Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista internacional de contaminación ambiental
versión impresa ISSN 0188-4999
Rev. Int. Contam. Ambient vol.29 no.1 Ciudad de México feb. 2013
Artículos
Oxidative stress responses and histological hepatic alterations in barbel, Barbus bocagei, from Vizela river, Portugal
Respuestas al estrés oxidativo y alteraciones hepáticas en el barbo común, Barbus bocagei, del río Vizela, Portugal
Francisco P. PEIXOTO1,2, João CARROLA1,2, Ana Maria COIMBRA1,2*, Conceição FERNANDES3, Paulo TEIXEIRA1,2, Luís COELHO1,4, Ivo CONCEIÇÃO1,4, Maria Manuel OLIVEIRA1,4 and António FONTAÍNHAS-FERNANDES1,2
1 School of Environment and Life Sciences (Escola de Ciências da Vida e do Ambiente-ECVA), University of Trás-os-Montes e Alto Douro (UTAD), Apartado 1013, 5001-801 Vila Real, Portugal.
2 Center for the Research and Technology of Agro-Environmental and Biological Sciences (Centro de Investigação e de Tecnologias Agro-Ambientais e Biológicas-CITAB), UTAD. *Corresponding author: acoimbra@utad.pt
3 Agrarian Superior School (Escola Superior Agrária, Instituto Politécnico de Bragança, Centro de Investigação de Montanha CIMO), Campus de Santa Apolónia, Apartado 1038, 5301-854 Bragança, Portugal.
4 Chemistry Research Center of Vila Real (Centro de Química de Vila Real-CQVR), UTAD.
Recibido agosto 2011,
Aceptado octubre 2012
ABSTRACT
Barbel (Barbus bocagei) a common species in Portuguese rivers was studied to assess the impact of water contamination on hepatic oxidative stress response, lipid peroxidation and histology. The Vizela River is a tributary of the Ave River, located in one of the most industrialized areas of Portugal. The oxidative stress biomarkers analyzed included superoxide dismutase, catalase, glutathione S-transferase, glutathione reductase, glucose 6-phosphate dehydrogenase and xanthine oxidase activities. Levels of reduced glutathione and lipid peroxidation were also evaluated. Except xanthine oxidase activity, that did not show any alteration, all the other enzymatic activities were increased in the liver of barbel captured in the Vizela River when compared with reference barbel. While, no differences were observed for glutathione reductase content, lipid peroxidation was higher in barbel from the Vizela River. Liver histological alterations were determined and their severity scored. Though lymphocyte foci were only observed in Vizela River barbel, macrophage aggregates were also present in reference barbel, although the severity score was higher in Vizela fish. The results of this study show that barbel liver oxidative stress responses, lipid peroxidation and histology are sensitive to the contaminants present in Vizela River water and are valuable biomarkers for monitoring purposes.
Key words: Oxidative stress enzymes, lipid peroxidation, fish, freshwater, liver histopathology.
RESUMEN
Barbos (Barbus bocagei), una especie común en los ríos portugueses, se utilizó para evaluar el impacto de la contaminación del agua en la respuesta hepática al estrés oxidativo, en la peroxidación lipídica y en la histología del órgano. El río Vizela es un afluente del río Ave, situado en una de las regiones más industrializadas de Portugal. Los biomarcadores de estrés oxidativo analizados fueron la actividad de las enzimas superóxido dismutasa, catalasa, glutatión S-transferasa, glutation reductasa, glucosa 6 fosfato deshidrogenasa y de la xantina oxidasa. Los niveles de glutatión reducido y de la peroxidación lipídica también fueron evaluados. Excepto la xantina oxidasa, que no mostró ninguna alteración, todas las otras actividades enzimáticas han sufrido incrementos en el hígado de los barbos capturados en el río Vizela, cuando se comparan con los barbos de referencia. No se observaron diferencias para el contenido de glutatión reductasa, pero la peroxidación lipídica fue mayor en los barbos del río Vizela. Las alteraciones en la histología hepática fueron identificadas y clasificadas de acuerdo con su gravedad. Mientras que los linfocitos de focos se observaron sólo en barbos del Río Vizela, los agregados de macrófagos también estuvieron presentes en barbos locales de referencia, aunque la gravedad de las alteraciones fue mayor en los peces del río Vizela. Los resultados de este estudio muestran que las respuestas de estrés oxidativo, la peroxidación lipídica y la histología hepática son sensibles a los contaminantes presentes en el agua del Río Vizela, demonstrando ser biomarcadores valiosos para propósitos de monitoreo.
Palabras clave: Enzimas de estrés oxidativo, peroxidación lipídica, peces, agua dulce, histopatología hepática.
INTRODUCTION
The hydrographical basin of the Ave River is located in Minho region (northwest of Portugal) and comprises an area of about 1390 km2, being limited up north by the Cavado River, east by the Douro River and south by the basin of Leça River. The Ave River has two main tributaries, the Este River on the right edge and the Vizela River on left edge. About 80 % of this basin is overpopulated. Textile industry is one of the most important economic activities in this region, with around 200 industrial units, that include both manufacturing and tanning units (Alves et al. 2009). For many decades both urban and industrial effluents were discharged directly into the river basin and the presence of heavy metals in sediments have already been reported. Soares et al. (1999) observed high values of Cr (0.36-0.67 g/kg volatile matter dry weight (DW)), Cu (0.46-1.03 g/kg volatile matter DW) and Zn (0.75-3.70 g/kg volatile matter DW). Ten years later, Alves et al. (2009) found values of Cr (2.85-4.45), Cu (0.62-0.69) and Zn (0.53-1.87). Recently, due to the European legal requirements and the increasing public awareness, Portugal has started to control the quality of industrial discharges. As result, norms, like ISO 14000 family, and legal contamination standards, such as the EU Water Framework Directive, are being implemented in Portugal. Despite this, several industrial effluents are still being discharged directly into streams without any treatment, and several heavy metals are present and can still be found in sediments (Alves et al. 2009).
Heavy metals are non-degradable and may bio-accumulate in organisms, possibly reaching toxic levels. This can constitute a threat to public and/or aquatic organisms' health due to chronic exposure to high concentrations (Fernandes et al. 2007, Fernandes et al. 2008a, Vieira et al. 2011).
Due to the high degree of pollution of the Ave River basin, the biota in this aquatic ecosystem has been progressively degraded. The Iberian barbel (Barbus bocagei) is one of the few fish species still widely distributed in this basin, being benthopelagic and with a versatile diet (Magalhães 1992), and thus susceptible to both, sediment and water column, contaminants.
The evaluation of biochemical and histological changes in fish liver has become an important tool to monitor the environmental exposure of fish to contaminants (Hinton & Lauren 1990, Deviller et al. 2005, Fernandes et al. 2008b, Carrola et al. 2009). Fish liver plays an important role in vital functions and is the major organ for accumulation, biotransformation, and excretion of contaminants in fish (Triebskorn et al. 1994, Triebskorn et al. 1997). Histopathology of fish liver is a monitoring tool, which allows the assessment of the environmental stressors effects, in fish. Indeed, it is one of the most reliable indicators of the health impairment induced by anthropogenic stressors in aquatic organisms (Fernandes et al. 2008b, Leonardi et al. 2009).
Heavy metal exposure, in aquatic ecosystems, is described as an enhancer of intracellular formation of reactive oxygen species (ROS), which can give rise to oxidative damage, as observed in flathead grey mullet (Mugil cephalus), flounder (Platichthys flesus) and Nile tilapia (Oreochromis niloticus) (Ferreira et al. 2005, Figueiredo-Fernandes et al. 2006). Thus, oxidative stress biomarkers can be employed in environmental monitoring programs (McCarthy and Shugart 1990, Fernandes et al. 2008c). Lipid peroxidation and protein oxidation are manifestations of oxidative damage induced by heavy metals (Livingstone et al. 1993, Ercal et al. 2001), and have a predictive importance as biomarkers of pollution (Collen et al. 2003, Bláha et al. 2004, Almroth et al. 2005). In addition, both antioxidant enzymes and non-enzymatic antioxidants have been successfully employed in aquatic monitoring studies (Figueiredo-Fernandes et al. 2006, Peixoto et al. 2006). ROS can be detoxified by an enzymatic defence system, comprising superoxide dismutase (SOD), catalase (CAT), and selenium-dependent glutathione peroxidase; or by a non-enzymatic system, with the scavenging action of reduced glutathione. Moreover, organic peroxides can be detoxified by the activity of glutathione S-transferase (GST) (Halliwell and Gutteridge 1999).
The aim of this study was to assess biochemical and histological biomarkers of exposure in the liver of the Iberian barbel (Barbus bocagei) captured in Vizela River. The values obtained will serve as base for future surveys on barbel populations in the Vizela River and evaluation of the impact of management policies. Lipid peroxidation in liver and hepatic activities of superoxide dismutase, catalase, glutathione S-transferase, glutathione reductase, glucose 6-phosphate dehydrogenase, xantine oxidase, and the amount of reduced glutathione were measured and compared with the respective activities in reference barbel. The biochemical evaluation was complemented with hepatic histological analyses and possible accumulation of metals in this organ was assessed.
MATERIALS AND METHODS
Fish sampling
Twenty-four barbel (Barbus bocagei) were captured in the Vizela River (41°22'16.85"N; 8°18'17.86'W) near the city of Caldas de Vizela. Fish were captured in the autumn of 2009 using pulsed DC backpack electrofishing equipment with a DC-500 V generator. Reference barbel were captured in Corgo River (41°17'14.62"N; 7°44'57.55'W), a low contaminated stream. Barbels, from both locations, were sacrificed at the same time, after being anaesthetized with 3-aminobenzoic acid ethyl ester.
Liver sections were frozen in liquid nitrogen and stored at -80 °C for biochemical analysis, or fixed in 10 % buffered formalin during 24-48 h for histology.
In addition, a pool of13 Vizela barbel was randomly selected and the livers sub-sampled and stored in eppendorfs at -20 °C, for metals analysis.
Biochemical analysis
All chemicals used in the enzymatic activity were of analytical purity from Sigma Chemical Co, except when indicated.
One gram of liver tissue was homogenized in 5 ml of ice-cold sodium phosphate buffer (100 mM, pH 7.4) and post-mitochondrial supernatant (PMS) was obtained after centrifugation at 10 500xg for 20 min at 4 °C.
Superoxide dismutase (SOD) activity was assayed according to Paya et al. (1992) with minor modifications (Peixoto et al. 2006). Nitrotetrazolium blue chloride (NBT) was used as detection molecule instead of cytochrome c. Assays were conducted in the presence of potassium phosphate buffer (100 mM, pH 7.0), hypoxanthine (10 mM), and NBT (10 mM). The reaction was initiated by the addition of xanthine oxidase (0.023 U/mol) to enzymatic extract at 25 °C. Activity was reported by its ability to inhibit 50 % reduction of NBT and the result is expressed as U/min/mg/protein.
Catalase (CAT) activity was assayed by the method Claiborne (1985). The reaction mixture consisted in potassium phosphate buffer 50 mM, pH 7.4, hydrogen peroxide19 mM and PMS 10 %. The reaction was carried out at 25 °C and the change in absorbance was recorded at 240 nm. CAT activity was calculated in terms of (μmol H2O2 consumed/ min/mg/protein. Glutathione reductase (GR) activity was assayed by the method of Carlberg and Mannervik (1975) as modified by Mohandas et al. (1984). The reaction system consisted of potassium phosphate buffer (100 M, pH 7.4), EDTA 0.5 mM, oxidized glutathione (GSSG) 1 mM, NADPH 0.1 mM and PMS 10 %. Enzyme activity was quantified at 25 °C by measuring the disappearance of NADPH at 340 nm and expressed as nmol NADPH oxidized/min/ mg/protein.
The activity of glucose 6-phosphate dehydrogenase (G6PD) was assayed by the method of Zaheer et al. (1965). The reaction mixture consisted of Tris-HCl buffer 50 mM, pH 7.6, nicotinamide adenine dinucleotide phosphate (NADP) 0.1 mM, glucose 6-phosphate 0.8 mM, MgCl2 8 mM (Merck, Mumbai), PMS 10 % and 2.1 mL distilled water. The change in absorbance at 25 °C was recorded at 340 nm and the enzyme activity was expressed as nmol NADP reduced/min/mg/protein.
Glutathione S-transferase (GST) activity was measured according to Habig et al. (1974) with minor modification. Reaction mixture contained 2 mL of potassium phosphate buffer 100 mM, triton X-100 10 %, 1-chloro-2, 4-dinitrobenzene (CDNB) 100 mM, and GSH 100 mM. Reaction was started at 25 °C by adding the sample and the absorbance was monitored at 340 nm. The GST activity was expressed in nmol CDNB/min/mg/protein (Uguz et al. 2003).
Xanthine oxidase (XOD) activity was assayed as described by Stirpe and Dellacor (1969). The reaction mixture containing 0.2 mL PMS diluted to 1 mL with phosphate buffer and was incubated for 5 min at 25 °C. The reaction was started by adding xanthine, kept at 25 °C for 20 min and stopped by the addition of ice-cold perchloric acid (10 %). After 10 min, 2.5 mL distilled water was added to it and the mixture was centrifuged at 4000 rpm for 10 min. The optical density of the supernatant was read at 290 nm. The activity of XOD was expressed as (mol uric acid formed/mg/protein.
Reduced glutathione (GSH) was determined by using the method of Jollow et al. (1974). PMS 10 % was precipitated with sulfosalicylic acid 4 % in 1:1 ratio. The samples were kept at 4 °C for 1 h and centrifuged at 1500 rpm for 15 min at 4 °C. The supernatant was used for GSH estimation. The assay mixture contained supernatant, phosphate buffer (100 mM, pH 7.4) and 5-5'-dithiobis-2-nitrobenzoic acid, DTNB (stocks 100 mM in 100 mM sodium phosphate buffer, pH 7.4) in total volume of 3 mL. GSH activity was determined spectrophotometrically by measuring reaction product at 412 nm and expressed as nmol of GSH consumed/mg/protein.
Peroxidative damage of lipids was determined according to the method of Utley et al. (1967) with some modifications proposed by Fatima et al. (2000). The liver, 1 g, was homogenized in 5 mL of chilled 100 mM potassium chloride solution. The assay mixture contained 0.67 % thiobarbituric acid (TBA), 10 % chilled trichloroacetic acid (TCA) and liver homogenate (10 %). The rate of LPO is expressed as nmol of thiobarbituric acid reactive substance (TBARS) formed per gram of tissue using a molar extinction coefficient of 1.56=105 M/cm and wavelength of 532 nm.
The protein content was determined according to Lowry et al. (1951), with bovine serum albumin as standard.
Liver histology
A liver sample/slice, 3 to 4 mm thick, from each barbel was fixed in 10 % buffered formalin during 24-48 h, at room temperature and processed for paraffin embedding. Then, 5 (im sections were cut in a rotary microtome (Leica RM 2135), stained with haematoxylin-eosin (H&E) and mounted for light microscopy (LM) scrutiny. Sections sampled from diverse blocks were studied. Microphotographies were taken with a Nikon 4500 Coolpix digital camera coupled to a Nikon Eclipse E 600 microscope. For each fish 20 fields were evaluated using a 200 x magnification.
The hepatic lesions/alterations were identified according to general diagnostic categories (Kohler et al. 2002, Lang et al. 2006).
The hepatic lesions (structures/cells that do not appear in healthy tissues) and alterations (changes in the number of structures/cells usually present in the tissue) were scored according to a scale of 8 grades, based on Matos et al. (2007), as described by Pinto et al. (2010). Briefly, each lesion/alteration score was assessed as a function of lesion/alteration frequency and its severity (extension of the lesion/alteration on each field), from zero to seven. Therefore, score zero is a tissue without any lesion/ alteration, score one represents a lesion/alteration with very low frequency and a low severity, while score seven represents extremely high frequency and severity.
Liver metal content
Liver metal content was assayed using the methodology described in Fernandes et al. (2008c). Briefly, for Al, Cr, Cu and Zn quantification, the tissue was lyophilized and digested overnight with nitric acid (supra pure grade) at 60 °C. Samples were analyzed in a graphite furnace atomic absorption spectrophotometer (UNICAMP 939 AA - GF90).
Blank determinations were done using the same procedure with Milli-Q50 water. Results were expressed in mg/kg dry weight (DW). The analytical accuracy and precision were checked using certified reference materials, i.e DOLT-3 and DORM-2 (National Research Council of Canada). The analyses of the reference materials were always within the certified intervals.
Statistical analysis
All statistical analyses were performed with SPSS statistical program. Quantitative differences for histological lesion/alteration score and enzymatic activities, between barbel from Vizela River and reference barbel, were tested by non-parametric Mann-Whitney U-Test. A 5 % significance level was employed throughout.
RESULTS
Enzyme profile and stress indicators
Hepatic enzymes activities and lipid peroxidation in fish liver are presented in Table I. Generally the higher activities were observed in fish collected from Vizela River, when compared to reference fish liver, with a 23 % increased of SOD, 31 % of CAT, 150 % of glutathione reductase activity and 47 % of glutathione S-transferase. Glucose 6-phosphate dehydrogenase showed an increase of 8 %. Xantine oxidase activity was similar in both groups of barbel.
In the same way, GSH content did not differed between the two groups (Table I). As for lipid peroxidation fish captured in Vizela River exhibited an increase of 41 % relative to the reference barbel (Table I).
Histology
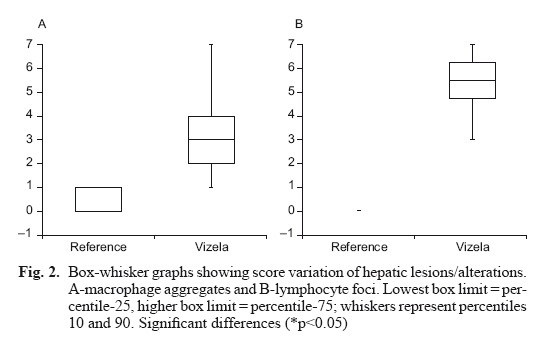
Two hepatic lesions were observed and identified (Fig. 1): macrophage aggregates, which were present on both groups (79 % of Vizela River barbel and 40 % of reference barbel), and lymphocyte foci that was only observed in Vizela River barbel (100 %). Regarding the lesions scores (Fig. 2), the macrophage aggregates and the lymphocytes foci showed significant differences between the reference and Vizela River barbels (p<0.05). In reference barbel, the median score for both macrophage aggregates and lymphocyte foci, was zero, while Vizela River barbel presented a score of three for macrophage aggregates and of five for lymphocyte foci (p<0.05).
Tissue metal content
Metals content in liver of barbel captured in the Vizela River ranged between 9-15 mg/kg DW for Al and 1.8-3.5 mg/kg DW for Cu and it was less than the detection limits of 0.026 mg/kg DW for Zn and 0.006 mg/kg DW for Cr. The liver metals content of the reference barbel were all below the detection limits (0.011 mg/kg DW for Al and 0.003 mg/kg DW for Cu).
DISCUSSION
In Portugal, there are frequent surveys on freshwater fish populations dynamic, including the Iberian barbel (Santos et al. 2004), and on ecosystems health and integrity (eg: Pinto et al. 2010, Varandas and Cortes 2010, Carvalho et al. 2011). However, the studies of exposure biomarkers, in freshwater species, are inexistent being almost exclusive of estuarine species (eg: Ferreira et al. 2005, Cunha et al. 2007, Gravato et al. 2010). The present study aimed to evaluate hepatic biochemical and histological biomarkers in barbel captured in a polluted river, as a base for future evaluation of the impact of management policies.
Many classes of environmental pollutants are known to increase the intracellular formation of ROS and several authors have already reported physiological alterations induced by ROS, in fish (Baker et al. 1997, 1998, Valavanidis et al. 2006).
Since induction of antioxidants represents a cellular defense mechanism to counteract toxicity of ROS, they have been extensively used in several field studies to assess the extent of pollution in rivers, lakes and coastal waters (Ferreira et al. 2005, Fernandes et al. 2008c).
SODs are a group of metalloenzymes that play a crucial antioxidant role and constitute the primary defense mechanism against the toxic effect of oxygen, in aerobic organisms. SOD catalyzes the dismutation of the superoxide anion radical to water and hydrogen peroxide, which afterwards is detoxified by CAT. Therefore, a simultaneous activity induction of SOD and CAT is usually an expected response. However, this relation is not always observed (Peixoto et al. 2006) and it is known to be species dependent (Ferreira et al. 2005). In the present study, the liver of barbel captured in the Vizela River presented high activity values of both, SOD and CAT, suggesting a "cooperative" mechanism of the two enzymatic systems.
In addition to SOD and CAT, which are considered the major antioxidant enzymes, there are others that may be useful biomarkers. These enzymes serve as a backup function by replenishing GSH from glutathione disulfide (GSSG) through the enzyme GR and the reducing equivalent is provided by the enzyme G6PD.
In the present study, higher values of GR and G6PD were observed in the liver of barbel captured in the contaminated location. Several authors reported that fish exposed to pollutants present higher GR activity due to higher peroxidative components (Peixoto et al. 2006, Sturve et al. 2008). Equally, the increase of G6PD activity should be related to the increase of NADPH production, an important cofactor necessary to recycle reduced glutathione through glutathione reductase activity, in order to minimize the oxidative stress condition. Thus, reflecting an adaptation to oxidative conditions to which fish has been exposed to (Lenartova et al. 1997).
Furthermore, detoxification enzymes, and especially GST, help to eliminate reactive compounds by conjugation with glutathione and subsequent elimination; thereby protecting cells against ROS induced damage (Matos et al. 2007). GST catalyzes the conjugation of electrophilic compounds with the tri-peptide glutathione and is a determinant enzyme for herbicide detoxification (Villarini et al. 1995, Peixoto et al. 2008). In Vizela barbel, GST activity was double compared to the one in reference barbel, this may indicate that in Vizela River fish are exposed to a higher load of compounds, present in textile industry effluents.
GSH is an effective protector, capable of quenching oxyradicals, and is an essential cofactor for GPx and GST activity (Ross 1988). Despite the observed increase in GST and GR activities in liver of barbel captured in Vizela River, GSH content was not increased. However, even if GSH was being synthesized de novo in barbel captured in Vizela River, since the oxidative stress condition were higher in these fish, GSH could be being used by GSH, GR and oxidized to GSSG, which would result in a decrease on GSH content. This situation could justify the similar GST content observed between the two barbel populations.
Xanthine oxidase (XO) catalyzes the conversion of xanthine to uric acid. Uric acid, an excretory product of purine catabolism, can act as a scavenger of ROS such as OH⋅ and O2-. Therefore uric acid can protect DNA and cellular membranes from ROS-mediated damage (Stinefelt et al. 2005). In the present work XO activity was similar in the liver of both groups of barbel.
When fish are submitted to oxidative stress conditions, fatty acid peroxidation can occur. Indeed, increased ROS production and subsequent oxidative damage has been associated with pollutant-mediated mechanisms of toxicity in fish liver (Livingstone et al. 1993). Malondialdehyde (MDA) production is a well-known oxidation product of polyunsaturated fatty acids, influencing cell membrane fluidity as well as the integrity of biomembranes (Ercal et al. 2001, Almroth et al. 2005), and can be used as an indicator of lipid peroxidation. The present study revealed high levels of lipid peroxidation, measured as MDA, suggesting that antioxidant enzymes stimulation was not capable of preventing hepatic lipid peroxidation, probably induced by water contamination.
This study also put in evidence the histopatological liver alterations observed in barbel from Vizela River: macrophage aggregates and foci of lymphocytes. Liver lesions have been classified and scored, according to their relative importance, as indicators of contaminant exposure. Macrophage aggregates are related to storage of foreign material, such as parasitic infestations, and although it can be observed in fish living in low contaminated sites (Stentiford et al. 2003), their prevalence and intensity can be used as a potential biomarker to environmental contamination (Couillard and Hodson 1996). In the present study, prevalence of macrophage aggregates in Vizela River barbel was higher than the one observed in reference barbel. Furthermore, when looking to macrophage aggregates median score in barbel, there is a clear difference between the two groups of barbel, probably related to water contamination.
The hepatic foci of lymphocites, observed only in fish from Vizela River, could be the result of chronic inflammatory conditions, by both, infectious and non-infectious causes. This may reflect a depleted immunological status due to contaminated water exposure.
Several studies have established a causal relationship between metals concentrations and fish liver histopathological alterations (Au 2004). The injuries are often dependent upon time of exposure to metals (Yang and Chen 2003, Au 2004, Olojo et al. 2005).
Metal accumulation in fish organs reflects bio-availability and exposure. In the present study, liver of barbel captured in Vizela River presented low metals levels. This could be due to the low bioavailability of metals and/or occasional effluent discharges from the textile factories. Furthermore, the textile effluents carry other contaminants rather than metals and, although Portugal is required to control industrial discharges, several effluents, located in the study area, are still discharging in the river bed without any type of treatment.
Several studies showed a growing interest in the use of bioindicators and biomarkers for assessment and monitoring of the ecological systems (Braunbeck and Võlkl 1993, Vethaak and Wester 1996) in addition to traditional biomonitoring studies, as a way to understand the real bio-effects of pollution in wildlife (Burger et al. 2007), namely in fish (Kirby et al. 2007).
In conclusion, although being non specific responses, antioxidant enzymes activity and liver histopathology are useful tools to evaluate the impact of industry wastewater. Therefore, these exposure biomarkers can be used to assess the future impact of management policies on Vizela River fish.
REFERENCES
Almroth B.C., Sturve J., Berglund A. and Forlin L. (2005). Oxidative damage in eelpout (Zoarces viviparus), measured as protein carbonyls and TBARS, as biomarkers. Aquat. Toxicol. 73, 171-180. [ Links ]
Alves C.M., Boaventura R.R.A.R. and Soares H.M.VM. (2009). Evaluation of heavy metals pollution loadings in the sediments of the Ave River Basin (Portugal). Soil Sediment Contam. 18, 603-618. [ Links ]
Au D.W.T. (2004). The application of histo-cytopathological biomarkers in marine pollution monitoring: areview. Mar. Pollut. Bull. 48, 817-834. [ Links ]
Baker R.T.M., Handy R.D., Davies S.J. and Snook J.C. (1998). Chronic dietary exposure to copper affects growth, tissue lipid peroxidation, and metal composition of the grey mullet, Chelon labrosus. Mar. Environ. Res. 45, 357-365. [ Links ]
Baker R.T.M., Martin P. and Davies S.J. (1997). Ingestion of sub-lethal levels of iron sulphate by African catfish affects growth and tissue lipid peroxidation. Aquat. Toxicol. 40, 51-61. [ Links ]
Bláha L., Kopp R., Simková K. and Mares J. (2004). Oxidative stress biomarkers are modulated in silver carp (Hypophthalmichthys molitrix Val.) exposed to microcystin-producing cyanobacterial water bloom. Acta Vet. Brno. 73, 477-482. [ Links ]
Braunbeck T. and Völkl A. (1993). Toxicant-induced cytological alterations in fish liver as biomarkers of environmental pollution? A case study on hepatocellular effects of dinitro-o-cresol in golden ide (Leuciscus idus melanotus). In: Fish - ecotoxicology and ecophysiology (H.W. Braunbeck T., H. Segner Eds.). VCH, Weinheim, pp. 55-80. [ Links ]
Burger J., Fossi C., McClellan-Green P. and Orlando E.F. (2007). Methodologies, bioindicators, and biomarkers for assessing gender-related differences in wildlife exposed to environmental chemicals. Environ. Res. 104, 135-152. [ Links ]
Carlberg I. and Mannervik B. (1975). Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 250, 5475-5480. [ Links ]
Carrola J., Fontainhas-Fernandes A., Matos P. and Rocha E. (2009). Liver histopathology in brown trout (Salmo trutta f.fario) from the Tinhela River, subjected to mine drainage from the abandoned Jales Mine (Portugal). Bull. Environ. Contam. Tox. 83, 35-41. [ Links ]
Carvalho L., Cortes R. and Bordalo A. (2011). Evaluation of the ecological status of an impaired watershed by using a multi-index approach. Environ. Monit. Assess. 174, 493-508. [ Links ]
Claiborne A. (1985). Catalase activity. In: Handbook of methods for oxygen radical research (R. A. Greenwald, Ed). Boca Raton, FL, pp. 283-284. [ Links ]
Collen J., Pinto E., Pedersen M. and Colepicolo P. (2003). Induction of oxidative stress in the red macroalga Gracilaria tenuistipitata by pollutant metals. Arch. Environ. Con. Tox. 45, 337-342. [ Links ]
Couillard C.M. and Hodson P.V. (1996). Pigmented macrophage aggregates: A toxic response in fish exposed to bleached-kraft mill effluent? Environ. Toxicol. Chem. 15, 1844-1854. [ Links ]
Cunha I., Neuparth T., Caeiro S., Costa M.H. and Guilhermino L. (2007). Toxicity ranking of estuarine sediments on the basis of Sparus aurata biomarkers. Environ. Toxicol. Chem. 26, 444-453. [ Links ]
Deviller G., Palluel O., Aliaume C., Asanthi H., Sanchez W., Nava M.A.F., Blancheton J.P. and Casellas C. (2005). Impact assessment of various rearing systems on fish health using multibiomarker response and metal accumulation. Ecotox. Environ. Safe. 61, 89-97. [ Links ]
Ercal N., Gurer-Orhan H. and Aykin-Burns N. (2001). Toxic metals and oxidative stress Part I: Mechanisms involved in metal-induced oxidative damage. Current Topics in Medicinal Chemistry. 1, 529-539. [ Links ]
Fatima M., Ahmad I., Sayeed I., Athar M. and Raisuddin S. (2000). Pollutant-induced over-activation of phagocytes is concomitantly associated with peroxidative damage in fish tissues. Aquat. Toxicol. 49, 243-250. [ Links ]
Fernandes C., Fontainhas-Fernandes A., Peixoto F. and Salgado M.A. (2007). Bioaccumulation of heavy metals in Liza saliens from the Esmoriz-Paramos coastal lagoon, Portugal. Ecotoxicol. Environ. Saf. 66, 426-431. [ Links ]
Fernandes C., Fontainhas-Fernandes A., Cabral D. and Salgado M.A. (2008a). Heavy metals in water, sediment and tissues of Liza saliens from Esmoriz-Paramos lagoon, Portugal. Environ. Monit. Assess. 136, 267-275. [ Links ]
Fernandes C., Fontainhas-Fernandes A., Rocha E. and Salgado M.A. (2008b). Monitoring pollution in Esmoriz-Paramos lagoon, Portugal: Liver histological and biochemical effects in Liza saliens. Environ. Monit. Assess. 145, 315-322. [ Links ]
Fernandes C., Fontainhas-Fernandes A., Ferreira M. and Salgado M.A. (2008c). Oxidative stress response in gill and liver of Liza saliens, from the Esmoriz-Paramos coastal lagoon, Portugal. Arch. Environ. Contam. Toxicol. 55, 262-269. [ Links ]
Ferreira M., Moradas-Ferreira P. and Reis-Henriques M.A. (2005). Oxidative stress biomarkers in two resident species, mullet (Mugil cephalus) and flounder (Platichthysflesus), from a polluted site in River Douro Estuary, Portugal. Aquat. Toxicol. 71, 39-48. [ Links ]
Figueiredo-Fernandes A., Fontainhas-Fernandes A., Peixoto F., Rocha E. and Reis-Henriques M.A. (2006). Effects of gender and temperature on oxidative stress enzymes in Nile tilapia Oreochromis niloticus exposed to paraquat. Pest. Biochem. Phys. 85, 97-103. [ Links ]
Gravato C., Guimarães L., Santos J., Faria M., Alves A. and Guilhermino L. (2010). Comparative study about the effects of pollution on glass and yellow eels (Anguilla anguilla) from the estuaries of Minho, Lima and Douro Rivers (NW Portugal). Ecotox. Environ. Safe. 73, 524-533. [ Links ]
Habig W.H., Pabst M.J. and Jakoby W.B. (1974). Glutathione S-transferases - First enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130-7139. [ Links ]
Halliwell B. and Gutteridge H. (1999). Free radicals in biology and medicine. University Press, Oxford, 936 p. [ Links ]
Hinton D.E. and Lauren D.J. (1990). Liver structural alterations accompanying chronic toxicity in fishes: Potential biomarkers of exposure. In: Biomarkers of environmental contamination (J.F. McCarthy and L.R. Shugart, Eds.). Lewis Publishers, Boca Raton FL, pp. 17-57. [ Links ]
Jollow D.J., Mitchell J.R., Zampaglione N. and Gillette J.R. (1974). Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacol. 11, 151-169. [ Links ]
Kirby M.F., Smith A.J., Rooke J., Neall P., Scott A.P. and Katsiadaki I. (2007). Ethoxyresorufin-O-deethylase (EROD) and vitellogenin (VTG) in flounder (Platich-thysflesus): System interaction, crosstalk and implications for monitoring. Aquat. Toxicol. 81, 233-244. [ Links ]
Kohler A., Wahl E. and Soffker K. (2002). Functional and morphological changes of lysosomes as prognostic biomarkers of toxic liver injury in a marine flatfish (Platichthys flesus (L.)). Environ. Toxicol. Chem. 21, 2434-2444. [ Links ]
Lang T., Wosniok W., Barsiene J., Broeg K., Kopecka J. and Parkkonen J. (2006). Liver histopathology in Baltic flounder (Platichthysflesus) as indicator of biological effects of contaminants. Mar. Pollut. Bull. 53, 488-496. [ Links ]
Lenartova V, Holovska K., Pedrajas J.R., Martinez Lara E., Peinado J., Lopez Barea J., Rosival I. and Kosuth P. (1997). Antioxidant and detoxifying fish enzymes as biomarkers of river pollution. Biomarkers 2, 247-252. [ Links ]
Leonardi M., Tarifeno E. and Vera J. (2009). Diseases of the Chilean flounder, Paralichthys adspersus (Steindachner, 1867), as a biomarker of marine coastal pollution near the Itata River (Chile): Part II. Histopathological lesions. Arch. Environ. Contam. Toxicol. 56, 546-556. [ Links ]
Livingstone D.R., Lemaire P., Matthews A., Peters L., Bucke D. and Law R.J. (1993). Prooxidant, antioxidant and 7-Ethoxyresorufin O-Deethylase (EROD) activity responses in liver of Dab (Limanda limanda) exposed to sediment contaminated with hydrocarbons and other chemicals. Mar. Pollut. Bull. 26, 602-606. [ Links ]
Lowry O.H., Rosebrough N.J., Farr A.L. and Randall R.J. (1951). Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265-275. [ Links ]
Magalhães M.F. (1992). Feeding ecology of the Iberian cyprinid Barbus bocagei Steindachner, 1865 in a lowland river. J. Fish Biol. 40, 123-133. [ Links ]
Matos P., Fontainhas-Fernandes A., Peixoto F., Carrola J. and Rocha E. (2007). Biochemical and histological hepatic changes of Nile tilapia Oreochromis niloticus exposed to carbaryl. Pest. Biochem. Phys. 89, 73-80. [ Links ]
McCarthy J.F. and Shugart L.R. (1990). Biological markers of environmental contamination. In: Biomarkers of environmental contamination (J.F. McCarthy and L.R. Shugart, Eds.). Lewis Publishers, Boca Raton FL, pp. 3-14. [ Links ]
Mohandas J., Marshall J.J., Duggin G.G., Horvath J.S. and Tiller D.J. (1984). Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney - possible implications in analgesic nephropathy. Biochem. Pharmacol. 33, 1801-1807. [ Links ]
Olojo E.A.A., Olurin K.B., Mbaka G. and Oluwemimo A.D. (2005). Histopathology of the gill and liver tissues of the African catfish Clarias gariepinus exposed to lead. Afr. J. Biotechnol. 4, 117-122. [ Links ]
Paya M., Halliwell B. and Hoult J.R. (1992). Interactions of a series of coumarins with reactive oxygen species. Scavenging of superoxide, hypochlorous acid and hydroxyl radicals. Biochem. Pharmacol. 44, 205-214. [ Links ]
Peixoto F., Alves-Fernandes D., Santos D. and Fontainhas-Fernandes A. (2006). Toxicological effects of oxyfluorfen on oxidative stress enzymes in tilapia Oreochromis niloticus. Pest. Biochem. Phys. 85, 91-96. [ Links ]
Peixoto F.P., Gomes-Laranjo J., Vicente J.A. y Madeira V.M.C. (2008). Comparative effects of the herbicides dicamba, 2,4-D and paraquat on non-green potato tuber calli. J. Plant Physiol. 165, 1125-1133. [ Links ]
Pinto A., Varandas S., Coimbra A., Carrola J. and Fontaínhas-Fernandes A. (2010). Mullet and gudgeon liver histopathology and macroinvertebrate indexes and metrics upstream and downstream from a wastewater treatment plant (Febros River-Portugal). Environ. Monit. Assess. 169, 569-585. [ Links ]
Ross D. (1988). Glutathione, free-radicals and chemo-therapeutic agents. Mechanisms of free-radical induced toxicity and glutathione-dependent protection. Pharmacol. Therapeut. 37, 231-249. [ Links ]
Santos J.M., Godinho F., Ferreira M.T. and Cortes R. (2004). The organisation of fish assemblages in the regulated Lima basin, Northern Portugal. Limnologica 34, 224-235. [ Links ]
Soares H.M.V.M., Boaventura R.A.R., Machado A.A.S.C. and da Silva J.C.G.E. (1999). Sediments as monitors of heavy metal contamination in the Ave river basin (Portugal): multivariate analysis of data. Environ. Pollut. 105, 311-323. [ Links ]
Stentiford G.D., Longshaw M., Lyons B.P., Jones G., Green M. and Feist S.W. (2003). Histopathological biomarkers in estuarine fish species for the assessment of biological effects of contaminants. Mar. Environ. Res. 55, 137-159. [ Links ]
Stinefelt B., Leonard S.S., Blemings K.P., Shi X.L. and Klandorf H. (2005). Free radical scavenging, DNA protection, and inhibition of lipid peroxidation mediated by uric acid. Ann. Clin. Lab. Sci. 35, 37-45. [ Links ]
Stirpe F. and Dellacor E. (1969). The regulation of rat liver xanthine oxidase. Conversion in vitro of enzyme activity from dehydrogenase (Type D) to oxidase (Type O). J. Biol. Chem. 244, 3855-3863. [ Links ]
Sturve J., Almroth B.C. and Forlin L. (2008). Oxidative stress in rainbow trout (Oncorhynchus mykiss) exposed to sewage treatment plant effluent. Ecotox. Environ. Safe. 70, 446-452. [ Links ]
Triebskorn R., Köhler H.-R., Honnen W., Schramm M., Adams S.M. and Müller E.F. (1997). Induction of heat shock proteins, changes in liver ultrastructure, and alterations of fish behavior: are these biomarkers related and are they useful to reflect the state of pollution in the field? J. Aquat. Ecosyst. Stress Rec. (Formerly Journal of Aquatic Ecosystem Health). 6, 57-73. [ Links ]
Triebskorn R., Kohler H.R., Flemming J., Braunbeck T., Negele R.D. and Rahmann H. (1994). Evaluation of bis(tri-n-butyltin)oxide (Tbto) neurotoxicity in rainbow-trout (Oncorhynchus mykiss). 1. Behavior, weight increase, and tin content. Aquat. Toxicol. 30, 189-197. [ Links ]
Uguz C., Iscan M., Erguven A., Isgor B. and Togan I. (2003). The bioaccumulation of nonyphenol and its adverse effect on the liver of rainbow trout (Onchorynchus mykiss). Environ. Res. 92, 262-270. [ Links ]
Utley H.G., Bernheim F. and Hochstei. P (1967). Effect of sulfhydryl reagents on peroxidation in microsomes. Arch. Biochem. Biophys. 118, 29-32. [ Links ]
Valavanidis A., Vlahogianni T., Dassenakis M. and Scoullos M. (2006). Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotox. Environ. Safe. 64, 178-189. [ Links ]
Varandas S.G. and Cortes R.M. (2010). Evaluating mac-roinvertebrate biological metrics for ecological assessment of streams in northern Portugal. Environ. Monit. Assess. 166, 201-221. [ Links ]
Vethaak A.D. and Wester P.W. (1996). Diseases of flounder Platichthys flesus in Dutch coastal and estuarine waters, with particular reference to environmental stress factors. 2. Liver histopathology. Dis. Aquat. Organ. 26, 99-116. [ Links ]
Vieira C., Morais S., Ramos S., Delerue-Matos C. and Oliveira M.B. (2011). Mercury, cadmium, lead and arsenic levels in three pelagic fish species from the Atlantic Ocean: intra- and inter-specific variability and human health risks for consumption. Food Chem. Toxicol. 49, 923-932. [ Links ]
Villarini M., Moretti M., Scassellati-Sforzolini G., Monarca S., Pasquini R., Crea M.G. and Leonardis C. (1995). Studies on hepatic xenobiotic-metabolizing enzymes in rats treated with insecticide deltamethrin. J. Environ. Pathol. Toxicol. Oncol. 14, 45-52. [ Links ]
Yang J.L. and Chen H.C. (2003). Serum metabolic enzyme activities and hepatocyte ultrastructure of common carp after gallium exposure. Zool. Stud. 42, 455-461. [ Links ]
Zaheer N., Tewari K.K. and Krishnan P.S. (1965). Exposure and solubilization of hepatic mitochondrial shunt dehydrogenases. Arch. Biochem. Biophys. 109, 646-648. [ Links ]














