Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista internacional de contaminación ambiental
versión impresa ISSN 0188-4999
Rev. Int. Contam. Ambient vol.28 no.3 Ciudad de México ago. 2012
Artículos
Evaluation of the removal of arsenic and cadmium from aqueous solution using natural rhyolitic sediments and metallurgical wastes
Evaluación de la remoción de arsénico y cadmio de soluciones acuosas utilizando sedimentos riolíticos naturales y desechos metalúrgicos
Luis Gerardo MARTÍNEZ JARDINES1, Francisco MARTÍN ROMERO1*, Margarita Eugenia GUTIÉRREZ RUIZ2 and Águeda Elena CENICEROS GÓMEZ3
1 Departamento de Geoquímica, Instituto de Geología, Universidad Nacional Autónoma de México *Autor responsable; fmrch@geologia.unam.mx
2 Grupo de Biogeoquímica Facultad de Química, Universidad Nacional Autónoma de México
3 Departamento de Química Analítica, Facultad de Química, Universidad Nacional Autónoma de México
Recibido octubre 2011,
aceptado marzo 2012
ABSTRACT
The use of natural materials abundant, efficient and inexpensive for use in stabilization of contaminants is in development, so some sorbent materials for removal of Cd (II) on aqueous solutions in the range of 10-100 mg/L and for As (III) and As (V) in the range of 1-500 mg/L have been investigated. The sorbent materials studied are indigenous rhyolitic sediments and metallurgical wastes from San Luis Potosi, México. Mineralogical analysis showed that rhyolitic sediments are characterized by the occurrence of clay minerals, while the metallurgical wastes are characterized by Fe-bearing minerals as ammoniojarosite, K-jarosite, hematite and goethite. The experimental results showed that the rhyolitic sediments had high removal efficiency (94-99 %) for Cd (II); while As (III) was barely removed (5-18 %) and As (V) was not retained by these natural geological materials. By contrast, the removal of As (III) and As (V) by metallurgical wastes had an efficiency of 88 and 77 %, respectively. However, these wastes were not able to remove Cd (II). The experimental results were fitted to the Linear, Langmuir, and Freundlich isotherm models to obtain the characteristic parameters of each model. The Linear model for As (III) on rhyolitic sediments, as well as the Langmuir model for Cd (II) on rhyolitic sediments and As (III) and (V) on metallurgical wastes, were found to well represent the measured sorption data.
Key words: arsenic, cadmium, rhyolitic sediments, metallurgical waste, sorption isotherm.
RESUMEN
El uso de materiales naturales, abundantes, eficientes y de bajo costo para utilizarlos en métodos de estabilización de contaminantes se encuentra en desarrollo por lo que se investigó la eficiencia de algunos materiales para la retención en solución acuosa de Cd (II) en concentraciones de 10 a 100 mg/L y para As (III) y As (V) en concentraciones de 1 a 500 mg/L. Los materiales evaluados son sedimentos riolíticos y residuos metalúrgicos que provienen de San Luis Potosí, México. La composición mineralógica de los sedimentos riolíticos indica la presencia de minerales de arcilla, mientras que los residuos metalúrgicos se caracterizan por la presencia de minerales de hierro como son: jarosita, amoniojarosita, goetita y hematita. Los resultados de este estudio indican que los sedimentos riolíticos son capaces de retener Cd (II) con una eficiencia del 9499 %. Sin embargo, tienen una eficiencia baja (5-18 %) para la retención de As (III) y no tienen capacidad de retención de As (V). Los residuos metalúrgicos tienen mucha capacidad de retención de As (V) y As (III) con 88 y 77 % de eficiencia, respectivamente; pero no tienen capacidad de retener Cd (II). Los resultados experimentales se ajustaron con los modelos de isotermas de sorción Lineal (Kd), Langmuir y Freundlich, con el fin de obtener los parámetros característicos de cada modelo. Los datos experimentales mostraron un ajuste adecuado a los modelos Lineal Kd para As (III) y de Langmuir para el caso de Cd (II) en los sedimentos riolíticos y para As (III) y As (V) en los residuos metalúrgicos.
Palabras clave: arsénico, cadmio, sedimentos riolíticos, residuo metalúrgico, isoterma de sorción.
INTRODUCTION
Arsenic and cadmium are toxic to plants and animals because of their mobility and solubility under environmental conditions, and their affinity for proteins, lipids and others cell components.
Arsenic (As) contamination of soils and water is mainly related to mining and metal processing, as well as the use of agricultural pesticides containing this element (Lin and Puls 2000, Wang and Mulligan 2006, Gerente et al. 2010). Arsenic is found in soils and natural waters, mainly in the form of arsenate (As (V)) and arsenite (As (III)). The distribution between dissolved As (III) and As (V) is dependent on redox potential and pH. Under oxidizing conditions, the predominant specie is As (V), which exists as deprotonated oxyanions of arsenic acid (H2AsO4-, HAsO42- and AsO43-). Under reducing conditions, As (III) is thermodynamically stable and exists in solution as arsenious acid, a neutral, uncharged molecule (H3AsO30) that only forms deprotonated oxyanions at pH > 9.2 (H2AsO3- and HAsO32-) (Manning and Goldberg 1997, Sadiq 1997).
The As (III) species are more toxic than As (V). At the pH of most natural soils and water, As (III) is electrically neutral and consequently electrostatically is not strongly adsorbed on most mineral surfaces as the negatively charged As (V) oxyanions (Brewster1992).
Cadmium (Cd) contamination of soils and water is mainly associated with industrial activity usually involving metal coatings, inadequate handling of nickel-cadmium batteries, use of phosphate fertilizers, mining and metal processing, etc. (Mulligan et al. 2001, Tiller 1989). Under acidic conditions, Cd is predominantly present as soluble Cd (II), it is soluble in aqueous solution at pH values <10. So is readily treatable by alkali precipitating as hydroxide.
Arsenic and Cd contamination of soils and water is a serious and recurring problem in many countries, including México, which requires intervention in order to decrease human health and environmental risks. Different methods aimed at chemical stabilization for the treatment of soils and water contaminated with As oxyanions and heavy metal cations have been developed.
Arsenic stabilization processes include immobilization by sorption in solid phases such as oxides and hydroxides of iron, aluminum and manganese as well as organic matter (Wang and Mulligan 2006, Daus et al. 2004).
For heavy metals, including Cd, sorption has been reported on activated carbon (Mohan and Singh 2002), zeolites and clays (Celis et al. 2000). Clays can act as sorbents for heavy metal cations because of their negative charge, large surface area associated with small particle size, high cation exchange capacity, low cost and occurrence in most soils and sediments.
In order to apply the stabilization methods in real situations, the selected sorbent materials must be abundant, inexpensive and efficient. Activated carbon and activated alumina are sorbent materials whose effectiveness has been amply demonstrated (Trivedi and Axe 2001, Mohan and Singh 2002); however, their use is limited due to their high costs. Due to this limitation, at the present time a number of technologies employing other natural sorbent materials are being developed. Examples include: montmorillonite for Cd (II) (Malferrari et al. 2007); kaolinite for Cd (II), Cu (II), Pb (II) and Zn (II) (Srivastava et al. 2005); bentonite for Cu (II) and Zn (II) (Veli 2007); basaltic volcanic slag for Zn (II) (Kwon et al. 2005); industrial wastes for Cd (II), Cu (II), Pb (II) and Zn (II) (Ciccu et al. 2003) and limestone and calcareous shales for As (V) (Romero et al. 2004, 2011). Recently, biosorbents derived from food industry waste (Gerente et al. 2010), agriculture (Mohan and Singh 2002) and biomass (Beesley and Marmiroli 2011) have also been used as sorbent materials.
This research was conducted with the aim of assessing As and Cd removal by natural rhyolitic sediments and metallurgical wastes from San Luis Potosi, México, as sorbent materials. These materials were characterized and sorption experiments were carried out using batch-leaching tests to determine their capacity to remove Cd (II) in the range of 10100 mg/L, and As (III) and (V) in the range of 1-500 mg/L from aqueous solutions.
MATERIALS AND METHODS
Sampling sorbent materials
The sampling of the sorbent materials was done in an area near to San Luis Potosi City, México. Seven samples of rhyolitic materials (S1-S7) from different natural deposits and one sample of metallurgical wastes (MW) named "jarosite" (a mixture of different compounds) stored in an impoundment of the electrolytic zinc refinery, were collected.
At each sampling site, five sub-samples of 10 kg were taken within a circle area of 25 m radius. The five sub-samples were mixed and quartered to prepare 8 composite samples. All solid samples were stored in hermetically sealed plastic bags to minimize dust contamination during transport to the laboratory for chemical and mineralogical analyses.
Characterization of sorbent materials
Samples were air dried, disaggregated and sieved through a 2-mm mesh and homogenized. The pH values were determined in solid suspensions (1:5 solid:water) following the EPA 9045 method (US EPA 1995) using a Beckman model Φ 720 pH meter and a glass electrode.
Also the pH value at the point of zero charge (PZC or pHpzc), at which the surface charge becomes neutral was calculated using the zeta potential values determined with a Zeta-Meter System 3.0+. The zeta potential was determined using a suspension containing 100 mg of homogenized solid samples and 500 mL of NaCl solution 0.01 M, as electrolyte equilibrated for 30 minutes. The zeta potential was measured at each pH values from 1 to 11, which were previously adjusted stirring the suspension with 0.1 M NaOH or 0.1 M HCl. The PZC of the samples were estimated by plotting the zeta potential as a function of pH and determining the pH through interpolation when zeta potential =0.
For chemical and mineralogical analysis, the homogenized samples were pulverized in an agate mortar to 200 mesh. The finely-milled samples were analyzed using a Portable X-ray Fluorescence analyzer NITON XL3t, according to the EPA 6200 method (US-EPA 2006). The mineral composition of samples was determined by X-ray Diffraction (XRD) using a Shimadzu XRD-6000 diffractometer, equipped with a Ni filter, copper tube and monochromator. All samples were analyzed at angular interval 2θ from 4 ° to 70 ° and a speed of 2 °/min, operated at 40 kV voltage and with 30 mA applied potential.
Experimental sorption experiments
Sorption experiments on rhyolitic sediments and metallurgical wastes were carried out in batch systems, using solutions of As (III), As (V) or Cd (II). The solid suspensions were prepared using 100 mL of As (III), As (V) or Cd (II) solutions and 5.0 g of sorbent materials. Sample suspensions were equilibrated for 18 ± 0.25 hours in batch reactors at room temperature (23 °C(+/- 1 °C)) and continuously shaken at 200 rpm, at constant ratio (solid:solution, 1:20). In the case of As (III), in order to prevent its oxidation, the experiments were conducted in a nitrogenous atmosphere.
Solutions of As (III), As (V) and Cd (II) were prepared from stock standard solutions of As2O3, Na2HAsO4.7H2O and CdCl22.5 H2O, respectively. The initial soluble concentration of these ions in the tested suspensions was 50 mg/L. Batch experiments were carried out in order to acquire data for constructing sorption isotherms for sorbent materials. For rhyolitic sediments, concentrations of As (III) and As (V) were used between 1 and 75 mg/L, and for the metallurgical wastes, from 2.5 to 500 mg/L. To test the sorption of Cd (II) in both materials five concentrations of this cation were used from 10 to 100 mg/L. To prevent hydroxide precipitation of Cd (II), the Cd (II) solution used has pH =4.0.
After the equilibration time, the final pH was measured and the suspension was centrifuged and filtered through a 0.45 μm membrane, transferred to a vial, and stored in the dark at 4 °C until chemical analyses were performed. The soluble concentrations of As (III), As (V) and Cd (II) were determined by atomic absorption spectrometry (AAS) using a Varian Spectra 110A model. Soluble concentrations of As (III) and As (V) were determined using hydride generation-AAS in samples with concentrations below 3 mg/L (detection limit: 5μg/L). The samples with concentrations >3 mg/L of As were measured using AAS-flame (detection limit: 3 mg/L). The Cd (II) in all samples was also measured by AAS-flame (detection limit of 0.02 mg/L).
A quality control was implemented including blanks, spiked blanks and duplicate samples Precision and accuracy were better than 10 % for all the analyzed elements.
RESULTS AND DISCUSSION
Mineralogical and chemical analyses of sorbent materials
The mineralogical and chemical compositions of rhyolitic sediments and the metallurgical wastes are presented in Tables I, II, respectively. The results show that rhyolitic sediments and metallurgical wastes are chemically and mineralogically different.
XRD analysis showed that the mineralogical composition of rhyolitic sediment samples (S1- S7) is dominated by quartz (SiO2), plagioclase [(CaAl) (SiAl)4O8], cristobalite (SiO2), feldspar (KSi3AlO8) and clays, from the smectite and mica groups. Additionally, the S3 sample contains zeolite (CaAl2Si7O18 6H2O).
The pH values of rhyolitic sediments varied between 8.0 and 8.8. Chemically, these materials are characterized by the absence of potentially toxic elements (As, Cd, Cu, Pb, S) which were not detected and relatively low total concentrations of Zn (40-82 mg/kg), Fe (1.39-1.96 %), Ca (0.76-1.33 %), K (1.061.69 %) and Mn (0.01-0.03 %).
Mineralogical analyses of the metallurgical wastes showed that it is mainly composed by ammoniojarosite [(NH4)2Fe6(SO4)4(OH)12] and K-jarosite [KFe3(SO4)2(OH)6]. These metallurgical wastes contain minor amounts of gunningite [(Zn,Mn)SO4· H2O], gibsite, Al(OH)3, anglesite (PbSO4), quartz (SiO2), sphalerite (ZnS), hematite (Fe2O3) and goethite (FeOOH). This complex mineralogical composition is due to the fact that these industrial wastes were generated by the hydrometallurgical processing of Znsulfide concentrates from different polymetallic mines from México, under strongly hot and acidic conditions.
The metallurgical wastes had low pH value (pH = 2.7) and high total concentration of potentially toxic elements, mainly As (1 968 mg/kg), Cd (2 194 mg/kg), Cu (8 347 mg/kg), Pb (10 939 mg/kg) and Zn (72 332 mg/kg). It is important to emphasize that total concentrations of Fe (48.3 %), S (6.3 %) and Mn (2.4 %) are consistent with its mineralogy composition (Table I).
Point of zero charge (PZC) values
The PZC of rhyolitic sediments varied between 2 and 3 (Table II) which are comparable to the values reported for quartz and clays (Appelo and Postma 2005, Manning and Goldberg 1997). As mentioned previously, the rhyolitic sediments contain quartz and clay minerals from the smectite and mica groups, which were identified by DRX (Table I).
The PZC of the metallurgical wastes ("jarosite") is around 2.7. This low value is probably related to the presence of ammoniojarosite ((NH4)2Fe6(SO4)4(OH)12) and K-jarosite (KFe3(SO4)2(OH)6), which, as previously noted, are the predominant minerals identified by XRD in these wastes. This PZC value is similar to other reported values for jarosite minerals, which vary from 1.8 to 3.9 (Sadowski etal. 2001).
Removal of Cd (II), As (III) and As (V) by rhyolitic sediments and metallurgical wastes
The removal efficiency of the sorbent materials was determined in suspensions prepared with solutions of 50 mg/L of Cd (II), As (III) and As (V). The initial pH of the suspensions of rhyolitic sediments varied between 8.0 and 8.7; while the suspensions of the metallurgical wastes presented a constant pH = 2.7, as expected by the acid behavior of jarosite minerals.
The percentage removal was estimated using Equation 1. The experimental results are shown in Table III.
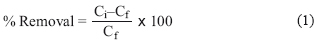
where Ci, is the initial concentration (mg/L) of Cd (II) , As (III) and As (V), before the interaction with the studying sorbent material.
Cf, is the final concentration (mg/L) of Cd (II), As (III) and As (V) after 18 h interaction with the sorbent materials.
The results indicate that the equilibrium pH depends on the sorbent material. In rhyolitic sediments the values varied from 6.6 to 8.8, and in the metallurgical wastes the pH was 3.9 for As (III) and 4.1 for As (V) and Cd (II) (Table III).
Rhyolitic sediments
The efficiency of the rhyolitic sediments to remove Cd (II) was very high (94-99 %), while As (III) was barely removed (5-18 %) and As (V) was not retained by this natural geological material.
The high efficiency of rhyolitic sediments for removal Cd (II) appears to be related to the presence of smectite, mica and zeolite, which, under equilibrium pH (pH = 6.6-8.0) reached during tests (Table III), must be negatively charged because the PZC values determined in these materials were low (PZC = 2-3), favoring, in this way, the retention of metal cations such as Cd (II), which has been widely reported in the literature (Celis et al. 2000, Srivastava et al. 2005, Rao et al. 2006, Malferrari et al. 2007, Veli 2007, Panuccio et al. 2009).
The inefficiency of the rhyolitic sediments to remove As (V) is likely related to the fact that at the pH of equilibrium (6.6-8.0) this element exists as deprotonated oxyanion of arsenic acid (HAsO42-), because the pKa values for arsenic acid are pK3a = 2.3, pK2a = 6.8, pK1a = 11.6. At equilibrium pH, the clay surfaces are negatively charged, so the oxyanions of As (V) are repelled.
The removal of As (III) by rhyolitic sediments varied from 5 to 18 %. The As (III) retention is probably due to the fact that it is present as non-charged arsenious acid (H3AsO30). This chemical specie is a pyramidal molecule consisting of three hydroxyl groups bonded to As (Bickmore et al. 2006) with a radius of 4.16 Å (Kim et al. 2004), a bond distance As-O of 1.79 Å and an angle of 100° 31' (Iakovleva 2003). The neutral arsenious acid exists in most of the pH range since its pKa is very high (pK3a = 9.2, pK2a= 12.1, pK1a= 13.1), and can approach the negative surfaces of clay minerals forming inner complexes with metals mainly with Al, since the greater reactivity of Al(OH)3 toward As (III) (Manning and Goldberg 1997).
Arsenite adsorption on clay minerals as kaolinite, illite and montmorillonite had been reported by Goldberg (2002). The adsorption increases with increasing solution pH up to a maximum near 7 to 8.5 (Goldberg 2000), that is the pH range of the experiments carried out in this work.
Metallurgical wastes
The major minerals in metallurgical wastes are ammoniojarosite and K-jarosite which, under equilibrium pH (pH = 3.1-4.9) reached during tests (Table III), must be negatively charged because the PZC determined in these materials was low (PZC = 2.7), favoring, in this way, the retention of metal cations and disfavoring the removal of negatively charged arsenic oxyanions. However, the experimental results showed that the removal As (III) and As (V) by metallurgical wastes was very high with an efficiency of 88 and 77 %, respectively.
A possible explanation about the high efficiency for removal As (III) and As (V) by metallurgical wastes is the presence of Fe-oxyhydroxides because of their high affinity for As (V) and As (III) (Manning and Goldberg 1997, Gimenez et al. 2007, Villalobos and Antelo 2011). Mineralogical analysis indicates that the metallurgical wastes contain minor amounts of hematite (Fe2O3) and goethite (FeOOH). Additionally, it is possible that Fe-oxyhydroxides could be formed under the equilibrium pH (pH = 3.1-4.9) reached during tests. Jarosite is stable at pH <3.0 (Babcan 1971; Baron and Palmer 1996). However, as pH increases jarosite is transformed to Fe-oxyhydroxides, represented as Fe(OH)3. The reported reaction between these two phases is:
KFe3(SO4)2(OH)6 + 3H2O = 3Fe(OH)3 + K+ + 2SO42- + 3H+
Another phenomenon that may be related with As (V) removal by metallurgical wastes is the arsenate/ sulfate substitution in jarosite minerals identified in these wastes. Some authors have reported the arsenate-for-sulfate substitution in jarosite-group minerals as an important control on arsenic mobility (Foster 1998, Asta et al. 2009).
Sorption Isotherms
The sorption isotherm is of great importance in the design of sorption systems. The sorption equilibrium is described by the sorption isotherms that are characterized by certain constants whose values express the surface properties and affinity of the sorbent. In order to investigate the sorption isotherm, three equilibrium models were analyzed: Linear, Langmuir and Freundlich isotherms, described by Equations 2, 3 and 4:

where "Cs", is the amount of sorbed ion per unit weight of solid (mmol/kg); "Ce", is the equilibrium concentration on solution (mmol/L) and "Kd", is the linear distribution coefficient (L/kg). The Linear isotherm is constructed by plotting "Cs" against "Ce"; if the data lie on a straight line then the linear model can be considered appropriate.

"K" is the Langmuir bonding energy coefficient and "Qmax" (mg/kg) is the adsorption maximum capacity. The Langmuir isotherm is constructed by plotting "Ce/Cs" against "Ce"; if the data lie on a straight line then the Langmuir model can be considered appropriate. Using least squares linear regression, the parameters "Qmax" (1/slope) and "K" (slope/ intercept), may be found.

"Kf" is the Freundlich distribution coefficient related to the total sorption capacity of the solid (L/kg) and "n" is a constant relating to adsorption intensity. When n = 1, the Freundlich isotherm simplified to the Linear isotherm (Equation 2). If data conform to the Freundlich model (Equation 4), a plot of "ln (Ce)" versus "ln (Cs)" should result in a straight line. Using a least squares linear regression, the parameters "1/n" (slope) and "Kf" (intercept) may be found.
Sorption isotherms of Cd (II) on rhyolitic sediments
Sorption isotherms of Cd (II) on rhyolitic sediments exhibited similarities in shape and the amount sorbed (Fig. 1A). According to the classification proposed by Giles (1960), these isotherms may be associated with group "L" and subgroup "2", indicating that the sorption sites of the theoretical monolayer have been saturated.
The experimental results were fitted to the Linear, Langmuir, and Freundlich isotherm models to obtain the characteristic parameters of each model (Table IV). Based on the coefficients of determination (r2), the three isotherm models appear to produce a reasonable adjustment for the sorption of Cd (II) on rhyolitic sediments. However, the Langmuir model yielded the highest coefficients of determination (r2= 0.9945 ± 0.0075), while for the Freundlich and Linear models the r2 values were 0.968 ± 0.020 and 0.873 ±0.030, respectively. Figure 1B shows plots comparing the adjusted Langmuir isotherm with the experimental data. The equation shows an excellent fit with the experimental data suggesting that the results could be explained by this model. There has been reported that clays can adsorb heavy metals via ion exchange reactions and by formation of inner-sphere complexes through Si-O and Al-O groups at the clay particle edge (Celis et al. 2000).
Results shown in Table IV indicate that rhyo-litic sediments have a maximum sorption capacity (Qmax) ranging between 18.5 and 26.0 mmol/kg and sorption energy values (K) ranging between 41.1 and 86.8. The S3 has the greatest sorption capacity for Cd (II) (Qmax = 26 mmol/kg), most likely due to the presence of zeolite which was identified by XRD, in addition to the clay minerals identified in all the other rhyolitic sediment samples (Table I). It is important to mention, that values reported for maximum sorption capacity on natural pure clays are 170 mmol/kg for sepiolite and 250 mmol/kg for montmorillonite (Celis et al. 2000).
Sorption isotherms of As (III) on rhyolitic sediments
Sorption isotherms of As (III) on rhyolitic sediments exhibited similarities in shape and amount sorbed (Fig. 2A). According to the classification proposed by Giles (1960), these isotherms can be associated with the group "C" subgroup "1", indicating that sorption sites have not been saturated.
The experimental results were fitted to the Lineal, Langmuir, and Freundlich isotherm models to obtain the characteristic parameters of each model (Table IV). Based on the coefficients of determination (r2), only the Linear isotherm and Freundlich appear to produce a reasonable model for sorption of As (III) by rhyolitic sediments. However, the highest coefficients of determination (r2= 0.959 ± 0.044) were obtained with the Linear model, while with the Freundlich and Langmuir models, the r2 values were 0.939 ± 0.028 and 0.276 ±0.175, respectively. Figure 2B shows plots comparing the adjusted Linear isotherm with the experimental data, demonstrating an excellent fit and suggesting that experimental data could be explained by this model.
Results shown in Table IV indicate that the rhyolitic sediments have a Kd ranging between 1.1 and 4.5 L/kg. Sample S2 is the rhyolitic material with the best properties for As (III) retention. The ability of rhyolitic sediments for As (III) sorption is related with the presence of clay minerals, identified in these natural geological materials. In the literature is reported that As (III) adsorption by clay minerals is minimal at low pH and increases with increasing pH (Wang and Mulligan 2006).
Sorption isotherms for As (III) and As (V) on metallurgical wastes
Sorption isotherms of As (III) and As (V) on metallurgical wastes exhibited similarities in shape and amount retained (Figures 3A1 and 3B1). According to the classification proposed by Giles (1960), these isotherms may be associated with group "L" and subgroup "2", indicating that sorption sites of the theoretical monolayer have been saturated.
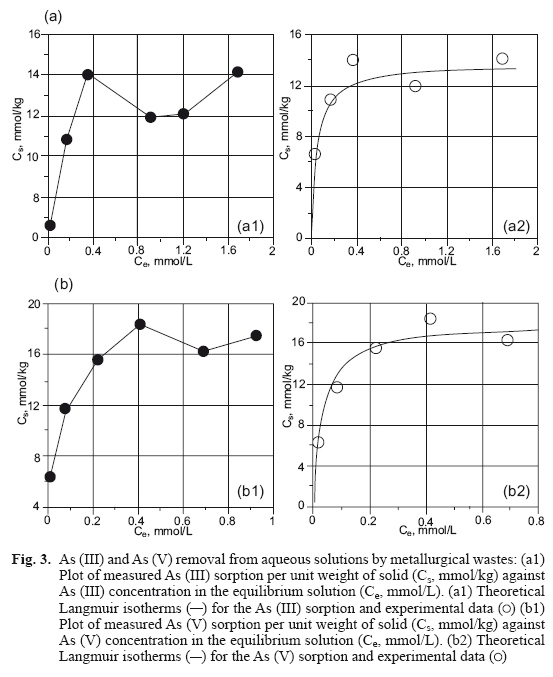
The experimental results were fitted to the same models used in the previous cases to obtain the characteristic parameters of each one (Table IV). Based on the coefficients of determination (r2), only the Langmuir model reasonably simulates the As (III) sorption by metallurgical wastes. The highest coefficients of determination (r2= 0.972) were obtained with this model, while with the Linear and Freundlich models, the r2 values were 0.414 and 0.05, respectively. In Figure 3A2 the adjustment for the Langmuir isotherm with the experimental data is compared. The equation shows an excellent fit with the experimental data for the Langmuir isotherm.
The r2 values for the sorption of As (V) by metallurgical wastes were also calculated applying the Langmuir, Freundlich and Linear models. The values obtained were 0.995, 0.898 and 0.55, respectively. These results suggest that both Langmuir and Freundlich isotherm models could explain the As (V) sorption by metallurgical wastes. However, Figure 3B2 shows an excellent fit with the experimental data for the Langmuir isotherm.
Results shown in Table IV indicate that the metallurgical wastes have a maximum sorption capacity (Qmax) of 13.6 mmol/kg for As (III) and 18 mmol/ kg for As (V) and energy values for sorption (K) of 21 y 31.8, respectively. The ability of metallurgical wastes for sorption of As (III) and As (V) is due to the presence of the jarosite and Fe-oxyhydroxides minerals because of their high affinity for these arsenic oxyanions. Pierce and Moore (1982) reported that As (V) is preferentially sorbed to Fe hydroxides between pH 4 and 7 with an optimal adsorption pH of about 4, whereas As (III) is preferentially sorbed onto Fe hydroxides between pH 7 and 10 with an optimal adsorption pH of about 7. Spectroscopic studies generally agreed that both As (III) and As (V) are specifically sorbed, forming inner-sphere complexes (Wang and Mulligan 2006). Goldberg and Johnston (2001) reported that amorphous Fe oxides has a maximum sorption capacity of 200 mmol/kg for As.
CONCLUSIONS
The results of this study indicate that the rhyolitic sediments had high removal efficiency (94-99 %) for Cd (II) in concentrations between 10-100 mg/L. However, these materials presented low removal efficiency (5-18 %) for As (III) in concentrations between 1-500 mg/L and they were not able to remove As (V) in the same concentrations range. Experimental data for the sorption of Cd (II) and As (III) were adjusted appropriately to the Langmuir model (r2= 0.9945 ± 0.0075) and the K Lineal model (r2= 0.959 ± 0.044), respectively. The efficiency of rhyolitic sediments for the removal of Cd (II) from aqueous solutions is most likely due to the presence of smectite, mica and zeolite. At the equilibrium pH (pH = 6.6-8.0) they were negatively charged, favoring the retention of metal cations such as Cd (II). The As (III) removal efficiency by these natural geological materials may be explained because at the equilibrium pH, the predominant species is an uncharged molecule (H3AsO30) which can approach to the negative surfaces of clay minerals and diffuse into the interlayer space of clays.
Metallurgical wastes had high removal ability for As (III) and As (V) in concentrations between 1-500 mg/L from aqueous solutions with an efficiency of 88 and 77 %, respectively. However, these wastes were not able to remove Cd (II). Experimental data for the sorption of As (III) and As (V) was adjusted appropriately to the Langmuir model with r2 values of 0.972 and 0.995, respectively. The high removal efficiency for As (III) and As (V) by metallurgical wastes is due to the presence of jarosite and Fe-oxyhydroxides minerals because of their high affinity for As (III) and As (V).
ACKNOWLEDGEMENTS
We express our gratitude to Industrial Minera México (IMMSA) for their financial and logistic support and CONACyT for LG-MJ PhD scholarship. The authors thank Guillermo Pérez, Heriberto Rosas, Inés Ramos Bautista, Raquel Domínguez Martínez and José Iván Morales Arredondo for their assistance in sampling, sample preparation and laboratory analysis. We also thank Teresa Pi Puig (Institute of Geology, UNAM) for XRD analyses and Jardine Wall and Alejandra Romo for the grammatical revision of this document.
REFERENCES
Appelo C.A.J. and Postma D. (2005). Geochemistry, groundwater and pollution, 2nd ed. A. A. Balkema, Leiden, The Netherlands. [ Links ]
Asta M.P., Cama J., Martinez M. and Giménez J. (2009). Arsenic removal by goethite and jarosite in acidic conditions and its environmental implications. J. Hazard. Mat. 171, 965-972. [ Links ]
Babcan J. (1971). Synthesis of Jarosite, KFe3(SO4)2(OH)6. Geol. Zb. 22 (2), 299-304. [ Links ]
Baron D. and Palmer C.D. (1996). Solubility of jarosite at 4-35°C. Geochim. Cosmochim. Acta. 60, 185-195. [ Links ]
Bickmore B.R., Rosso K.M., Tadanier C.J., Bylaskaand E.J. and Doud D. (2006). Bond-valence methods for pKa prediction. II. Bond-valence, electrostatic, molecular geometry, and solvation effects. Geochim. Cosmochim. Acta.70, 4057-4071. [ Links ]
Beesley L. and Marmiroli M. (2011). The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environ. Pollution. 159, 474-480. [ Links ]
Brewster M. (1992). Removing arsenic from contaminated waste water. Water Environ. Technol. 4, 54-57. [ Links ]
Celis R., Hermosín M.C. and Cornejo J.E. (2000). Heavy metal adsorption by functionalized clays. Environ. Sci. Technol. 34, 4593-4599. [ Links ]
Ciccu R., Ghiani M., Serci A., Fadda S., Peretti R. and Zucca A. (2003). Heavy metal immobilization in the mining-contaminated soils using various industrial wastes. Minerals Engineering. 16, 187-192. [ Links ]
Daus B., Wennrich R. and Weiss H. (2004). Sorption materials for arsenic removal from water: a comparative study. Water Res. 38, 2948-2954. [ Links ]
Foster A., Brown G., Tingle N.and Parks G. (1998). Quan-tative arsenic speciation in mine tailings using X-ray absorption spectroscopy. Am. Miner. 83, 553-568. [ Links ]
Gerente C., Andrés Y., McKay G. and LeCloirec P. (2010). Removal of arsenic(V) onto chitosan: From sorption mechanism explanation to dynamic water treatment process. Chem. Eng. J. 158, 593-598. [ Links ]
Giles C.H., McEwan T.H., Nakhawa S.N. and Smith D. (1960). Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 3973-3993. [ Links ]
Goldberg S. (2000). Competitive Adsorption of Inorganic Arsenic Species on Oxides and Clay Minerals. Preprint of extended Abstracts, vol. 40. No. 2 Symposia Paper presented before The Division of Environmental Chemistry. American Chemical Society, Washington, D.C. [ Links ]
Goldberg S. and Johnston C.T. (2001). Mechanisms of arsenic adsorption on amorphous oxides evaluated using macroscopic measurements, vibrational spectros-copy, and surface complexation modeling. J. Colloid Interface Sci. 234, 204-216 [ Links ]
Goldberg S. (2002). Competitive adsorption of arsenate and arsenite on oxides and clay minerals. Soil Sci. Soc. Am. J. 66, 413-421. [ Links ]
Gimenez J., Martínez M., De Pablo J., Rovira M. and Duro L. (2007). Arsenic sorption onto natural hematite, magnetite, and goethite. J. Hazard. Materials. 141, 575-580. [ Links ]
Iakovleva V.P. (2003). UV Spectrophotometric Studies of Arsenic (III) and Antimony (III) Aqueous Chemistry from 25 to 300 °C. A dissertation submitted to the Swiss Federal Institute of Technology Zurich. Degree of Doctor of Science. http://e-collection.library.ethz.ch/eserv/eth:27055/eth-27055-02.pdf [ Links ]
Kim Y., Kim Ch., Choy I., Rengarajaj S. and Yi J. (2004). Arsenic Removal Using Mesoporous Alumina Prepared via a Templating Method. Environ. Sci. Technol. 38, 924-931. [ Links ]
Kwon J.S., Yun S-T., Kim S-O., Mayer B. and Hutcheon I. (2005). Sorption of Zn(II) in aqueous solutions by scoria. Chemos. 60, 1416-1426. [ Links ]
Lin Y.Z. and Puls R.W. (2000). Adsorption, desorption and oxidation of arsenic affected by clay minerals and aging process. Environ. Geol. 39(7), 753-759. [ Links ]
Malferrari D.B., Brigatti M.F., Laurora A., Pini S. and Medici L. (2007). Sorption kinetics and chemical forms of Cd(II) sorbed by thiol-functionalised 2:1 clay minerals. J. Hazard. Mater. 143, 73-81. [ Links ]
Manning B. and Goldberg S. (1997). Adsorption and stability of arsenic(III) at the clay mineral-water interface. Environ. Sci. Technol. 31, 2005-2011. [ Links ]
Mohan D. and Singh K.P. (2002). Single and multicompo-nent adsorption of cadmium and zinc using activated carbon derived from bagasse-an agricultural waste. Water Res. 36, 2304-2314. [ Links ]
Mulligan C.N., Yong R.N. and Gibbs B.F. (2001). Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Eng. Geology. 60, 193-207. [ Links ]
Panuccio M.R., Sorgona A., Rizzo M. and Cacco G. (2009). Cadmium adsorption on vermiculite, zeolite and pumice: Batch experimental studies. J. Environ. Management 90, 364-374. [ Links ]
Pierce M.L. and Moore C.M. (1982). Adsorption of arsenite and arsenate on amorphous iron hydroxide, Water Res. 16, 1247-1253. [ Links ]
Rao G.P.C., Satyaveni S., Ramesh A., Seshaiah K., Murthy K.S.N. and Choudary N.V. (2006). Sorption of cadmium and zinc from aqueous solutions by zeolite 4A, zeolite 13X and bentonite. J. Environ. Management. 81, 265-272. [ Links ]
Romero F.M., Armienta M.A. and Carrillo A., (2004). Arsenic sorption by carbonate-rich aquifer material, a control on arsenic mobility at Zimapán, México. Arch. Environ. Contam.Toxicol. 47, 1-13. [ Links ]
Romero F.M., Núñez-Alvarez L., Gutiérrez M.E., Armien-ta M.A. and Ceniceros-Gómez A.E. (2010). Evaluation of the potential of indigenous calcareous shale for neutralization and removal of arsenic and heavy metals of acid mine drainage in Taxco mining area, México. Arch. Environ. Contam. Toxicol. 60, 191-203. [ Links ]
Sadiq M. (1997). Arsenic chemistry in soils: an overview of thermodynamic predictions and field observations. Water Air Soil Pollut. 93, 117-136. [ Links ]
Sadowski Z., Polowczyk I., Farbiszewska T. and Farbisze-wska-Kiczma J. (2001). Adhesion of jarosite particles to the mineral surface. Prace Naukowe Instytutu Górnictwa Politechniki Wroclawskiej. Konferencje rok: 95, 93-102 [ Links ]
Srivastava P.S., Singh B. and Angove M. (2005). Competitive adsorption behaviour of heavy metals on kaolinite. J. Colloid Interf. Sci. 290, 28-38. [ Links ]
Tiller K.G. (1989). Heavy metals in soils and their environmental significance. Adv. Soil Sci. 9; 113-142. [ Links ]
Trivedi P. and Axe L. (2001). Predicting divalent metal sorption to hydrous Al, Fe and Mn oxides. Environ. Sci. Technol. 35, 1779-1784. [ Links ]
US EPA (2006). Method 6200 and field portable X-ray fluorescence analysis for metals in soil. [ Links ]
Veli S. and Alyüz B. (2007). Adsorption of copper and zinc from aqueous solutions by using natural clays. J. Hazard. Mat. 149, 226-233. [ Links ]
Villalobos M. and Antelo J. (2011). A unified surface structural model for ferrihydrite proton charge, electrolyte binding, and arsenate adsorption. Rev. Int. Contam. Ambie. 27, 139-151. [ Links ]
Wang S. and Mulligan C.N. (2006). Occurence of arsenic contamination in Canada: sources, behaviour and distribution. Sci. Total Environ. 366, 701-721. [ Links ]














