Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista internacional de contaminación ambiental
versão impressa ISSN 0188-4999
Rev. Int. Contam. Ambient vol.28 no.2 Ciudad de México Mai. 2012
Artículos
Acute Toxicity of Ammonia on Macrobrachium tenellum (Smith) larvae
Toxicidad aguda del amonio en larvas de Macrobrachium tenellum (Smith)
Gerardo FIGUEROA–LUCERO, María Cecilia HERNÁNDEZ–RUBIO2* y Miguel de Jesús GUTIÉRREZ–LADRÓN DE GUEVARA2
1 Planta Experimental de Producción Acuícola. Departamento de Hidrobiología. DCBS. Universidad Autónoma Metropolitana–Iztapalapa. Av. San Rafael Atlixco 186, Colonia Vicentina. C. P. 09340. México, D. F.
2 Laboratorio de Hidrobiología Experimental, Depto. de Zoología, Escuela Nacional de Ciencias Biológicas, IPN. Prol. M. Carpio esq. Plan de Ayala s/n. Col. Sto. Tomás. 11340. México, D.F. Apdo. Postal 4–132. 06400. México. *Corresponding author; cecheru@yahoo.com.mx.
Recibido abril 2011,
aceptado enero 2012
ABSTRACT
The prawn shrimp Macrobrachium tenellum is a potential species for culture in México. The effect of ammonia on larvae was evaluated to provide basic information on safe levels for larviculture. A 72 h static assay was performed on 5 days old M. tenellum larvae. The nominal concentrations tested ranged from 2.89 to 185.48 mg NH4–N/L which represent 0.103 to 6.585 mg NH3–N/L at 20 g/L salinity, 28 °C and pH 7.79. LC50 for 12, 24, 48 and 72 h were 2.939 ± 0.505, 0.749 ± 0.301, 0.477 ± 0.163 and 0.409 ± 0.068 mg NH3–N/L, respectively. These results suggest that M. tenellum exhibits a slightly higher tolerance to ammonia in the zoea stage when compared to most of the prawn and shrimp species.
Keywords: river shrimp, prawn, zoea, tolerance.
RESUMEN
El langostino Macrobrachium tenellum es una especie potencial para cultivo, en México. El efecto del amonio en larvas se evaluó para proveer información básica de los niveles seguros para el cultivo en esta etapa de desarrollo. Un ensayo estático de 72 h se realizó con larvas de M. tenellum de cinco días de edad. Las concentraciones nominales probadas fueron desde 2.89 a 185 mg NH4–N/L, que equivalen a 0.103 hasta 6.585 mg NH3–N/L a 20 g/L de salinidad, 28 °C y pH 7.79. Las LC50 a 12, 24, 48 y 72 h fueron 2.939 ± 0.505, 0.749 ± 0.301, 0.477 ± 0.163 y 0.409 ± 0.068 mg NH3–N/L, respectivamente. Estos resultados sugieren que M. tenellum presenta una tolerancia ligeramente más alta al amonio en el estado de zoea que otras especies de langostinos y camarones.
Palabras clave: camarón de río, langostino, zoea, tolerancia.
INTRODUCTION
Macrobrachium tenellum (Smith) is a freshwater prawn, from the Pacific coast rivers of America. In Mexico, it is a commercially important resource, particularly in the state of Guerrero, and it is considered suitable for mass culture (Guzmán 1987) even through the capture and growth of wild postlarvae (Martínez et al. 1980).
Ammonia is the main excretory product in crustaceans (Hartenstein 1980, Cavalli et al. 2000). This is an end product of amino acid catabolism originated from excretion and organic matter decomposition. Crustaceans excrete 60–70 % of nitrogen as ammonia through their gills through passive diffusion and the rest is made up of small amounts of ammonic acid, urea and uric acid (Chen and Kou 1996). High ammonia concentrations in tanks stocked in high densities of larvae is a potential danger to aquatic organisms due to high toxicity (Chin and Chen 1987, Ostrensky and Wasielesky 1995), which may cause death or slow down prawn growth rate at sublethal levels (Wickins 1976, Armstrong et al. 1978, Daniels et al. 1992, Miranda–Filho et al. 2009). In aqueous solution, ammonia can be present in ionized (NH4+) and/or unionized (NH3) form, condition that is pH, temperature and salinity dependent.
In crustaceans, elevated ammonium concentration might produce hemolymph alcalinization as a consequence of increased internal concentration of ammonium (Campbell 1973, Chen and Kou 1993, Chen and Lin 1995, Mugnier and Justou 2004). Other reported effects are respiratory inhibition (Alcaraz et al. 1999, Malassen and Valenti 2005), reduction of osmoregulatory capacity (Young–Lai et al. 1991, Lin et al. 1993, Mugnier and Justou 2004) and reduction of survival (Mallasen and Valenti 2005, Naqvi et al. 2007, Schuler et al. 2010, Barbieri 2010, Liao et al. 2011).
In animals with gills, sensitivity to ammonia is greater during the early developmental stages, because gill surface ratio to body weight is bigger and also because the physiological detoxifying mechanisms are still immature (Rand and Petrocelli 1985).
By understanding tolerances of Macrobrachium tenellum (Smith) to ammonia its culture system can be improved to optimized survival. In this study LC50 values were obtained for larvae at various exposure times, to increase our knowledge about the water quality requirements of this species for aquaculture systems.
MATERIALS AND METHODS
A static bioassay was performed to assess the acute toxicity of ammonia (LC50 values) on M. tenellum larvae over a period of 72 h, with toxic renewal. The experiment was designed to assess the effect of different concentrations of ammonia on survival of larvae. The different concentrations of ammonia were obtained by first making a stock solution of reagent grade ammonium chloride (NH4Cl, BakerTM). Test concentrations of ammonia were then made up as total ammonia nitrogen (TAN) by measuring a specified quantity of NH4Cl, dissolving it in culture water in a volumetric flask and then making up the solution with more culture water to 5 L in a plastic container. Stock solutions with measured ammonia concentrations were then further diluted with brackish water (20 g/L, ReefsaltTM of SeachemTM) according to the individual concentrations required for each treatment. Concentrations tested ranging from 2.89 to 185.48 mg NH4–N/L (0.103, 0.206, 0.412, 0.823, 1.540, 3.292, 6.585 mg NH3–N/L). Ammonia was measured using a Hach Model DR–2000 spectrophotometer (Hach Company, Ames, Iowa, USA). The concentrations of unionized ammonia (NH3–N) were calculated according to the equations of Thurston, Khoo and Whitfield modified by Boueres (2001) based on salinity 20 g/L, water temperature 28 °C and pH 7.8. Each treatment was stocked with five days old larvae, at zoea III stage, obtained from a single ovigerous M. tenellum female reared in laboratory ponds. Three replicates of ten larvae each were used for treatment. For the assay, larvae were placed in 250 mL beakers containing 150 mL of test solution without aeration. Salinity was 20 g/L, pH 7.79 and temperature 28 °C. Larvae were fed on Artemia nauplii before and during the experiment. Food debris was removed from the beakers daily to prevent decomposition. Test solutions in the beakers was completely replaced every 24 h with new solution prepared fresh each day
Mortality of larvae was recorded after 1, 2, 3, 6, 12, 24, 48 and 72 h of exposure, following the parameters established by Armstrong et al. (1976), considering ceasing of the heartbeat as death sign for the first 24 hours, and opacity and lack of movement after 24 h.
Statistical analysis
One–way ANOVA was used to investigate the effect of ammonia concentration on survival and comparisons amongst means were made using T post hoc test (Sokal and Rholf 1981).
The reported LC50 values and 95% confidence limits were obtained on the statistical software EPA Probit Analysis Program ver. 1.5.
RESULTS
There was no mortality in control treatments during the experiment. Neither was observed any deaths, during the first six hours of exposure for all ammonia concentrations. All larvae exposed to 5.796 mg NH4–N/L (0.206 mg NH3–N/L) and 11.593 mg NH4–N/L (0.412 mg NH3–N/L) survived for 24 and 12 h, respectively.
However, exposure to total ammonia had a significant effect (ANOVA, P< 0.01) on larvae survival, causing mortality in concentrations as low as 2.898 mg NH4–N/L (0.103 mg NH3–N/L) at 48 h (Fig. 1).
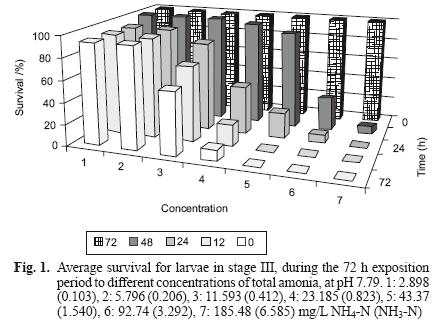
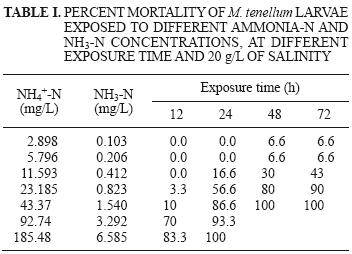
A mortality of 100 % was observed at concentrations of 43.37 mg NH4–N/L (1.540 mg NH3–N/L) and 92.74 mg NH4–N/L (3.292 mg NH3–N/L) after 48 h. and in 185.48 mg NH4–N/L (6.585 mg NH3–N/L) at 24 h (Table I).
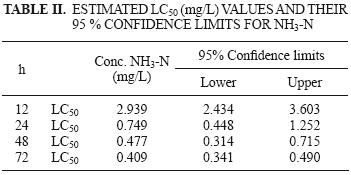
The LC50 values obtained decreased with increasing exposure time, from 75.95 mg NH4–N/L (2939 mg NH3–N/L) for 12 h, 23.98 mg NH4–N/L (0.749 mg NH3–N/L) for 24 h, 14.25 mg NH4–N/L (0.477 mg NH3–N/L) for 48 h, and 12.66 mg NH4–N/L (0.409 mg NH3–N/L) for 72 h exposure (Table II).
DISCUSSION
Previous studies have shown that ionized and unionized ammonia toxicity varies with water pH (Armstrong et. 1978), with development stage (Neil et al. 2005), and among decapods species (Allan et al. 1990), but also with temperature, salinity, atmospheric pressure and dissolved oxygen (Allan et al. 1990).
In ammonia toxicity assays with fishes, toxic concentrations are expressed as unionized ammonia only. Nevertheless, it has been shown that both forms of ammonia are toxic. At a higher pH, ammonia is predominantly in the unionized form and it is responsible for the toxicity; the opposite occurs at a low pH, when NH4 is the main form present (Armstrong et al. 1978). An increase in ammonia toxicity with increased pH has been reported in Macrobrachium rosenbergii and other crustaceans, during larval and juvenile stages (Noor–Hamid et al. 1994, Mallasen and Valenti 2005, Neil et al. 2005).
In brackish water species, salinity exerts an important effect on ammonia internal concentration. Research experiments have shown that sodium has a lower affinity than NH4 for the enzyme responsible for the active transport into the intracellular milieu. Apparently the Km values for Na+ transport are tenfold higher in marine species compared to freshwater species (Shaw 1960). Barbieri (2010) observed that Litopenaeus schmitti juveniles experienced an increase in susceptibility to TAN up 69 % as the salinity decreased from 35 g/L to 5 g/L for 96 h exposure.
The present study was performed at 20 g/L salinity (5 166 mg Na+/L), ammonia concentrations considered toxic ranged from 9.39 to 85.16 mg/L. The ratio of NH4+ to Na+ was 0.0018–0.016: 1. For Macrobrachium rosenbergii larvae (Armstrong et al. 1978), it was determined a 0.01–0.02: 1 ratio, at 12 g/L salinity. Those results agree with our results, since an increase from 12 to 20 g/L, approximately, produces a hundredfold increase in ammonia toxicity. In freshwater decapods larvae it has been determined an inverse relationship, Shaw (1960) obtained a ratio of 10:1.
Several toxic effects of ammonia on crustacean decapod larvae and adults have been reported. For M. rosenbergii larvae, development slowed down and mortality rate increased in alkaline water (pH 9) with increasing ammonia concentration and larval tolerance to high ammonia and pH levels decreased for the last zoeal stages (Mallasen and Valenti 2005). Ammonia stress has been associated with decreased haemolymph osmotic concentrations in Penaeus japonicus (Chen and Chen 1996), changes in nitrogenous excretion in M. rosenbergii adult prawns (Chen and Kou 1996), decreased survival and slowed down larval development in P. monodon (Noor–Hamid et al. 1994), changes in oxygen consumption (Alcaraz et al. 1999, Barbieri 2010) and decreased growth (Armstrong et al. 1978, Chen and Kou 1992).
Recent studies with other decapods species report that tolerance to ammonia decreases for the last zoeal stages or even in later development stages (Mallasen and Valenti 2005), but the opposite has also been found (Chin and Chen 1987, Ostrensky and Wasielesky 1995). In this experiment the tolerance to ammonia concentrations was tested on zoea III larvae in order to control for other environmental factors, i. e., larval nutritional status or damage associated to larvae handling during the rearing period.
These results suggest that M. tenellum exhibits a slightly higher tolerance to ammonia in the zoea stage when compared to most of the prawn and shrimp species complied in Ostrensky and Wasielesky (1995).
Sprague (1969, 1971) pointed out the effects of a given toxicant could be described in terms of "safe level", that can be obtained using an application factor of 0.1. According to our results, safe level would be below 0.6 mg/L for TAN and 0.075 NH3–N/L on the basis of the 24 h LC50 value at pH 7.79 and 20 g/L salinity for Macrobrachium tenellum larval rearing under controlled conditions. The results suggest TAN and unionized ammonia must be daily measured since a little increase, combined with an increase pH, could result in a high mortality.
ACKNOWLEDGMENTS
This research was partly supported by the project from Banco de Germoplasma de Recursos Genéticos Acuáticos y Fauna Silvestre: Etapa I, UAMI, partly by the project CONACyT–130200 from UAM–IPN (México) and partly by the project SIP–20111206 (Secretaría de Investigación y Postgrado, IPN, México).
REFERENCES
Alcaraz G., Espinoza V. and Vanegas C. (1999). Acute effect of ammonia and nitrite on respiration of Penaeus setiferus postlarvae under different oxigen levels. J. W. Aqua. Soc. 30, 98–106. [ Links ]
Allan G.L., Maguire G.B. and Hopkins S.J. (1990). Acute and chronic toxicity of ammonia to juvenile Metapenaeus macleayi and Penaeus monodon and the influence of low dissolved oxygen levels. Aquaculture 91, 265–280. [ Links ]
Armstrong D.A., Stephenson M.J. and Knight A.W. (1976). Acute toxicity of nitrite to larvae of the giant Malaysian prawn, Macrobrachium rosenbergii. Aquaculture 9, 39–46. [ Links ]
Armstrong D.A., Chippendale D., Knight A. W. and Colt J. E. (1978). Interaction of ionized and un–ionized ammonia on short–term survival and growth of prawn larvae, Macrobrachium rosenbergii. Biol. Bull., 154, 15–31. [ Links ]
Barbieri E. (2010). Acute toxicity of ammonia in white shrimp (Litopenaeus schmitti) (Burkenroad, 1936, Crustacea) at different salinity levels. Aquaculture 306, 329–333. [ Links ]
Boueres C.S. (2001). Calculation on un–ionized NH3 in saline waters. Apendix III. Chemistry Lab. Meth. Manual, Tallahassee. Flo. Dep. of Env. Protection. USA. [ Links ]
Campbell J.W. (1973). Nitrogen excretion. In: Comparative animal physiology. (C. L. Prosser, Ed.) W. B. Saunders Co., Philadelphia. USA, pp. 279–316 [ Links ]
Cavalli R.O., Vanden B.E. Lavens P., Thuy N.T.T., Wille M. and Sorgeloos P. (2000). Ammonia toxicity as a criterion for the evaluation of larval quality in the prawn Macrobrachium rosenbergii. Comp. Biochem. Endo. 125, 333–343. [ Links ]
Chen J.C. and Kou Y.Z. (1992). Effects of ammonia on growth and molting of Penaeus japonicus juvenile. In: Abstracts, Aquaculture '92 Marriots Orlando Center, Orlando, FL. [ Links ]
Chen J.C. and Chen C.T. (1996). Changes of osmotic and electrolyte concentrations in the haemolymph of Penaeus japonicus exposed to ambient ammonia. Comp. Biochem. Physiol. 114C, 35–38. [ Links ]
Chen J.C. and Kou Y.Z. (1993). Accumulation of ammonia in the haemolymph of Penaeus monodon juveniles. Comp. Biochem. Physiol. 101C, 453–458. [ Links ]
Chen J.C. and Kou C.T. (1996). Nitrogenous excretion in Macrobrachium rosenbergii at different pH levels. Aquaculture 144, 155–164. [ Links ]
Chin T.S. and Chen J.C. (1987). Acute toxicity of ammonia to larvae of the tiger prawn, Penaeus monodon. Aquaculture 66, 247–253. [ Links ]
Daniels W.H., D'abramo L.R., and Parseval L. (1992). Design and management of a closed, recirculating "clearwater" hatchery system for freshwater prawns, Macrobrachum rosenbergii De Man, 1879. J. Shell. Res. 11, 65–73. [ Links ]
Guzmán M. (1987). Biología, ecología y pesca del langostino Macrobrachium tenellum (Smith, 1871), en lagunas costeras del estado de Guerrero, México. Tesis de Doctorado en Ciencias del Mar. Instituto de Ciencias del Mar y Limnología, Colegio de Ciencias y Humanidades. UNAM. México. 306 p. [ Links ]
Jayasankar P. and Muthu M. F. (1983). Toxicity of ammonia to the larvae of Penaeus indicus H. Milne Edwards. Indian J. Fish., 30, 1–12. [ Links ]
Harstenstein R. (1970). Nitrogen metabolism in non–insect arthropods. In:. Comparative biochemistry of nitrogen metabolism. I. The invertebrates. (J.W–. Campbell, Ed.). Academic Press. New York. 299–372 [ Links ]
Liao Y.Y., Wang H.H. and Lin Z.G. (2011). Effect of ammonia and nitrite on vigour, survival rate, moulting rate of the blue swimming crab Portunus pelagicus zoea. Aquacult. Int. 19, 339–350. [ Links ]
Lin H.P., Thuet P., Trilles J.P., Mounet–Guillaume R. and Charmantier G. (1993). Effects of ammonia on survival and osmoregulation of various development stages of the shrimp Penaeus japonicus. Marine Biology 117, 591–598. [ Links ]
Mallasen M. and Valenti W.C. (2005). Larval development of the giant river prawn Macrobrachium rosenbergii at different ammonia concentrations and pH values. J. W. Aqua. Soc. 36, 32–41. [ Links ]
Martínez C., Chávez C. and Palomo G. (1980). Avances sobre el semicultivo del langostino Macrobrachium tenellum (Smith). "Memorias". Segundo Simposio Latinoamericano de Acuicultura, Departamento de Pesca, México. pp. 643–662. [ Links ]
Miranda–Filho K.C., Leaes Pinho G.L., Wasielesy W. Jr. and Bianchini A. (2009). Long term ammonia toxicity to the pink–shrimp Farfantepeneaeus paulensis. Comp. Biochem. Physiol. Part C 150, 377–382. [ Links ]
Mugnier C. and Justou C. (2004). Combined effect of external ammonia and molt stage on the blue shrimp Litopenaeus stylirostris physiological response. J. Exp. Mar. Biol. Ecol. 309, 35–46. [ Links ]
Naqvi A.A., Adhikari S., Pillai B.R. and Sarangi N. (2007). Effect of ammonia–N on growth and feeding of juvenile Macrobrachium rosenbergii (De–Man). Aquac. Res. 38, 847–851. [ Links ]
Neil L.L., Fotedar R. and Shelley C.C. (2005). Effects of acute and chronic toxicity of unionized ammonia on mud crab, Sylllla serrata (Forsskal, 1755) larvae. Aquac. Res. 36, 927–932. [ Links ]
Noor–Hamid S.R., Fortes R.D. and Parado–Estepa F. (1994). Effect of pH and ammonia on survival and growth of the early larval satges of Penaeus monodon Fabricius. Aquaculture 125, 67–72. [ Links ]
Ostrensky A. and Wasielesky Jr., W. (1995). Acute toxicity of ammonia to various life stages of the Sao Paulo shrimp, Penaeus paulensis Perez–Farfante, 1967. Aquaculture 132, 339–347. [ Links ]
Rand G. M and Petrocelli S.R. (1985). Fundamentals of aquatic toxicology. Hemisphere Publ. Washington, D.C. USA. [ Links ]
Regnault M. (1987). Nitrogen excretion in marine and freshwater crustacean. Biol. Rev. 62, 1–24. [ Links ]
Shaw J. (1960). The absorption of sodium ions by the crayfish Astacus pallipes Lereboullet. III. The effect of other cations in the external solution. J. Exp. Biol., 37, 548–556. [ Links ]
Shuler, D. J., Boardman, G. D., Kuhn, D. D. and Flick, G. J. (2010). Acute toxicity of ammonia and nitrite to Pacific White Shrimp, Litopenaeus vannamei, at low salinities. J. World Aquacult. Soc. 41, p. 438–446. [ Links ]
Sprague J.B. (1969). Measurement of pollutant toxicity to fish. 1. Bioassay methods for acute toxicity. Water Res., 3, 793–821. [ Links ]
Sprague J.B. (1971). Measurement of pollutant toxicity to fish. III. Sublethal effects and "safe" concentrations. Water Res. 5, 245–266. [ Links ]
Stirling H.P. (1985). Chemical and biological methods of water analysis for aquaculturists. Inst. of Aqua. Univ. Stirling. Great Britain, 119 p. [ Links ]
Sokal R. and Rohlf J. (1981). Biometry. W. H. Freeman San Francisco, 208–262. [ Links ]
Wickins J.F. (1976). The tolerance of warm–water prawns to recirculated water. Aquaculture 9, 19–37. [ Links ]
Young–Lai W.W., Charmantier–Daures M. and Charmantier G. (1991). Effect of ammonia on survival and osmoregulation in different life stages of the lobster Homarus americanus. Marine Biol. 110, 293–300. [ Links ]














