). Electron paramagnetic resonance study of the generation of reactive oxygen species catalyzed by transition metals and quinoid redox cycling by inhal-able ambient particulate matter. Redox Rep. 10, 37-51. [ Links ]
Valavanidis A., Vlahoyianni T. and Fiotakis K. (2005b). Comparative study of the formation of oxidative damage marker 8-hydroxy-2'-deoxyguanosine (8-OHdG) adduct from the nucleoside 2'-deoxyguanosine by transition metals and suspensions of PM in relation to metal content and redox reactivity. Free Radic Res. 39, 1071-1081. [ Links ]
Valavanidis A., Loridas S., Vlahogianni T. and Fiotakis K. (2009). Influence of ozone on traffic-related PM on the generation of hydroxyl radicals through a heterogeneous synergistic effect. J. Hazard Matter. 162, 886-892. [ Links ]
Valenzuela R., Contreras D., Oviedo C., Freer J. and Rodríguez J. (2008). Copper catechol-driven Fenton reactions and their potential role in wood degradation. Int. Biodeterior. Biodegradation. 61, 345-350. [ Links ]
Valgimigli L., Pedulli G.F. and Paolini M. (2001). Measurement of oxidative stress by EPR radical-probe technique. Free Radic Biol. Med. 31, 708-716. [ Links ]
Vega E., Reyes E., Ruiz H., García J., Sánchez G., Martínez-Villa G., González U., Chow J.C. and Watson J.G. (2004). Analysis of PM25 and PM10 in the atmosphere of Mexico City during 2000-2002. J. Air Waste Manag. Assoc. 54, 786-798. [ Links ]
Vega E., Ruíz H., Escalona S., Cervantes A., López-Veneroni D., González-Ávalos E. and Sánchez-Reyna G. (2011). Chemical composition of fine particles in Mexico City during 2003-2004. Atmos. Pollut. Res. 2, 477-483. [ Links ]
Veranth J.M., Moss T.A., Chow J.C., Labban R., Nichols W.K., Walton J.C., Watson J.G. and Yost G.S. (2006). Correlation of in vitro cytokine responses with the chemical composition of soil-derived PM. Environ. Health Perspect. 114, 341-349. [ Links ]
Wei Y., Han I.K., Shao M., Hu M., Zhang O.J. and Tang X. (2009). PM25 constituents and oxidative DNA damage in humans. Environ. Sci. Technol. 43, 4757-4762. [ Links ]
Wessels A., Birmili W., Albrecht C., Hellack B., Jermann E., Wick G., Harrison R.M. and Schins R.P. (2010). Oxidant generation and toxicity of size-fractionated ambient particles in human lung epithelial cells. Environ. Sci. Technol. 44, 3539-3545. [ Links ]
Yamazaki I. and Piette L.H. (1990). Stoichiometric measurements of the Fenton reaction, possible non-OH oxidizing intermediate by spin-trapping. Free Radic Biol. Med. 9, 42. [ Links ]














