Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista internacional de contaminación ambiental
versión impresa ISSN 0188-4999
Rev. Int. Contam. Ambient vol.30 no.1 Ciudad de México feb. 2014
Removal of methyl parathion and coumaphos pesticides by a bacterial consortium immobilized in Luffa cylindrica
Remoción de los plaguicidas metil paratión y coumafos por un consorcio bacteriano inmovilizado sobre Luffa cylindrica
David Antonio MORENO-MEDINA, Enrique SÁNCHEZ-SALINAS y Ma. Laura ORTIZ-HERNÁNDEZ*
Laboratorio de Investigaciones Ambientales, Centro de Investigación en Biotecnología, Universidad Autónoma del Estado de Morelos. Av. Universidad 1001, Col. Chamilpa, C.P. 62209, Cuernavaca, Morelos, México *Corresponding author; ortizhl@uaem.mx
Recibido febrero 2013,
aceptado octubre 2013
ABSTRACT
The constant application of pesticides has led to environmental and health problems in many regions of the world, as well as the storage of large amounts of pesticide waste and obsolete pesticides. For this reason, it is necessary to develop strategies for the disposal of pesticides that are ecofriendly and economically viable. Different treatment options exist, but in recent years, the application of biological systems has gained the greatest acceptance, because it promises the degradation and detoxification of pesticides without harming the environment or human health. In this study, we used a bacterial consortium that was isolated from agricultural soils to degrade a mixture of the organophosphate pesticides methyl parathion (MP) and coumaphos (COU). The efficiency of removal was evaluated using mineral salt medium supplemented with glucose, and the bacterial consortium was cultivated as free cells and immobilized on Luffa cylindrica fibers. To improve the structure of the fibrous network and to achieve greater retention of microorganisms, removal was also tested prior to fibers treatment with sodium hydroxide (NaOH). The results indicate that the microorganisms used had better growth as free cells. A removal of 54.88 % and 62 % for MP and COU, respectively was observed using the free cells; but when the cells were immobilized on loofa sponge fibers, the removal was increased to 98 and 100 % of those pesticides. This pesticide removal was the result of a combined effect among the activity of the microorganisms, the adhesion to the bacterial cells and the adsorption on the support material. We observed a strong and fast adsorption on the loofa sponge fiber, since removal obtained only with loofa fiber, did not present significant difference with immobilized microorganisms. Further studies are needed to understand the processes that occur with adsorbed pesticides and whether there was subsequent desorption and degradation.
Key words: pesticides, biodegradation, immobilized cells, biofilm, Luffa cylindrica.
RESUMEN
La aplicación constante de plaguicidas ha traído como resultado problemas ambientales y de salud pública en muchas regiones del mundo, además del almacenamiento de grandes cantidades de residuos y de plaguicidas obsoletos. Por esta razón, es necesario desarrollar estrategias económica y ambientalmente viables para el tratamiento y disposición final de los plaguicidas. Existen diferentes opciones, aunque recientemente la aplicación de sistemas biológicos ha ganado gran aceptación debido a la posibilidad de lograr la degradación y detoxificación de los plaguicidas sin causar daños al ambiente o a la salud. En este estudio se utilizó un consorcio bacteriano aislado de suelos agrícolas para degradar una mezcla de paratión metílico (PM) y coumafos (COU), dos plaguicidas organofosforados. Se evaluó la eficiencia de remoción del consorcio utilizando un medio de sales minerales con adición de glucosa, además de que el consorcio fue cultivado en suspensión e inmovilizado en fibras de Luffa cylindrica. Para mejorar la estructura de la red fibrosa de la planta y lograr una mayor retención de los microorganismos, la remoción se probó con y sin un pretratamiento con hidróxido de sodio. Los resultados indican que los microrganismos utilizados presentaron mejor crecimiento en suspensión y se observó una remoción del 54.88 % y 62 % de PM y COU, respectivamente; sin embargo, cuando las células fueron inmovilizadas en las fibras de Luffa cylindrica, la remoción se incrementó a 98 % y 100 % respectivamente. Esta remoción fue el resultado de un efecto combinado entre la actividad de los microorganismos, la adhesión a las células bacterianas y la adsorción al material de soporte. Se observó una rápida y fuerte adsorción sobre las fibras del material soporte, ya que la remoción obtenida sólo con la fibra, no presentó diferencias significativas con los microorganismos inmovilizados. Se requieren mayores estudios para entender los procesos que ocurren con los plaguicidas adsorbidos y su posible desorción y degradación posterior.
Palabras clave: plaguicidas, biodegradacion, células inmovilizadas, biopelícula, Luffa cylindrica.
INTRODUCTION
Increasing population, growing demand for food, agricultural mechanization and the need to control a variety of new pests have resulted in an increased production and consumption of pesticides. In addition to increasing agricultural production, the benefits of pesticides for controlling pests and disease vectors are undeniable. However, every year, pesticides must be applied at higher doses, and this intensive use of pesticides has irreversible adverse effects on the environment and human health (Soares etal. 2003, Recena etal. 2006). A pesticide is any substance or mixture of substances intended to prevent, destroy or control pests, which cause damage or otherwise interfere in the production, processing, storage, transportation or marketing of food, agricultural products and wood. Vectors or intermediate hosts of human and animal diseases that can be supplied to animals in order to fight against any organism inside or on their bodies are also considered pesticides (Athia 2006).
It is estimated that approximately 85 % of pesticides used worldwide are used for agriculture (Cervantes 2010). In 2007, 2.36 million tons of active pesticides were consumed for agricultural purposes worldwide (USEPA 2011). The continuous application of pesticides has increased their concentration in soil and water, which leads to their entry into the food chain (Ortiz-Hernández et al. 2011). Dispersion mechanisms have also increased the level of environmental risk for the occupationally exposed population and the inhabitants of surrounding villages. On the other hand, liquid and solid waste and obsolete products that are stored or disposed of in an inappropriate manner have resulted in significant environmental liabilities, which in most cases are not reported to the appropriate authority (Sánchez-Salinas and Ortiz-Hernández 2011).
Pesticides of different chemical structures are toxic and persistent in the environment. Currently, organophosphates (OP) are used worldwide as pesticides or chemical warfare agents because of their high toxicity toward insects, mammals and other animals (Theriot and Grunden 2011). These compounds have a basic structure that consists of ester or thiol derivatives of phosphoric, phosphonic or phosphoramidic acids (Vilanova and Sorgob 1999, Ortiz-Hernández and Sánchez-Salinas 2010). Their mechanism of action involves the irreversible inhibition of acetylcholinesterase, a key enzyme of the central nervous system, and thus, they affect non-target organisms (Singh and Walker 2006). Like all pesticides, intensive use of OP pesticides can lead to their accumulation in the environment and may affect ecosystems and human health.
To mitigate the problem of contamination by OPs, treatments have been developed to detoxify and/or degrade these pesticides through physical, chemical and/or biological processes. In biological processes, biological systems (whole cells or isolated enzymes) are used to catalyze chemical reactions that transform the pesticide into simpler and less toxic compounds or, better yet, mineralize them into molecules.
Biological methods are gaining interest due to their simplicity, high efficiency and cost effectiveness compared to other methods (Chandran and Das 2011). Biodegradation of OP pesticides provides a cheap and efficient solution for their final disposal or for the treatment of agricultural soils, contaminated water or polluted ecosystems. To date, a number of different microorganisms have been identified, and the enzymes involved in OP degradation have been studied (Singh 2009, Ortiz-Hernández et al. 2011,).
In order to optimize biological treatment, different strategies have been developed. Among them, cell immobilization has been a successful approach that ensures that the catalytic activity of the biological processes is maintained for longer periods (Manohar et al. 2001, Cheng et al. 2003, Yáñez-Ocampo et al. 2009). Immobilization consists in restricting cell mobility within a defined space of a material with particular characteristics.
There are two types of processes for cell immobilization: those based on physical retention (entrapment and inclusion membrane) and those based on chemical bonds, such as biofilm formation (Kennedy and Cabral 1983). In cell immobilization methods, various inorganic (clays, silicates, glass and ceramics) and organic (cellulose, starch, dextran, agarose, alginate, chitin, collagen, keratin, etc.) supports are employed (Arroyo 1998).
The most widely studied method for the practical application of immobilized cell techniques is entrapping cells in polymer gels, such as alginate and carrageenan. However, this method has been limited by problems with gel stability and by the mass transfer limitations of gel beads. Entrapment in natural polymeric gels has become the preferred technique for the immobilization of cells, due to the toxicity problems associated with the synthesis of polymeric materials (Lusta et al. 1990). However, biodegradable natural supports have been used more frequently in the treatment of sewage contamination (Garzón-Jiménez 2009).
Several reports indicate that a variety of materials provide the necessary features to immobilize microorganisms (Jin et al. 1998, Iqbal and Saeed 2006, Barragán et al. 2007, May-Esquivel et al. 2008, Garzón-Jiménez 2009). Pattanasupong et al. (2004) reported on the use of various plant fibers as supports for immobilizing a bacterial consortium to degrade xenobiotics. The use of the natural structural materials, such as the petiolar felt-sheath of palm, for cell entrapment has added another dimension to a variety of immobilization matrices (Iqbal and Saeed 2006, Iqbal and Edyvean 2007). The advantages of such biostructures are their reusability, no toxicity, mechanical strength and open spaces within the matrix for growing cells, which avoids rupture and diffusion problems (Akhtar et al. 2004). These findings led us to search diverse plant sources for other types of biomaterials that may be used for cell entrapment.
Among these materials, the fibers of Luffa cylindrica L. (sponge loofa) have been used as a natural support to immobilize various microorganisms. Among them Chlorella sorokiniana, Porphyridium cruentrum, Penicillium cyrlopium, and Funalia trogii, for nickel and cadmium II treatment, as well as for use in dyes and chlorinated substances (Akhtar et al. 2004, Mazmanci and Ünyayar 2005, Alluri et al. 2007). Furthermore, the use of Luffa sp. to immobilize fungal biomass for metal biosorption processes was previously reported by Iqbal and Edyvean (2007).
Loofa (Luffa cylindrica) grows well in both tropical and subtropical climates, and the sponges are produced in large quantities in México, where they are currently used for bathing and dish washing. The sponges are light, cylindrical in shape and made up of interconnecting voids within an open network of matrix support materials. Due to their random lattice of small cross sections and very high porosity, they have great potential as carriers for cell immobilization (Ogbonna et al. 1994). The sponges are strong, chemically stable, and composed of interconnecting voids within an open network of fibers. Because of their random lattice of small cross sections and high porosity, the sponges are suitable for cell adhesion (Phisalaphong et al. 2007).
This study aimed to evaluate the removal efficiency of a bacterial consortium immobilized in a fibrous network of Luffa cylindrica on a mixture of methyl parathion (MP) and coumaphos (COU) pesticides.
MATERIALS AND METHODS
Pesticides
To simulate a residue of OP pesticide mixtures, two compounds that are authorized as OP pesticides for agricultural and livestock use in Mexico were selected, methyl parathion (O,O-dimethyl O-4 nitrophenyl phosphorothioate) and coumaphos
(O,O-Diethyl O-3-chloro-4-methyl-2-oxo-2H-chromen-7-yl phosphorothioate). Both pesticides were purchased from Chemservice (99 % purity) (http://web1.chemservice.com). Reactive grade ethyl acetate was used as a solvent for pesticide extraction; HPLC grade methanol from Mallinckrodt Baker Inc. (Phillipsburg, NJ, USA) was used to inject samples into the gas chromatograph. All other chemicals were of reagent grade and were obtained from J.T. Baker, Mexico City.
Bacterial consortium
The bacterial consortium that was used in this work was isolated from agricultural soils of the Morelos state in central Mexico, which has a long history of pesticide usage. Yáñez-Ocampo et al. (2009) previously reported the characteristics of this consortium.
Culture medium composition
To obtain bacterial biomass as a source of inoculum, soy tripticasein (ST), both agar and broth methods were used (Bioxon, Becton Dickinson from Mexico). For bacterial growth kinetics and pesticide removal, we used a mineral salts medium (MSM) at pH 7.00 ± 0.05; its composition per liter was 0.82 g of K2HPO4, 0.19 g of KH2PO4, 0.20 g of MgSO4.7H2O, 2.00 g of KNO3 and 0.99 g of (NH4)2SO4. Then, 2 mL of a trace solution containing 2.8 g/L H3BO3, 2.55 g/L MnSO4, H2O, 0.20 g/L CuSO4 5 H2O, 2.43 g/L CoCl2 6 H2O and 0.25 g/L ZnSO4 7 H2O was added to the MSM. In addition, glucose (0.1 %, w/v) was added to the culture medium as a co-substrate (Yáñez-Ocampo et al. 2009).
Material used to support the immobilization of cells
Loofa sponge, a natural material consisting of a fibrous network obtained from the matured dried fruit of Luffa cylindrica, was used to immobilize bacterial cells. The fruits of this Cucurbitaceae plant have a fibrous endocarp structure that is composed of cellulose (60 %), hemicellulose (30 %) and lignin (10 %) (Mazali and Alves 2005). Loofa sponge is a good support matrix for microbial cell immobilization due to its high degree of porosity, high specific pore volume, stable physical properties, biodegradability and lack of toxicity for microorganisms (Liu et al. 1999, Iqbal and Saeed 2004, Nabizadeth et al. 2008). In addition, this material is low cost, which is another advantage for developing countries. Because of these excellent characteristics, its use as an immobilization carrier is feasible.
Preparation of loofa sponge
Plant material (loofa sponge) obtained from the matured dried fruit of Luffa cylindrica was used as an immobilization carrier. Given that each loofa sponge has a different structure, we selected sponges of similar size and structure, i.e., similar in diameter, position, size of the hollows and pore size of the fibrous network. The native loofa sponge (about 70-80 cm in length) has a complex structure, differing between the core and peripheral regions. The sponge was cut into appropriate segments for experimental use.
The fruits of Luffa cylindrica were dried to obtain the fibrous network (FN). This procedure was carried out in a dryer, at 70 °C for approximately 4 days. Once the fruits were dry, the peel was removed, and they were washed with distilled water and then a solution of sodium lauryl sulfate (0.2 %) in order to remove excess biomass. Washing was carried out three times for 60 minutes at 60 °C, and the FN was dried again until it reached a constant weight. For ease of handling, the sponge was cut into pieces of approximately 1.0 x 1.5 x 0.4 cm and 0.1 cm thick. The average weight of 100 pieces of sponge was 32.24 ± 6.89 mg. The sponge pieces were sterilized at 120 °C.
In addition, we used a second preparation method for the sponge pieces. According to Ghali et al. (2009) and Bal et al. (2004), the structural characteristics of sponges improve when they are subjected to pretreatment with NaOH. Therefore, NaOH 5 % and anthraquinone were used as catalysts (Bal et al. 2004, Ghali et al. 2009). This treatment was carried out at 80 °C for 3 hours with constant stirring, and the sponge pieces were subsequently washed three times with deionized water. Finally, the pieces were dried at 70 °C and stored in desiccators until further use (Iqbal and Edyvean 2007). We performed a sterilization of the sponge pieces prior to their use as supports.
Cells immobilization
For sponge colonization, the bacterial consortium was inoculated into Erlenmeyer flasks with 50 mL of ST broth containing pieces of sponge and a mixture of pesticides (1 %). To stimulate the formation of biofilm, the flasks were incubated for 5 days at 28 °C and 100 rpm. Bacterial growth was measured by optical density to reach the stationary phase of the consortium. Then, the loofa pieces were washed with sterile 0.5 % NaCl to remove the free cells. The immobilized biomass in FN was used for further kinetics.
Electron microscopy
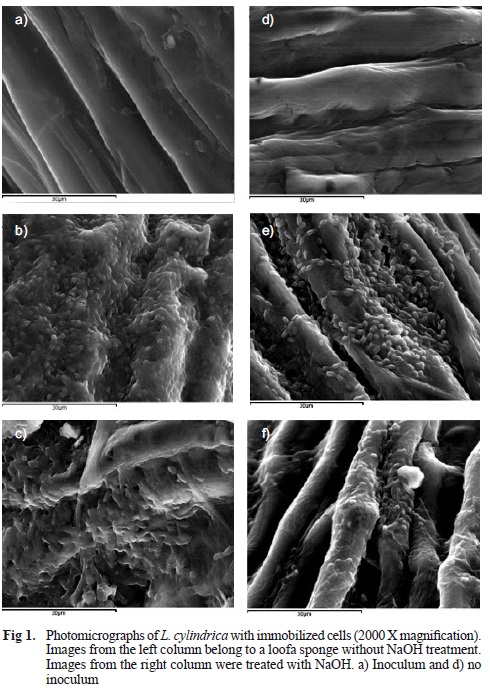
To confirm bacterial immobilization in the Luffa cylindrica sponges, the pieces were collected and treated using the Wu (2003) method (2.5 % glutaral-dehyde treatment for 48-72 h, with repeated dehydrations using 20 % acetone for a half-hour period, 50 % acetone for 1 h and 100 % acetone for 7 hours at 4 ºC). These samples were observed with a JSM 6400 JEOL Scanning Electron Microscope (SEM) (Fig. 1).
Kinetics of bacterial growth and removal of the mixture of pesticides with free cells
For removal experiments, three 125 mL sterile Erlenmeyer flasks were supplemented with both pesticides dissolved in filter-sterilized methanol. The methanol was evaporated to dryness, and then the flasks were supplemented with 35 mL of MSM and 5><105 ± 101 CFU/mL of the biomass. Previous testing indicated that low bacterial activity degraded the mixture of pesticides when supplied as a sole carbon source (data not shown); therefore, we decided to measure the growth kinetics with the addition of glucose (0.1 %), in order to enhance pesticide biodegradation. The initial pesticide concentration was 25 mg/L and 5 mg/L for MP and COU, respectively. MSM with pesticides and without the inoculum and MSM with the inoculum and without pesticides were used as controls. All flasks were incubated on a shaking platform for 72 hours at 125 rpm and 30 °C. All treatments were performed in triplicate. Pesticide removal and bacterial growth were measured immediately after inoculation and several different time intervals.
Adhesion of pesticides to the bacteria cells was also tested. The consortium was cultured with ST broth without pesticides for 24 hours; then the culture was sterilized at 121 °C for 35 min. Thereafter, pesticides mixture (MP-COU) was added and incubated for 7 days. Pesticides were then extracted and quantified as described below (Ortiz-Hernández et al. 2003). Variations between pre- and postincubation of concentration of pesticides was 8.56 % for MP and 7.34 % for MP and COU, respectively, which was taken in consideration for the pesticide removal.
Kinetics of bacterial growth and removal of the pesticide mixture with immobilized cells in loofa sponges
The removal of MP and COU by immobilized cells in loofa sponges was analyzed. Pesticides were provided as a principal carbon source as follows: three 125 mL sterile Erlenmeyer flasks were supplemented with both pesticides dissolved in filtersterilized methanol. The methanol was evaporated to dryness, and then the flasks were supplemented with 35 mL of MSM, glucose (0.01 %) and immobilized cells in loofa sponges, with and without sodium hydroxide treatment, as described above. The initial pesticide concentration was 25 mg/L and 5 mg/L for MP and COU, respectively. In addition, two groups of Erlenmeyer flasks were used as controls: one was inoculated with immobilized bacteria without pesticides and the other with pesticides and loofa fiber without immobilized bacteria.
In order to quantify the growth of the immobilized cells, the loofa sponge biofilm was first separated to obtain free cells. The MSM was removed from the loofa sponge, and a solution of 0.5 % NaCl was added, and the mixture was stirred gently; then, a phosphate buffer (0.4 M, pH 7) was added, and the mixture was stirred gently for 2 minutes. This procedure ensured that the immobilized bacteria in the loofa sponge were sent into solution and that they were removed from the loofa sponge for later quantification. Following this procedure, 1 mL was taken to measure the number of colonies by viable count (CFU/mL). Separately, a scanning electron microscope was used to verify the separation of the biofilm, and we ensured that all microorganisms previously immobilized on loofa sponge were released into the culture medium.
Analytical methods
For pesticides quantification, 1 mL aliquots were collected from each experimental flask and placed in glass tubes. One milliliter of ethyl acetate was added as an extracting agent, and the mixture was homogenized for three minutes using a vortex and allowed to stand for two minutes. The organic phase was recovered, and it was filtered through a glass funnel packed with glass fiber (Whatman GF/B) and anhydrous sodium sulfate. The extract was collected in amber vials; this procedure was repeated three times, and 1 mL of ethyl acetate was mixed with the organic phase each time. Finally, the content of the vials was evaporated. These samples were reconstituted in 1 mL of HPLC grade methanol for analysis (Yáñez-Ocampo et al. 2009). Thus, extracted pesticides were quantified on a gas Trace GC chromatograph coupled to a Polaris Q Thermo Finnigan mass spectrometer (GC-MS) using the EPA8141 method under the following conditions: equity column-5; 30 m > 0.25 mm ID; 0.25 (jm, oven at 120 °C (3 min) and at 270 °C at 5 °C /min, injector 250 °C, MSD detector, scan range 45-450 amu, 325 °C transfer line, helium flow 30 cm/s @ 120 °C, injection 1.0 μL, splitless (0.3 min), splitless liner, and double taper.
Statistical analysis
An analysis of variance (a = 0.05) for each variable (growth and degradation) was calculated. The mean value of each treatment was analyzed by multiple comparison of means, using Tukey's test. We performed a logarithmic transformation (Y = log10X) for microbial growth data (CFU/mL). Data were analyzed using the SAS version 9.0 (2002) statistical package.
RESULTS
Treatment of Luffa cylindrica
The average weight of 100 pieces of sponge was 32.24 ± 6.84 mg, and the average weight of the pieces after treatment was 24.58 ± 5.13 mg. Therefore, a weight loss of 23.75 % was observed after treatment. The morphological structure and variability of the material treated with NaOH-AQ were analyzed with a scanning electron microscope (SEM). Observations suggest that the fibers consist of a relatively long central channel (lumen) with small cells, 4 to 30 microns in diameter, which are attached through a wall of approximately 2 to 3 microns in diameter, forming a complex network within a growth axis of the fruit endocarp. Ghali et al. (2009) and Bal et al. (2004) reported that the structural features and absorbency of the sponge improve when it is subjected to a treatment with sodium hydroxide (NaOH) (Fig. 1).
Growth kinetics of cells in the suspension and immobilized
The growth behavior of the bacterial consortium in the suspension is shown in figure 2. Growth observed in the suspension was likely due to the combined action of glucose and the pesticide. To compare consortium growth with the different treatments, we carried out an analysis of variance including the different treatments and the different culture times during growth kinetics. Significant statistical differences were found only at 72 hours of growth. Tukey's test revealed that the growth was enhanced when pesticides were missing from the culture medium (P = 0.0074, a = 0.05). The controls did not show growth.

After releasing the FN microorganisms to the culture medium, growth of the immobilized cells was quantified by the CFU/mL of the culture. Figure 3 shows the results of the bacterial consortium growth. During the first 48 hours, the number of microorganisms found on the loofa sponge remained virtually unchanged, but after that time, the microorganisms began to grow, especially those immobilized in the loofa sponge without pretreatment. The ANOVA indicated that after 48 hours the growth was different (P = 0.0001, a = 0.05), which was greater on untreated fibers. This suggests that the consortium used may more easily form a biofilm on the fiber with its original components. In addition, there was an increase in growth when the pesticide was present. For FN, regardless of treatment, the immobilized microorganisms remained unchanged over time. However, treatment with NaOH maintained the viability of the biofilm for a longer period, independently of the presence or absence of the pesticide mixture.

Removal of pesticides mixture from culture medium
Figure 4 shows the percentage of the MP-COU mixture removed by the consortium in the suspension compared to the immobilized consortium. The removal percentage in the suspension cultures, in the case of MP, reached 55 %. However, we observed a loss of 22 % in the control treatment, which may be due to adhesion of pesticides to the bacteria cells, abiotic losses or by errors in pesticide extraction prior to quantification. In the case of COU in the suspension, removal was 62 % when bacteria were present, with a loss of15 % in the control treatment (without bacterial consortium). However, pesticide removal was better following treatment with the bacterial consortium than without it. After 72 h, the pesticide concentration reached 13.75 mg/L and 1.82 mg/L of MP and COU, respectively.
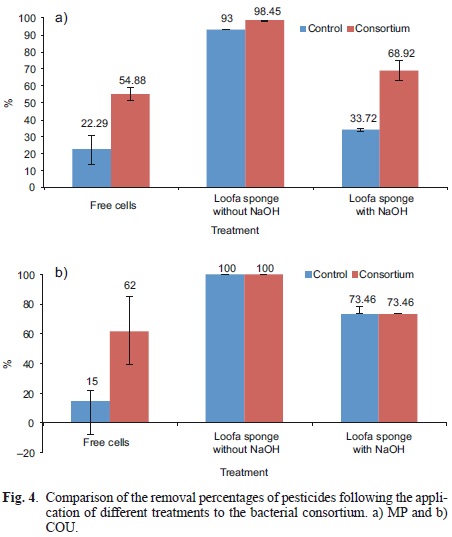
Pesticide removal with the immobilized consortium is shown in figure 4. Due to the high removal percentages, differences were observed when the FN was not treated with NaOH. However, the results show that these differences can be attributed to the FN rather than the activity of bacteria. Most of the MP and COU were not recovered from the culture medium, regardless of the absence or presence of microorganisms. These results suggest an adsorption process on the surface of the FN, which can be very rapid. MP concentration was removed at 93 and 98 % in the control treatment and the immobilized consortium, respectively, and COU was removed entirely.
When the loofa sponge was treated with NaOH, we observed significantly lower pesticide removal. This was likely because the adsorption on the treated FN was lower. The concentrations obtained at the end of treatment were 10.07 and 1.25 mg/L for MP and COU, respectively. The removal of MP following treatment with the consortium was significantly different (P = 0.0086, a = 0.05), while in the case of COU, the treatments did not differ significantly (P = 0.277).
The abiotic losses of these pesticides may occur due to adsorption by the support material, regardless of the presence or absence of the inoculum, as previously reported for the adsorption of methylene blue, crude oil and malachite green and even other xeno-biotics (Annunciado et al. 2005, Demir et al. 2008, Altinisik et al. 2010). We found that loofa sponges without pretreatment provided increased removal of both MP and COU.
Overall assessment of pesticide removal by the consortium
After measuring growth and removal kinetics, an overall comparison of all the treatments was performed. For the statistical analysis, three variables were included: microbial growth, removal of MP and removal of COU. We used an analysis of variance, and when the results were statistically significant, Tukey's test was applied.
For bacterial growth, we performed a logarithmic transformation of the data (Log10X, where X = number of CFU/mL). The ANOVA results of the last values of the growth kinetics revealed significant differences in the growth of the bacterial consortium in response to the different treatments (P = 0.0057, a = 0.05). We observed that cells in the suspension showed the best growth, and there were no significant differences in the presence or absence of the pesticide (Table I). However, it is important to note that the cultures with immobilized cells showed prolonged viability, which could be advantageous for practical applications of remediation.
For the statistical analysis of MP and COU removal (%), arcsine transformation of data was performed (arcsin V X, where X = percentage of pesticide removal). The ANOVA results revealed significant differences between the treatments (P = 0.0002, a = 0.05) (Table I).
The results of this analysis confirm the data mentioned above, and we concluded the following:
The growth of bacterial consortium was higher in suspension and only showed statistical differences after 72 hours of culture, being higher when pesticides were absent.
In accordance to Tukey's test, after 48 hours the growth of immobilized consortia was significantly greater on fiber loofa sponge without pretreatment. The treatment with NaOH maintained the viability of the biofilm for a longer period, independently of the presence or absence of the pesticide mixture.
MP removal is more efficient when using the FN whether in presence or absence of microorganisms, which suggests a strong and fast adsorption on Loffa cylindrica fibers.
Similar behavior was observed for COU, although differences were found when the consortium was present, indicating a lower adsorption as a result of treatment and higher microorganism activity.
DISCUSSION
The enzymatic degradation of organophosphorus pesticides as a treatment strategy for their final disposal has gained attention over the last 20 years, because it has advantages over other treatments; namely, it does not generate additional waste, and it is relatively cheaper than physical and chemicals treatments. However, it remains important to develop strategies for its implementation, such as identifying low cost solutions, especially in developing countries. Here, we attempted to optimize a system for degrading a mixture of pesticides that are generated as a waste at the end of their use. We developed a simple procedure for the immobilization of bacteria by adsorption on the surface of the fibrous network of Luffa cylindrica, which is inexpensive and readily available in central Mexico.
Microorganisms can be immobilized in different matrices, whether the cells are entrapped in the support material or an adsorption phenomenon occurs. Immobilized microbial cells have mainly been used to produce useful chemicals and for xenobiotic degradation. The material used for immobilization must be inert, non-toxic to cells, and preferably economical and practical. The main advantages of using biomass immobilization techniques are; the high cell density achieved, the reuse of cells is enabled, high operational stability and an increased rate of degradation of the substance being processed, also, the washing of biomass cells is avoided, which is advantageous in continuous processes (Kadakol et al. 2011).
Yáñez-Ocampo et al. (2011) and Yáñez-Ocampo et al. (2009) studied the use of a consortium of bacteria immobilized in volcanic rock as an alternative for the degradation of organophosphorus pesticides, and they found that this method had a high rate of MP removal (66 and 70 %). In this study, we removed 100 % of the MP and COU added to the culture medium. Various studies have demonstrated that biofilms formed on substrates, used for immobilization of microorganisms, are less sensitive to environmental changes, such as temperature and pH, compared with bacteria cultured in suspension and in presence of metabolic products and toxic substances (Ohandja and Stuckey 2006). Importantly, we found that the highest removal was obtained by the activity of the fibers, rather than the activity of microorganisms. This is likely because the adsorption is very fast and strong.
Experiments testing the removal of MP with immobilized cells indicated that the sponge fibers contribute to the removal of the pesticide from culture media. Thus, abiotic losses of this pesticide can be attributed to adsorption into the support material and adhesion to the bacterial cell. These results suggest that MP and COU are removed from the media mainly by adsorption and not by degradation, since a removal in the control (culture media with fiber sponge without bacteria) could be observed. Furthermore, pesticide degradation experiments revealed that the removal of COU was more complete than MP. This could be due to the characteristics of the pesticide and/or the different concentrations used in the assay.
Pattanasupong et al. (2004) reported the use of fiber Luffa sp. as a support material for immobilization of a bacterial consortium to simultaneously degrade two pesticides, the fungicide carbendazim and the herbicide 2,4-Dichlorophenoxyacetic acid (2,4-D). Similar results were obtained after treatment with biofilm immobilized on Luffa sp., without pretreatment, which presented the highest values of MP and COU removal.
The present study suggests that it is possible to increase the efficiency of MP and COU degradation using cells immobilized on a loofa sponge compared with free cells in a suspension. Thus, immobilized microbial technology is a very versatile approach that could be used for the degradation of toxic pollutants from industrial effluents. Moreover, the loo-fa-immobilized cells systems have been efficiently used for the treatment of wastewaters containing toxic metals, dyes and chlorinated compounds, and the technology has been used to develop biofilms for the remediation of domestic and industrial waste-waters rich in inorganic and organic matter (Saeed and Iqbal 2013).
Further studies are needed to thoroughly elucidate the mechanisms by which pesticides are adsorbed on FN, whether the adsorbed pesticides are likely to be degraded or whether they stay in the FN. Only until then it will be possible to recommend potential applications for the degradation of pesticides in specific locations.
CONCLUSIONS
In order to degrade pesticides, it is important to search for materials with favorable characteristics for the immobilization of cells, including aspects such as physical structure, ease of sterilization, the possibility of repeated use, and affordable.
In this study, the microbial consortium immobilized on loofa sponges exhibited a high ability to remove both MP and COU. The use of an immobilized consortium is thought to be more advantageous than the use of free-living consortium when applied for the bioremediation of water contaminated by these pesticides. Loofa sponges are considered one of the most suitable supports for this application, since they are renewable, biodegradable, and affordable (and thus viable for use in developing countries), in addition, the microbial immobilization method is simple. Although the degradative ability of this consortium was not distinguished by the adsorption that occurred in the FN and to the bacteria cells, the system developed could be applied to degrade pesticides and develop further studies to ensure its subsequent desorption and degradation. Information about the effect of various environmental factors on the degradation of MP and COU would facilitate its application.
ACKNOWLEDGEMENTS
We would like to thank Dr. Andrés Aguilar Negrete from the Instituto de Ciencias Físicas (UNAM) for his collaboration with SEM micrographs. We also thank Dr. Rosa Cerros Tlatilpa from Laboratorio de Sistemática, Facultad de Ciencias Biológicas, UAEM, for taxonomic identification of Luffa cylindrica.
REFERENCES
Akhtar N., Iqbal J. and Iqbal M. (2004). Removal and recovery of nickel (II) from aqueous solution by Loofa sponge immobilized biomass of Chlorella sorokiniana characterization studies. J. Hazard. Mater. 108, 85-94, [ Links ]
Alluri H.K., Ronda S.R., Settalluri V.S., Singh J., Suryanarayana B. and Venkateshwar P. (2007). Biosorption: An eco-friendly alternative for heavy metal removal. Afr. J. Biotechnol. 6, 2924-2931. [ Links ]
Altinisik A., Gur E. and Seki Y. (2010). A natural sorbent, Luffa cylindrica for removal of a model basic dye. J. Hazard. Mater. 179, 658-664. [ Links ]
Annunciado T.R., Sydenstricker T.H.D. and Amico S.C. (2005). Experimental investigation of various vegetable fibers as sorbent materials for oil spills. Mar. Pollut. Bull. 50, 1340-1346. [ Links ]
Arroyo M. (1998). Inmovilización de enzimas. Fundamentos, métodos y aplicaciones. Ars. Pharmaceutica. 39, 23-39. [ Links ]
Athia A.M. (2006). Risk assessment of occupational exposure to pesticides. In, Comparative Risk Assement and Envirobmental Decision Making (I. Linkov and A.B. Ramadan, Eds). Kluwer Academic Publishers, Dordrecht, pp.349-362. [ Links ]
Bal K.E. and Bal Y., Lallam A. (2004). Gross morphology and absorption capacity of cell-fibers from the fibrous vascular system of Loofa (Luffa cylindrica). Text. Res. J. 74, 241-247. [ Links ]
Barragán-Huerta B., Costa-Pérez C., Peralta-Cruz J., Barrera-Cortés J., Esparza-García F. and Rodríguez-Vázquez R. (2007). Biodegradation of organochlorine pesticides by bacteria grown in microniches of the porous structure of green bean coffee. Int. Biodeter. Biodegr. 59, 239-244. [ Links ]
Cervantes M.R. (2010). Plaguicidas en Bolivia: sus implicaciones en la salud. Agricultura y medio ambiente. Revista Virtual REDESMA. 4, 1-12. [on line]. http://revistavirtual.redesma.org/vol9/pdf/tapa.pdf. 06/11/2012 [ Links ]
Chandran P. and Das N. (2011). Degradation of diesel oil by immobilized Candida tropicalis and biofilm formed on gravels. Biodegradation. 22, 1181-1189. [ Links ]
Cheng K., Wu J., Yang W., Hwang S. (2003). Evaluation of effective diffusion coefficient and intrinsic kinetic parameters on azo dye biodegradation using PVA-im-mobilized cell beads. Biotechnol. Bioeng. 83, 821-832. [ Links ]
Demir H., Top A., Balkose D. and Ülkü S. (2008). Dye adsorption behavior of Luffa cylindrica fibers. J. Hazard. Mater. 153, 389-394. [ Links ]
Garzón-Jiménez R.C. (2009). Cinética de degradación de colorantes textiles de diferentes clases químicas por hongos y bacterias inmovilizados sobre fibra de Agave tequilana Webber var. Azul. Tesis de licenciatura. Pontificia Universidad Janveriana. Facultad de Ciencias. Bogotá, Colombia, 124 pp. [ Links ]
Ghali L., Msahli S., Zidi M. and Sakli F. (2009). Effect of pre-treatment of Luffa fibres on the structural properties. Mater. Lett. 63, 61-63. [ Links ]
Iqbal M. and Edyvean. R.G.J. (2007). Ability of Loofa sponge-immobilized fungal biomass to remove lead ions from aqueous solution. Pak. J. Bot. 39, 231-238. [ Links ]
Iqbal M. and Saeed A. (2006). Entrapment of fungal hyphae in structural fibrous network of papaya wood to produce a unique biosorbent for the removal of heavy metals. Enzyme Microbiol. Tech. 39, 996-1001. [ Links ]
Iqbal M. and Saeed A. (2004). Novel method for cell immobilization and its application for production of organic acid. Lett. Appl. Microbiol. 40, 178-182. [ Links ]
Yum J.K. and Walter J.P. (1998). Biodegradation kinetics of chlorophenols in immobilized-cell reactors using a white-rot fungus on wood chips. Water Environ. Res. 70, 205-213. [ Links ]
Kadakol J.C., Kamanavalli C. M. and Shouche Y. (2011). Biodegradation of carbofuran phenol by free and immobilized cells of Klebsiella pneumoniae ATCC13883T. World J. Microbiol. Biotechnol. 27, 25-29. [ Links ]
Kennedy J.F. and Cabral J.M.S. (1983). Immobilized enzymes. In Solid Phase. Biochemistry (W.I-I. Scouten. Ed), Wiley, New York. Pp. 245-293. [ Links ]
Liu Y.K, Seki M. and Furusaki S. (1999). Plant cell immobilization in loofa sponge using two-way bubble circular system. J. Chem. Eng. Jpn. 32, 8-14. [ Links ]
Lusta K.A., Starostina N.G. and Fikhte B.A. (1990). Immobilization of microorganisms: cytophysiological aspects. In: Proceedings of an International Symposium: Physiology of Immobilized Cells (J.A.M. De Bont, J. Visser, B. Mattiasson and J. Tramper Eds.). Elsevier. Amsterdam, The Netherlands. pp. 557-562. [ Links ]
Manohar S., Kim C.K. and Karegoudar T.B. (2001). Enhanced degradation of naphthalene by immobilization of Pseudomonas sp. Strain NGK1 in polyurethane foam. Appl. Microbiol. Biotechnol. 55, 311-316. [ Links ]
May-Esquivel F., Ríos-González L.J., Garza-García Y. and Rodríguez-Martínez J. (2008). Performance of a packed reactor with Opuntia imbricata for municipal wastewater treatment. Environ. Res. J. 2, 238-245. [ Links ]
Mazali I.O. and Alves O.L. (2005). Morphosynthesis: high fidelity inorganic replica of the fibrous network of loofa sponge (Luffa cylindrica). An. Acad. Bras. Cien. 77, 25-31. [ Links ]
Mazmanci M. and Ünyayar A. (2005). Decolorization of reactive black 5 by Funalia trogii immobilized on Loofa cylindrical sponge. Process. Biochem. 40, 337-342. [ Links ]
Nabizadeth R., Naddafi K., Mesdaghinia A. and Nafez A.H. (2008). Feasibility study of organic matter and ammonium removal using loofa sponge as a supporting medium in an aerated submerged fixed-film reactor (ASFFR). Electron J. Biotechnol. 11, 1-9. [ Links ]
Ogbonna J.C., Liu Y.C., Liu Y.K. and Tanaka H. (1994). Loofa (Luffa cylindrica) sponge as a carrier for microbial cell immobilization. J. Ferment. Bioeng. 78, 437-442. [ Links ]
Ohandja D.G. and Stuckey D.C. (2006). Development of a membrane-aerated biofilm reactor to completely mineralize perchloroethylene in wastewaters. J. Chem. Technol. Biot. 81, 1736-1744. [ Links ]
Ortiz-Hernández M.L., Quintero-Ramírez R., Nava-Ocampo A. and Bello-Ramírez A.M. (2003). Study of the mechanism of Flavobacterium sp. for hydrolyzing organophosphate pesticides. Fundam. Clin. Pharmacol. 17, 717-723. [ Links ]
Ortiz-Hernández M.L. and Sánchez-Salinas E. (2010). Biodegradation of the organophosphate pesticide tetrachlorvinphos by bacteria isolated from agricultural soils in México. Rev. Int. Contam. Ambient. 26, 27-38. [ Links ]
Ortiz-Hernández M.L., Sánchez-Salinas E., Olvera-Velona A. and Folch-Mallol J.L. (2011). Pesticides in the environment: Impacts and its biodegradation as a strategy for residues treatment. In: Pesticides-Formulations, Effects, Fate (M. Stoytcheva Ed.). InTech. Rijeka, Croatia. 551-574. [ Links ]
Pattanasupong A., Nagase H., Sugimoto E., Hori Y., Hirata K., Tani K., Nasu M. and Miyamoto K. (2004). Degradation of carbendazim and 2,4-dichlorophenoxyacetic acid by immobilized consortium on loofa sponge. J. Biosci. Bioeng. 98, 28-33. [ Links ]
Phisalaphong M., Budiraharjo R., Bangrak P., Mongkolkajit J. and Limtong S. (2007). Alginate-Loofa as carrier matrix for ethanol production. J. Biosci. Bioeng. 104, 214-217. [ Links ]
Recena M.C., Caldas E., Pires D. and Rose P.E. (2006). Pesticides exposure in Culturama, Brazil. Knowledge, attitudes, and practices. Environ. Res. 102, 230-236. [ Links ]
Saeed A. and Iqbal M. (2013). Loofa (Luffa cylindrica) sponge: Review of development of the biomatrix as a tool for biotechnological applications. Biotechnol. Prog. 29, 573-600. [ Links ]
Sánchez Salinas E. and Ortiz Hernández M.L. (2011). Riesgos y estrategias en el uso de plaguicidas. Inventio 14, 21-27. [ Links ]
Singh B.K. (2009). Organophosphorus-degrading bacteria: ecology and industrial applications. Nat. Rev. Microbiol. 7, 156-164. [ Links ]
Singh B.K. and Walker A. (2006). Microbial degradation of organophosphorus compounds. FEMS Microbiol. Rev. 30, 428-471. [ Links ]
Soares W., Almeida R.M. and Moro S. (2003). Rural work and risk factors associated with pesticide use in Minas Gerais, Brazil. Cad. Saude Publica. 19, 1117-1127. [ Links ]
Theriot C.M. and Grunden A.M. (2011). Hydrolysis of organophosphorus compounds by microbial enzymes. Appl. Microbiol. Biotechnol. 89, 35-43. [ Links ]
USEPA (2011). Pesticide database. [on line]. http://www.epa.gov/opp00001/pestsales. [ Links ]
Vilanova E. and Sorgob M.A. (1999). The role of phos-photriesterases in the detoxication of organophosphorus compounds. Crit. Rev. Toxicol. 29, 21-57. [ Links ]
Wu J. Y., Chen K.C., Chen C.T. and Hwang S.C. (2003). Hydrodinamics characteristics of immobilized cell beads in a liquid-solid fluidized-bed bioreactor. Biotechnol. Bioeng. 83, 583-594. [ Links ]
Yañez-Ocampo G., Sánchez-Salinas E., Jiménez-Tobon G. A., Penninckx M. and Ortiz-Hernández M.L. (2009). Removal of two organophosphate pesticides by a bacterial consortium immobilized in alginate or tezontle. J. Haz. Mat. 168, 1554-1561. [ Links ]
Yáñez-Ocampo G., Sánchez-Salinas E. and Ortiz-Hernández M.L. (2011). Removal of methyl parathion and tetrachlorvinphos by a bacterial consortium immobilized on tezontle-packed up-flow reactor. Biodegradation 22, 1203-1213. [ Links ]














