Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista internacional de contaminación ambiental
versión impresa ISSN 0188-4999
Rev. Int. Contam. Ambient vol.31 no.1 Ciudad de México feb. 2015
Modified procedure to assess DNA breakage in spermatozoa by means of the comet assay
Modificación al procedimiento para determinar rompimientos de ADN de espermatozoides por medio del ensayo cometa
Griset Martínez-Luna1, Julieta Castillo-Cadena1 and Jorge Humberto Serment-Guerrero2*
1 Facultad de Química, Universidad Autónoma del Estado de México, Paseo Colón esq. Paseo Tollocan s/n, CP 50100, Toluca, Estado de México
2 Departamento de Biología, Instituto Nacional de Investigaciones Nucleares, Carretera México-Toluca s/n, km 36.5, La Marquesa, Municipio de Ocoyoacac, CP 52750, Estado de México *Corresponding author: jorge.serment@inin.gob.mx
(Received May 2014;
accepted January 2015)
ABSTRACT
The comet assay is a relatively inexpensive, fast, sensitive and reliable method to detect DNA breakage upon individual cells. The most widely used cell type in this technique is lymphocyte, because they are easy to obtain and handle, and because they are continually exposed to xenobiotics that enter into the body. However, is important to consider the possibility to use other cell types for very specific purposes. Sperm cells are of special interest because they could be used as a biomonitor to risk assessment in populations occupationally exposed to xenobiotics. Besides, the fact that there are not functional DNA repair mechanisms in these cells could increase the sensitivity of the system. We present here several modifications to the comet assay methodology to evaluate DNA breakage in sperm cells with reliable results.
Key words: genotoxicity, DNA breakage, sperm, microelectrophoresis.
RESUMEN
El ensayo cometa es un método relativamente simple, rápido, sensible y confiable para detectar rompimientos en el ADN en células individuales. Las células más comúnmente utilizadas en esta técnica son los linfocitos, ya que son fáciles de obtener y manejar y además porque se encuentran expuestas continuamente a los xenobióticos que llegan a entrar al cuerpo. Sin embargo, es importante el considerar la posibilidad de utilizar otros tipos celulares para propósitos específicos. Las células espermáticas son de especial interés ya que pueden servir como biomonitores de riesgo en poblaciones ocupacional-mente expuestas. Además, el hecho de que este tipo de células no tienen mecanismos funcionales de reparación de lesiones en el material genético podría incrementar la sensibilidad del ensayo. En el presente trabajo se muestran algunas modificaciones a la técnica del cometa para evaluar rupturas en el ADN de espermatozoides con resultados confiables.
Palabras clave: genotoxicidad, rupturas de ADN, esperma, microelectroforesis.
INTRODUCTION
Genotoxicity is defined as any change in basic DNA chemical structure as a result of the interaction of harmful chemical or physical agents with genetic material. These changes will finally lead to three possible outcomes: cell death, total repair or imperfect repair that may result in the appearance of mutations. Those genetic alterations would be transmitted to subsequent generations through germ cells such as sperm (Clayson and Grant 1992). Thus, it is imperative to assess and identify xenobiotic agents capable of producing such DNA damage. Genotoxicity bioassays are usually employed to evaluate the risk to human health in order to establish prevention and control regulations (Paules et al. 2011).
One of the most popular genotoxicity tests is the single cell gel microelectrophoresis, commonly called comet assay, a sensitive, fast and inexpensive method to examine DNA breakage in individual cells (Berwick and Vineis 2000, Collins 2004).This assay has positioned itself in a privileged position into the battery of biomonitoring studies used to evaluate hazardous agents in environmental toxicology (Hartman et al. 2003), in occupational exposure to xenobiotics and as a biomarker capable to demonstrate dose-effect relationships (Jakubowski and Trzcinka-Ochocka 2005).
The comet assay allows the detection of single or double strand DNA breakage or alkaline-labile sites in individual cells, with relatively high sensitivity. Briefly, cells are mixed with low melting point agarose (LMP) to form a thin gel on top of a microscope slide and then lysed in situ to remove all cellular membranes and proteins. Then, slides are put into an electropho-resis cell and a voltage is applied. Fragmented DNA will migrate out of the lysed cell towards the anode (Nadin et al. 2001), forming a structure similar to a comet (Hughes et al. 1996, Collins 2004).
Although a wide range of cell types can be used, lymphocytes has been traditionally used in this technique, because of the ease to obtain and also because these cells are circulating in the peripheral blood as part of the immune response; therefore they are continually exposed to xenobiotics that enter into the body either through the skin, oral, digestive or respiratory mucosae, so a high degree of DNA damage could be assessed by these cells (Moller et al. 1989, Orson et al. 1989, McCarthy et al. 1990, Rödl et al. 1990). Indeed, the damage to individual somatic cells such as lymphocytes, as measured by the comet assay, detects changes in DNA structure that can lead to genomic instability.
In germ cells, such as spermatozoa, which carry genetic information passed from one generation to the next, is important to evaluate individuals with occupational exposure to detect possible DNA damage that could lead to malformations in future generations. Another possible advantage in using this kind of cells is that they can be considered as packages of DNA, so for in vitro test repair events could be avoided, increasing the sensitivity of the system. However, even though several methodologies have been reported, we couldn't reach consistent results with any of them, therefore we tried to improve the protocol proposed by Hauser and co-workers (Hauser et al. 2007) for comet assay with human spermatozoa, making changes that permit obtaining reliable results.
MATERIAL AND METHODS
The procedure for evaluation of DNA damage in human sperm with the general modifications is shown below.
Chemicals and solutions
Glucose, potassium chloride and sodium hydroxide were purchased from J.T. Baker; sodium chloride was from BDH; Dithiothreitol (DTT) was from Gibco BRL; proteinase K was from Thermo Lab, and the rest of chemicals were purchased from Sigma-Aldrich. Casiopeina III-Ea was synthesized, purified and provided by Dr. Lena Ruiz-Azuara, from the school of Chemistry, UNAM (Ruiz-Ramirez et al. 1991, Ruiz-Azuara 1993) and used as a positive control (Serment-Guerrero et al. 2011).
Semen sample collection
Samples were obtained from three different healthy donors who were properly informed about the purposes of the investigation and voluntarily consented to participate. Semen was obtained by masturbation after a recommended abstinence period of 48 hours, and collected in a sterile plastic cup. Liquefaction was performed at 37 °C for 60 minutes, and semen was diluted 1:20 in Beltsville Thawing Solution (BTS) (glucose 0.2M; Na2 EDTA 3 mM; NaHCO3 0.015 M; citrate Na3.2H2O 0.02 M and KCl 0.01 M, pH 7.2) (Erikkson and Martinez-Rodriguez 2000, Mapeka et al. 2012), and frozen at -20 °C (Pursel and Johnson 1975) for later analysis. When needed, samples were thawed by gently shaking in a water bath at 37 °C for 10 minutes and semen was immediately processed for comet assay.
In vitro exposure and treatments
A 100 mM stock solution of the casiopeina was prepared in 1% dimethyl sufoxide (DMSO), and subsequent dilutions were made in sterile milliQ water. To induce DNA damage in spermatozoa, 180 uL of 1:20 sperm-BTS dilution (10 uL of semen with 190 uL of BTS) were placed in a microtube with 10 μL of the proper dilution of Casiopeina III-Ea (each sample was made in duplicate), the mixture was then incubated in a 37 °C water bath for 30 minutes and centrifuged for 10 minutes at 2500 rpm. The supernatant was discarded and the pellet suspended in 180 μL of BTS.
Comet assay
Clear slides were previously prepared with 90 uL of Type I: Low EEO agarose at 0.6 % and let to dry at 37 °C, then a second layer was applied and the drying step was repeated.
Cell suspension was mixed with an equal volume of 1% low melting point agarose to reach a final concentration of 0.5 %, then 90 μL of this mixture were applied on previously prepared slides and topped with a cover slip, placed on a metal tray and put in the refrigerator for 5 minutes to solidify. Afterwards, cover slip was removed and slides were immersed in cold lysis solution (2.5 M NaCl, 10 mM Tris, 100 mM Na2.EDTA, 10 % DMSO, 1 % N-lauroyl sarcosine, 1% Triton X-100, pH 10.0) for one hour. Afterwards, slides were withdraw from the lysis solution and 60 μL of a solution of proteinase K (1 mg/mL) and DTT (5 mM) in the same lysis solution reported above were applied and incubated overnight (18-20 hours) at 37 °C in a humidity chamber. Slides were placed on a horizontal electrophoresis cell, covered with electrophoresis solution (0.3 M NaOH, 1 mM Na2.EDTA) for 20 minutes to allow DNA unwinding and then a current was applied (20 V, 300 mAmp, 15 minutes at 4 °C).
Later, slides were removed from the electrophoresis chamber, gently rinsed three times with neutralization buffer (0.4 M Tris-HCl; pH 7.5), staining with 60 μL of ethidium bromide (20 μg/ml) and finally observed under a Hund Wetzlar epifluorescence microscope with a 40x objective using a 515-560 nm (green light) exciting filter. Two slides were prepared for each treatment. One hundred randomly selected cells were scored by means of the Comet Assay IV Analyzer (Perceptive Instruments Inc.). DNA breakage was evaluated as percentage of cells with fragmented DNA and/or tail moment, which is the relation between comet tail length and DNA fluorescence intensity (Collins 2004).
RESULTS AND DISCUSSION
Currently, this laboratory is interested in monitoring the effect of xenobiotics in spermatozoa from occupationally exposed populations, so it was essential to have a reliable comet assay procedure to assess DNA breakage. However, when the standard methodology report by Tice and co-workers (2000) was used, no good results were obtained. Alternatively, the one reported by Hauser et al. (2007), specifically designed for spermatic cells was tested, unsuccessfully. Therefore, several modifications were made to try to improve the procedure to make it functional in these facilities.
In the first place, the use of clear slides instead of frosted slides was implemented, in order to reduce the background fluorescence. The standard methodology recommended the use of a first layer of normal agarose, a second layer of LMP mixed with cells and a final third layer of LMP (Tice et al. 2000). In this work, we used two layers of Type I: Low EEO; once dried, the agarose crystals serve as a frosting that allow the next layer of LMP agarose to become firmly attached to the slide. Another advantage of using this technique is that the preparation of slides is faster, so treated cells can go into the lysis solution in less time.
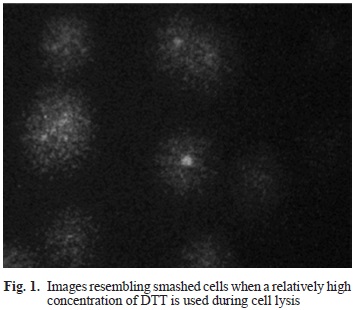
The lysis solution reported by Tice and co-workers has proved to be quite functional for lymphocytes and some other cell types. However, it does not work upon spermatozoa, since this kind of cells has a plasmatic membrane with a unique lipid and protein composition, that allows for very little or no substance exchange, therefore it is necessary to use a mucolytic agent capable of lysing these cells to liberate the genetic material (Davies-Morel 1999). Previous reports proposed a lysis procedure in which the use of proteinase K (Hughes et al. 1996) and RNase (Hauser et al. 2007) were included. At first, the technique was implemented using only proteinase K, but lysis did not occur, therefore DTT was added as a mucolytic agent, which is able to disrupt the protein disulfide bonds (-SS-) that are present in sperm, allowing fully deployment and separation of protein subunits of a multimeric protein. DTT is routinely used in DNA extraction for forensic purposes to make accessible the chromatin of the sperm head (Bart-mazt et al. 1994, González-Estrella et al. 1994) and was used in the comet assay before (Donnelly et al. 1999, Enciso et al. 2009). First, DTT (40 mM,20 mM and 10 mM) and proteinase K (1 mg/mL) were added straight into the lysis solution and incubated at 4 °C for 60, 90, 120 and 180 minutes (Kaymak et al. 2012). However, lysis was not good. Besides, the addition of DTT in the lysis solution increased dramatically the cost of the assay, so 60 mL of a DTT/proteinase K solution were applied directly on the slides after 1 hour in standard lysis solution, and further incubated for 18-20 hours in a humidity chamber at 37 °C. Under microscope, images resembling "smashed" cells instead of comets were found (Fig. 1), indicating that lysis actually occurred but DNA migration did not, most probably because the electric charge of the DTT interfered with the negative charge of DNA.
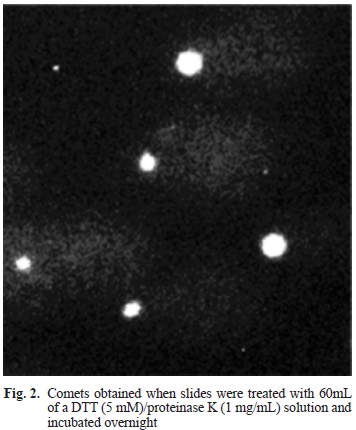
The amount of DTT showed to be very important. In total, 11 different concentrations of DTT were tested, but when solutions of 1 mM or lower were used, lysis did not occurred, whereas when solutions of 100 mM or higher were employed, DNA migration was no good. At 5 mM of DTT lysis was excellent and so was migration (Fig. 2).
Another parameter that changed in this work was the diluent used for semen. When the classical PBS solution was used (Hauser et al. 2007) controls were not good, with more than ten percent of comets in untreated cells, so results could not be trustworthy. When the BTS solution reported by Pursel and Johnson (1976) results were superb. Moreover, BTS has a dual advantage, since it acts as a handling solution and is excellent for cryopreservation of semen. Indeed, frozen cells with more than three weeks at -70 °C, produced results in the comet assay that were highly correlated with results from freshly obtained samples.
Cell concentration is an important issue in this technique, since too many cells will result in comet overlapping, whereas too few will take too long for scoring. The cell concentration average of the samples used in this work has 7×107 cells/mL, so routinely a 1:20 dilution of the ejaculated in BTS was prepared and from there 100 uL aliquots were used per treatment, reaching a final concentration of 1×105 cells/mL approximately.
The electrophoresis time was also adjusted. The original procedure indicated 20 minutes at 20 V and 300 mA, however, spermatozoa are haploid cells (n) with half of the genetic material contained in any other cell type. When an electrophoresis time of 20 minutes was used the comets showed long tails, yet unusually low tail moment values were obtained and dose response correlation was not good. Hughes and co-workers (1996) reported an electrophoresis time of 10 minutes, but comets were rather small and hence diminished the sensitivity of the assay. Finally, the electrophoresis time was reduced to 15 minutes with good results (Fig. 3).
To verify the optimal performance of the improved methodology described above experiments were carried out using a molecule recently reported as an actual genotoxic (Serment-Guerrero et al. 2011), casio-peina III-Ea ([Cu(4,7-dimethyl-1,10-phenanthroline) acac]NO3), figure 4a). Casiopeina is the generic name of a group of compounds, with a central cooper atom bound to organic ligands designed to be used as antineoplastics. Different concentrations of CasIII-Ea were apply to a suspension of spermatozoa diluted in BTS for 30 minutes at 37 °C, then mixed with LMP agarose and lysed a described above. Experiments were scored by means of the Comet Assay IV (Perceptive Instruments, UK) and tail moment was taken into account. Under microscope, comets in which tail increased along with the concentration were found (Fig. 4b). The results confirmed the genotoxic activity of CasIII-Ea and, moreover, showed that this improved methodology is more sensitive, regarding the results obtained when lymphocytes were used, most probably due to the lack of DNA repair mechanisms in spermatic cells (Serment-Guerrero et al. 2011).
CONCLUSIONS
Overall, the sum of the small modifications introduced to the comet assay technique resulted in a reliable methodology for the use of sperm cells. The use of the BTS solution for both cryopreservation and dilutions minimized the breakage upon untreated cells. To apply a solution of DTT/proteinase K directly on the slides permitted an optimal disruption of spermatic cells membrane without increasing significantly the cost of the assay. Due to relatively low amount of DNA in spermatic cells the tail moment was not a representative parameter for genotoxicity since, as stated above, genetic material in the tail was too dispersed. By reducing the electrophoresis time to 15 minutes, a good correlation between tail moment and the dose applied were found. This was demonstrated by the use of CasIII-Ea; results showed an increase in tail moment along with the concentration of this compound. Under the conditions described here, the use of spermatic cells seems to increase the sensitivity of the comet assay, compared with others types of cells.
ACKNOWLEDGMENTS
This project was partially funded by the UAEMex, Project Agreement No. 3452/2013CHT and by the Instituto Nacional de Investigaciones Nucleares. Studies were carried out according to the regulations of the Reglamento de la Ley General de Salud en Materia de Investigación para la Salud (Official Mexican Regulations in the field of health research) and approved by the corresponding institutional technical committee.
REFERENCES
Barmatz M.J., Karabinus D. and Dalkin B.L. (1994). Dithiothreitol effects of human sperm quality. J. Urol. 52, 2287-2290. [ Links ]
Berwick M. and Vineis P. (2000). Markers of DNA repair and susceptibility to cancer in humans: an epidemiologic review. J. Natl. Cancer Inst. 92, 874-897. [ Links ]
Bravo-Gómez M.E., García-Ramos J.C., Gracia-Mora I. and Ruíz-Azuara L. (2009). Antiproliferative activity and QSAR study of cooper (II) mixed chelate [Cu(N-N) (acetylacetonato)] NO3 and [Cu(N-N)(glycinato)] NO3 complexes, (Casiopeinas ®). J. Inorg. Biochem. 103, 299-309. [ Links ]
Clayson D.B. and Grant D.L. (1992). The assessment of mutagenicity. Health protection branch mutagenicity guidelines. Environ. Mol. Mutagen. 21, 15-37. [ Links ]
Collins A.R. (2004). The comet assay for DNA damage and repair. Mol. Biotechnol. 26, 249-261. [ Links ]
Davies-Morel M.C.G. (1999). Equine artificial insemination. Wallingford, Oxon: CAB International. [ Links ]
Donnelly E.T., McClure N. and Lewis S.E.M. (1999). The effect of ascorbate and alpha-tocopherol supplementation in vitro on DNA integrity and hydrogen peroxide induced DNA damage in human spermatozoa. Mutagenesis 14, 505-511. [ Links ]
Enciso M., Sarasa J., Agarwal A., Fernández J.L. and Gosálvez J. (2009). A two-tailed Comet assay for assessing DNA damage in spermatozoa. Reprod. Biomed. online 18, 609-616. [ Links ]
Erikkson B.M. and Martínez-Rodríguez H. (2000). Effect of freezing and thawing rates on the post-thaw viability of boar spermatozoa frozen in Flat Packs and Maxi-straws. Anim. Reprod. Sci. 63, 205-20. [ Links ]
González-Estrella J.A., Coney P., Ostash K. and Karabinus D. (1994). Dithiothreitol effects on the viscosity and quality of human semen. Fertil. Steril. 62, 1238-1246. [ Links ]
Hartmant A., Agurell E., Beevers C., Brendler-Schwaab S., Burlinson B., Clay P., Collins A., Smith A., Speit G., Thybaud B. and Tice R.R. (2003). Recommendations for conducting the in vivo alkaline comet assay. 4th International Comet Assay Workshop. Mutagenesis 18, 45-51. [ Links ]
Hauser R., Meeker J.D., Singh N.P., Silva M.J., Ryan L., Duty S. and Calafat A.M. (2007). DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites. Hum. Reprod. 22, 688-695. [ Links ]
Hughes C.M., Lewis S.E., McKelvey-Martin V.J. and Thompson W. (1996). A comparison of baseline and induced DNA damage in human spermatozoa from fertile and infertile men, using a modified comet assay. Mol. Hum. Reprod. 2, 613-619. [ Links ]
Jakubowski M. and Trzcinka-Ochocka M. (2005). Biological monitoring of exposure: trends and key developments. J. Occupational Health 47, 22-48. [ Links ]
Kaymak C., Kadoiglu E., Coskun E., Basar H. and Basar M. (2012). Determination of DNA damage after exposure to inhalation anesthetics in human peripheral lymphocytes and sperm cells in vitro by comet assay. Hum. Exp. Toxicol. 31, 1207-1213. [ Links ]
Mapeka M.H., Lehloenya K.C. and Nedambale T.L. (2012). Comparison of different extenders and storage temperature on the sperm motility characteristics of Kolbroek pig semen. S. Afr. J. Anim. Sci. 42, 530-534. [ Links ]
McCarthy M.A., Michalsky J.P., Sears E.S. and Mc-Combs C.C. (1990). Inhibition of polyamine synthesis suppresses human lymphocyte proliferation without decreasing cytokine production of interleukine 2 receptor expression. Immunopharmacology 20, 11-20. [ Links ]
Moller J., Hoffman B., Langhoff E., Damgard-Jacobsen K., Odum N., Dickmeiss E., Ryder L.P., Thastrup O., Scharff O. and Foder B. (1989). Inmunodeficiency after allogenic bone marrow transplantation in man. Effect of phorbol ester (phorbolmyristate acetate) and calcium ionophore (A23187) in vitro. Scand. J. Immunol. 30, 441-447. [ Links ]
Nadin S., Vargas-Roig L. M. and Ciocca D.R. (2001). A silver staining method for single-cell gel assay. J. Histochem. Cytochem. 49, 1183-1186. [ Links ]
Orson F.M., Saadeh C.K., Lewis D.E. and Nelson D.L. (1989). Interleukin 2 receptor expression by T cells in human aging. Cell Immunol. 124, 278-291. [ Links ]
Paules S.R., Aubrecht J., Corvi R., Garthoff B. and Kleinjans J.S. (2011). Moving forward in human cancer risk assessment. Environ. Health Perspect. 119, 739-743. [ Links ]
Pursel VG. and Johnson L.A. (1975). Freezing of boar spermatozoa: Fertilizing capacity with concentrated semen and a new thawing procedure. J. Anim. Sci. 40, 99-102. [ Links ]
Rödl S., Fuchs G., Khoshsorur G., Iberer F. and Tscheliess-nigg K.H. (1990). Lipoprotein-induced modulation of cyclosporine-a-mediated immunosuppression. Eur. J. Clin. Invest. 20, 248-252. [ Links ]
Ruiz-Azuara L. (1996). Process to obtain new mixed copper aminoacidate from methylate phenanthroline complexes to be used as anticancerigenic agents. USA, Patent No. 5,576,326 (07/628,628). 1992. [ Links ]
Ruíz-Ramírez L., Gracia M.I., Moreno E. R., Gasque L., Huerta L., Mayet L., Ortiz V. and Lomeli C. (1991). The antitumor activity of several transition metal complexes. J. Inorg. Biochem. 43, 615. [ Links ]
Serment-Guerrero J., Cano-Sánchez P., Reyes-Pérez E., Velázquez-García F., Bravo-Gómez M.E. and Ruiz-Azuara L. (2011). Genotoxicity of the cooper antineoplastic coordination complexes casiopeinas®. Toxicol. In Vitro 25, 1376-1384. [ Links ]
Tice R.R., Agurell E., Anderson D., Burlinson B., Hartmann A., Kobayashi H., Miyamae Y., Rojas E., Ryu J. C. and SasakiY.F. (2000). Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 35, 206-221. [ Links ]














