Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista internacional de contaminación ambiental
versión impresa ISSN 0188-4999
Rev. Int. Contam. Ambient vol.27 no.1 Ciudad de México feb. 2011
Utilization of by–products from the tequila industry. Part 10. Characterization of different decomposition stages of Agave tequilana Webber bagasse using FTIR spectroscopy, thermogravimetric analysis and scanning electron microscopy
Utilización de subproductos de la industria tequilera. Parte 10. Caracterización de diferentes etapas de descomposición del bagazo de Agave tequilana Webber usando espectroscopía FTIR, análisis termogravimétrico y microscopía electrónica de barrido
Gilberto Íñiguez1*, Alex Valadez2, Ricardo Manríquez1 and María V. Moreno2
1 Universidad de Guadalajara, Departamento de madera, celulosa y papel, km 15.5 carretera Guadalajara–Nogales, Las Agujas, Mpio. de Zapopan, Jalisco, Apartado Postal 52–93, C.P. 45020, Guadalajara, Jalisco, México
2 Unidad de Materiales, Centro de Investigación Científica de Yucatán, A.C. Calle 43 # 130, Chuburná de Hidalgo, Mérida Yucatán Mexico, C.P. 97200
Recibido abril 2010
Aceptado enero 2011
ABSTRACT
Composting evolution of two different agave bagasse provided by two tequila factories was monitored at 0, 28, 56, 84, 112 and 126 days using Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA) and scanning electron microscopy (SEM) to assess their degree of decomposition. Stages of decomposition were assessed using their IR spectral pattern since the characteristic bands undergo changes during the biological treatment of the agave bagasse. Additionally, the samples were analyzed using the temperature range from 40 to 600 ºC in nitrogen atmosphere in order to assess the changes. Agave bagasses (without composting) had the higher mass loss percentage in TGA and these losses diminished as the composting process progressed. The DTG curves showed two peaks which can be attributed to the degradation of the hemicelluloses and cellulose. Scanning electronic microscope observations showed significant changes in the structure of the vascular bundle in agave bagasse samples after 126 days of composting and massive fungal invasion that led to the cracking of the fiber. Weakening the structure of the vascular bundles of the agave bagasse by composting can improve water retention capacity of bagasse that is to be used as a substrate for greenhouse vegetable production.
Key words: composting evolution, tequila residues, biodegradation.
RESUMEN
En este trabajo se estudió la evolución durante el compostaje de diferentes bagazos de agave proveniente de dos fabricas de tequila, al tomar muestras los días 0, 28, 56, 84, 112 y 126, para valorar su grado de descomposición mediante espectroscopía infrarroja de transformadas de Fourier, análisis termogravimétrico (TGV) y microscopía electrónica de barrido. Los estados de descomposición fueron valorados teniendo como referencia el espectro patrón en infrarrojo ya que las bandas características del bagazo de agave presentaron cambios durante el tratamiento biológico. Adicionalmente las muestras fueron sometidas al análisis termogravimétrico de 40 a 600 ºC en atmósfera de nitrógeno para valorar los cambios de descomposición por temperatura. Las muestras de bagazo de agave sin compostaje tuvieron en el análisis TGV el porcentaje más alto de pérdida de masa, pérdidas que fueron disminuyendo conforme avanzó el proceso de compostaje. Las curvas derivadas del análisis TGV mostraron dos picos que se atribuyeron a la degradación de la hemicelulosa y celulosa. Observaciones en el microscopio electrónico de barrido, mostraron cambios significativos en la estructura del haz vascular en las muestras de bagazo de agave después de 126 días de compostaje y la invasión masiva de hongos que llevó a desquebrajamiento de la fibra. Debilitando la estructura del haz vascular del bagazo de agave mediante el compostaje puede mejorar la capacidad de retención de agua del bagazo, para ser utilizado como sustrato en la producción de hortalizas en invernadero.
Palabras clave: evolución del compostaje, residuos del tequila, biodegradación.
INTRODUCTION
Agave bagasse is the residual fiber remaining after cooked agave heads are shredded, milled and the sugar water–extracted. The bagasse is primarily the rind and fibrovascular bundles dispersed throughout the interior of the agave head. It represents about 40 % of the total weight of the milled agave on a wet weight basis. Bagasse is available all year in only two main regions of the tequila producing areas in México: the Tequila region and the Jalisco Highlands. In recent years, the tequila market has grown and gained international recognition. Market growth is expected to continue due to the recently recognized "tequila origin denomination" by the European Union, which means more tequila will be produced with a substantial improvement of the process and tequila quality. This will mean even more bagasse, increasing the disposal problems for the tequila companies.
In recent years, the largest tequila companies have adopted the composting process as the only way to manage and dispose of agave bagasse. The composting process has been carried out empirically, by trial and error. In order to optimize the process and improve utilization of the composting product, it is important to generate knowledge about the physical and chemical transformations undergone by the bagasse during composting, either as compost for soil improvement or as a substrate for greenhouses.
The stabilization of organic waste matter is a crucial requirement before being used as compost or substrate for plant growth. The quality of compost mainly depends on the level of organic matter stability (Wu et al. 2000). Application of non–stabilized organic material soils could affect both crop growth and the environment because of the presence of phytotoxic compounds (Butler et al. 2001). During composting, the most biodegradable organic compounds are degraded and partly converted into humic–like substances (Hsu and Lo 1999, Sánchez Monedero et al. 1999, Wu and Ma 2002). Several indexes and methods have been proposed for evaluating compost stability (Bernal et al. 1998, Itävaara et al. 2002, Wu et al. 2000, Wu and Ma 2001). However, to date, there is no single method that can be successfully used alone for characterizing composts from different organic residues (Barberis and Nappi 1996, Chen et al. 1996, Itävaara et al. 2002) due to the widely different chemical characteristics of organic wastes. The utilization of different parameters and indexes that focus on the different properties provided by composting materials can give a more complete picture of the degree of transformation achieved by the organic materials. The composition of the organic matter in waste materials is very complex due to the wide range of chemical compounds and the variety of decomposed and synthesized products. Organic matter and inorganic compounds can build up organic–mineral complexes. The separation of organic substances is not possible without chemical changes. New analytical methods and established methods with new applications provide insight into the entire sample of material and its chemical properties, contributing to a better understanding of the decomposition and stabilization processes taking place. Spectroscopic techniques, including Fourier transform infrared (FTIR) spectroscopy and thermogravimetric analysis (TGA), are among the more promising tools for characterizing the chemical transformation of organic matter. Infrared spectroscopy is based on the interaction of infrared light with matter and is sensitive to the presence of chemical functional groups (Hesse et al. 1995, Smith 1999). Humic substances originating from composts were characterized by Chen et al. (1996), Filip et al. (2000), and Zach and Schwanninger (1999). FTIR spectra revealed the transformation of organic matter during a composting process (Chen and Inbar 1993). FTIR spectroscopy was applied for assessing compost maturity (Smidt et al. 2002). Thermogravimetry is a technique in which the weight change is measured during the incremental heating of the sample. The first derivative of the TG trace (DTG) permits a better resolution: it does not contain any new information. However, it clearly identifies the temperatures at which mass loss is at a maximum, as well as superimposed transformations appear more clearly as DTG peaks. Thermogravimetry and scanning calorimetry (DSC) were used to assess compost stability and maturity (Blanco and Almendros 1994, 1997, Dell'Abate et al. 1998, 2000, Dell'Abate and Tittarelli 2002).
The basic principle of the scanning electron microscope (SEM) is to scan the specimen with a finely focused electron beam of keV energy. The electrons interact with the atoms of the material and produce signals containing information about the sample's surface topography, composition and other properties. SEM has been used, for instance, for the analyses of microbial matrix formation on both the surface and the inside of a clay residue used during composting (Jolanun and Towprayoon 2010) and for the analyses of formation of struvite crystals in a simulated food waste aerobic composting process (DU Xian–yuan et al. 2010).
The aim of this work is to investigate the structural transformations and thermal characterization of compost samples from two different agave bagasse residues at different stages of composting, combining FTIR spectroscopy, TGA and SEM, in order to find out the applicability of these methods in operational processes under realistic conditions.
METHODS
Materials and pretreatment
Agave bagasse, supplied by two tequila factories, La Codradía and La Regional, were used for the characterization of different composting stages where the principal difference between bagasses was the fermentable sugars extraction system. Compost samples of the La Regional tequila factory originated from an agave bagasse obtained as follows: agave heads of tequilana Weber plants were cooked for nine hours in a steel autoclave, cooled and shredded. The shredder had a set of horizontally aligned wedge–shaped cutters arranged in the form of an arrow head, moving against another fixed set of identical cutters. The shredded material passed through a series of four follow–up operations for washing and pressure extraction of the fermentable sugars. Compost samples of the La Cofradía tequila factory originated from an agave bagasse obtained as follows: agave heads of tequilana Weber plants were cooked for 36 hours in brick ovens, cooled and cut in a shredder similar to that described above. The shredded material passed through two fiber–pith separators consisting of horizontal stainless steel cylinders with a central shaft equipped with several sets of blades arranged perpendicular to each other to facilitate pith detachment and to ensure adequate movement of the shredded material through the cylinder. Through valves at the top of the cylinder, water is injected for washing the material. At the bottom, sieves allow the juices and the detached pith to be collected and fermented. The follow–up treatment was similar to that at the La Regional tequila factory.
Agavae bagasse, the organic fibrous byproduct emanating from both processes, was subsequently composted for 126 days and sampled at defined time intervals, and analyzed by scanning electron microscopy, FTIR and thermogravimetric analyses.
Composting conditions
The composting process of La Cofradía and La Regional agave bagasse has been reported by Íñiguez et al. (2009). These two sources of bagasse were composted for 126 days in four piles, two for each bagasse source. Ammonium nitrate (NH4NO3) was used as nitrogen source to adjust the agave bagasse C:N ratio to 25:1, to favor the fibrous residue biodegradation (Willson 1989, Rynk 1992). Every week, the piles were moved to facilitate aeration and added with water as needed to keep water content of about 40–65 %, recommended for proper composting (Rynk 1992). The maximum temperatures of the piles reached on day 23 were 59 ºC and 60 ºC for La Cofradía and La regional bagasse respectively. After 126 days of composting, both bagasses had an earthy smell with a dark brown color.
Sampling and sample preparation
During the composting process, compost samples were taken on days 0, 28, 56, 84, 112 and 126. On every sampling date, nine samples were taken, three near the bottom, three at the middle and three near the top of each composting pile. These samples were mixed into a composite sample which was air–dried for further chemical and physical analyses.
Analytical methods (measurement parameters)
Thermogravimetry
Thermogravimetric analysis (TGA) was carried out in order to evaluate the thermal stability of the samples using a thermal analyzer Perkin Elmer model TGA 7. In order to obtain good reproducibility the material was ground with a pestle and an average of approximately 7 mg of each sample was used. The samples were heated from 40 to 600 ºC with a heating rate of 10 ºC min—1 under nitrogen atmosphere with a nitrogen flow rate of 20 mL min—1.The weight loss and its derivative (DTG) as a function of temperature were recorded.
FTIR spectroscopic investigations
For spectroscopic investigations, the material was ground with a pestle in order to obtain good reproducibility of the recorded spectra. Two milligrams of the sample were mixed with 200 mg KBr (FT–IR grade) and pressed into a pellet. The pellet was immediately measured after preparation in the spectrometer using the transmission mode. The measurements were carried out in the mid–infrared range 4000 cm–1 to 400 cm–1 with a Nicolet model Protege 460 Magna FTIR spectrometer equipped with OMNIC software. The resolution was set to 4 cm—1; 100 scans were recorded, averaged for each spectrum, and corrected against ambient air as background.
Scanning electron microscopy (SEM)
The morphology of the composted bagasse samples were observed using a scanning electron microscope JEOL JSM 6360 operated at 20 keV. The samples were coated with gold prior the analysis.
2.4.4. Chemical determinations
The pH and conductivity were measured on aqueous extracts of 1g of each sample treated with 50 mL of distilled water CWMI (1976). Ash, moisture and total Kjeldahl nitrogen (TKN) were determined as described in the Standard Methods for the Examination of Water and Wastewater (APHA 1985). Total organic carbon (TOC) content was calculated by the following equation (Golueke 1977): % TOC = (100 — % ash residue)/1.8. Ash residue was measured after ignition at 550 ºC for 2 hours in a muffle furnace. The C:N ratio was computed on the basis of these analyses. Total Na, Cu, Be, Al, Ba, Cd, Ca, Cr, Pb, Co, Fe, Mg, Mn, Mo, Ni, P, K, Ag, Zn, Sb, Tl and V were measured by ICP–AES after digestion with an aqua regia procedure according to TMECC (2001).
RESULTS AND DISCUSSION
Characterization using FTIR spectroscopy
The most important bands found in the spectra of the original agave bagasses and their usual behavior during degradation are compiled in table I. The arrows indicate the development of bands during the composting process. In figures 1 and 2 a great resemblance can be observed between the four FTIR spectra for the original agave bagasse collected from the tequila factories La Regional (pile 1 and pile 2) and La Cofradía (pile 1 and pile 2) and their resulted composts. Changes in the IR spectra of each sample represent different stages of composting process (0, 28, 56, 84, 112 and 126 days). A broad band centered at 3400 cm—1 is due to the stretching vibrations of bonded and non–bonded hydroxyl groups as well as water in the samples. This band was more marked before initiating the composting period. At 28 days and after this time, the OH band tends to decrease and broaden, indicating that an environmental change in the groups has been initiated according to the composting mechanism (Smidt and Meissl 2007). At zero time (before composting) figures1 and 2 are practically similar for the two samples of agave bagasse (La Cofradía pile 1 and pile 2, La Regional pile 1 and pile 2). The results are not surprising given that the two bagasse samples have their origin from the same variety of agave plant (Agave tequilana Weber blue). The fermentable sugars extraction system is the principal difference between the two lignocelullosic residues, as mentioned in materials and methods. However, despite this difference, their chemical composition is basically the same, differing only quantitatively; for example, their cellulose, hemicellulose and acid detergent lignin content (Íñiguez et al. 2011). The decrease of the C–H stretching bands corresponding to aliphatic methylene bands at 2920 and 2850 cm—1 can be an indicator of the degradation process. These aliphatic methylene groups are part of many organic molecules present in the samples. Their degradability varies in a wide range. Mineralization, volatilization or transformation of easily degradable molecules and metabolites cause the most significant decrease of these bands within the first weeks. The decrease of the aliphatic methylene bands were more marked in the spectra of the sample La Regional pile 1 in comparison with La Regional pile 2 (see Fig. 2). Nevertheless, according to these bands, the composting process was more homogeneous for the La Cofradía agave bagasse since the aliphatic methylene bands for pile 1 and 2 samples were very similar (Fig. 1). The weak band at 1740 cm—1 is caused by hemicellulose; this indicates the C=O stretch in non–conjugated ketones, carbonyls and in ester groups (Owen and Thomas 1989, Hergert 1971, Bodîrlău and Teacá 2009). It appears as a shoulder in the fresh La Regional and La Cofradía agave bagasse samples (pile 1 and 2). However, it disappeared completely after 84 days of composting in the La Regional bagasse indicating decomposition of those early metabolic products. In contrast, in La Cofradía samples this band did not disappear completely. This phenomenon probably was due to the different composting conditions between both bagasses. Unfortunately, a microbiological analysis of both bagasses (La Cofradía and La Regional) was not carried out. The band at 1640 cm—1 (Figs. 1 and 2) that is also attributed to the C=O stretching vibration, is due to carboxylates (Smith 1999), amides I (Naumann et al. 1996, Smith 1999) and to the C=C stretching vibration of alkenes and aromatic rings (Smith 1999, Hesse et al. 1995, Nanny and Ratasuk 2002) showing a divergent behavior. It means that this band represents both synthesis and decomposition in the same composting process. Formation of carboxylates due to the release of carboxylic acids from decomposed lipids contributes to the rise as well. When degradation of substances that absorb at this wavenumber exceeds synthesis, the band height decreases. Aromatic skeletal vibration from lignin and lignocellulose absorbs at 1510–1520 cm—1 (Faix 1991). Biowaste materials are identified by this weak but characteristic band. Despite its small size, it is hardly overlapped by other bands and, therefore, is an indicator for biogenic waste of the agave bagasse and its composts. On the other hand, the development of the band at 1510–1520 cm—1 after 28 (La Cofradía pile 1, La Regional pile 1), 56 (La Cofradía pile 2) and 84 days (La Regional pile 2) of composting was probably due to the amide formation (overlapped amide II band formed at 1546 cm—1 (Grube et al. 1999, Smith 1999). These amide compounds can be promoted by the addition of ammonium nitrate (as a nitrogen source) to the agave bagasse at the beginning of the composting in order to adjust the C:N rate to 30:1. The added nitrate is visible as a narrow and constant band height at 1384 cm—1 (Figs.1 and 2) in all samples after the 28 day of composting (Smidt et al. 2002, Smith 1999, Zaccheo et al. 2002). Moreover this band seems to remain unaffected or with small changes during the composting process. This fact can explain the weak presence of amide II observed at 1546 cm—1. The C–N band of aromatic amines at 1320 cm—1 (Smith 1999) observed in both figures 1 and 2 were shown clearly at 28, 56, 84, 112 and 126 days of composting as the result of the microbial activity. The C–O–C and C–O vibrations of polysaccharides content in the initial agave bagasse samples are found in the region between 1200 cm—1 and 900 cm—1 (Tan 1993, Grube et al. 1999). At the 28th day of composting, this band (Figs. 1 and 2) tended to flatten and lengthen, indicating polysaccharides started being assimilated by the microorganisms present in the compost which increases the temperature in the pile. Furthermore, the weak band (shoulder) at 1160 cm—1 and 1080 cm—1 (at the beginning of composting, figure 1 and 2) can also be assigned to polysaccharides as reported by Grube et al. (1999), who found absorption bands of glycogen at these IR frequencies. This finding was confirmed by the addition of starch or polysaccharides containing components (miscanthus, straw) to the waste material (Smidt 2001). Finally, the band at 1080 cm—1 can be attributed to aromatic C–H bending the plane (Bodîrlău and Teacá 2009).
Characterization using thermogravimetric analysis
Figures 3(a) and 4(a) show the thermograms for parent and composted La Cofradía and La Regional agave bagasse samples (piles 1 and 2) after 0, 28, 56, 84, 112 and 126 days of composting. These figures show the percentage of residual weight (percentage of total sample weight) as a function of temperature. It can be seen that both piles (pile 1 and 2) of both kinds of parent agave bagasses undergo a major mass percentage of weight loss in the range of 50–600 ºC. It is also evident that, as the number of composting days increase, the total weight loss in the range 50–600 ºC decreases steadily; i.e., the residual weight at 600 ºC increases monotonically. Several authors have pointed out that this behavior is consistent with the compost stabilization process (Dell'Abate et al. 1998, Melis and Castaldi 2004, Gómez et al. 2007, Droussi et al. 2009), Som et al. 2009, Tsui and Juang 2010. Finally, the classical dehydration process (Wielage et al. 1999, Wang et al. 2007, Carballo et al. 2008, Korosec et al. 2009) is observed in all curves in the range 50–150 ºC; which represents around 3 to 5 % of the total weight of the samples. The derivative thermogravimetry (DTG) curves are shown in figures 3(b) and 4(b) for parent and composted La Cofradía and La Regional agave bagasses (piles 1 and 2) at 0, 28, 56, 84, 112 and 126 days of composting. In the DTG profiles, two principal ranges can be distinguished between 150 and 600 ºC, which indicate the highest losses of organic matter. The first range lies between 150 and 400 ºC and the second one appears between 400 and 600 ºC. The peaks in the first range can be attributed to the pyrolysis of carbohydrates such as hemicelluloses and cellulose, whereas the peak in the range between 400 and 600 ºC are due to lignin pyrolysis (Grandmaison et al. 1987, Aggarwal et al. 1997, Sun et al. 1998, Álvarez et al. 2005, Mohan et al. 2006, Yang et al. 2007, Kumar et al. 2008, Luangkiattikhun et al. 2008, Cagnon et al. 2009). These are the main components present in the agave tequilana bagasses. It is evident from figures 3 and 4 that, as the composting time increases, the thermal behavior of the samples taken from the several piles changes as a result of the composting process. Table II summarizes the weight loss (% of total sample) corresponding to the 150–400 ºC and 400–600 ºC ranges and content (%) of sample char residue at 600 ºC obtained from the thermograms of figures 3(a) and 4(a). It can be seen that, as the composting time increases, the weight losses in the carbohydrates thermal decomposition range decrease steadily, whereas the losses in the lignin range remain practically constant. On the other hand, the char yield also increases with composting time. According to the literature this trend suggests a progressive transformation of the biomass in the macromolecules known as humified matter. Thus we obtain the increase in molecular weight, stability, and aromatization degree during the composting process (Sharma 1990, Dell'Abate et al. 1998, Dell'Abate et al. 2000, Li et al. 2001, Ranalli et al. 2001, Zaccheo et al. 2002, Melis and Castaldi 2004, Dresboll and Magid 2006, Spaccini and Piccolo 2007, 2009, Carballo et al. 2008, Droussi et al. 2009, Som et al. 2009).
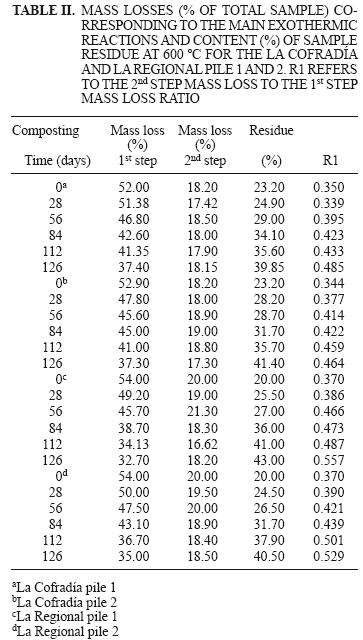
In order to evaluate the composting process, we can use the ratio between the weight loss associated with the first and the second degradation steps (R1). The R1 was previously identified as a reliable parameter for evaluating the level of maturation of organic matter in composts (Dell'Abate et al. 2000, Mondini et al. 2003, Marhuenda–Egea et al. 2007). This value indicates the relative amount of the most thermally stable fraction of the organic matter with respect to the stable one. The R1 ratio increases during composting (Table II) thus revealing the high sensitivity of this parameter to the chemical changes induced by the bio–transformation of organic materials. The variation in the R1 ratio and char residues (%) during the composting process are similar for pile 1 and pile 2 of each parent bagasse; however, it can be seen that the apparent stability of the compost obtained from the La Regional bagasse is slightly higher than the La Cofradía one.
Characterization using scanning electron microscopy
Scanning electron microscopy (SEM) micrographs of uncomposted (day 0) and composted (day 126) La Cofradía and La Regional agave bagasse fibers are shown in figures 5 and 6. Both fibers (Figs. 5a and 6a) show the characteristic surface topography reported in the literature for hard fibers like sisal (Barkakaty 1976) and henequén (Valadez–González et al. 1999). Figure 5a shows intact vascular bundles at the beginning of the composting process covered by parenchyma cells which are highlighted by a white contour. In figure 5b a still intact vascular bundle is shown at the end of 126 days of composting process, covered by lightly degraded parenchyma cells showing pitted cell walls (white contour), fungal hyphae and exposed fibers. Figure 5b shows the presence of fungal hyphae and spores at the surface of partially degraded parenchyma cells with strongly pitted and locally cracked cell walls. Figure 6a, as in figure 5a, shows intact vascular bundles at the beginning of the composting process covered by parenchyma cells of different shape (see white contour). Figure 6b presents vascular bundles at the end of the 126 composting period showing degraded surface, fungal hyphae and exposed vascular and fibrous tissue. Figure 6c shows a magnified area indicated in figure 6b that shows an exposed vessel with spiral thickening, fractures and breakdown areas of cell walls, and fungal hyphae.
Table III presents some chemical characteristics of the composts obtained of the agave bagasse from the tequila factories La Cofradía and La Regional. Both composts presented low pH values (5.5 and 5.8) in comparison to other composts. For instance Íñiguez et al. (2005) reported pH values of 9.2, 9.1, 9.1 and 9.1 for composts prepared with uncooked agave bagasse, uncooked agave bagasse composted with tequila vinasses, uncooked agave bagasse enriched with urea, and uncooked agave bagasse enriched with urea composted with tequila vinasses. For the C:N ratio it can be concluded both studied composts were mature composts. Harada et al. (1981) concluded that a mature compost product should have a C:N ratio of less than 20:1. (Before composting (0 day) the C:N ratio was 25:1 for both agave bagasse) (Íñiguez et al. 2011). Practically in all the chemical elements analyzed, except for Na and Be, the elements concentration was higher for compost produced with La Regional agave bagasse that compost produced with La Cofradía agave bagasse. This is due to the different methods of obtaining the agave bagasse described in the methods section where there is a very marked difference in the extraction system of fermentable sugars. This marked difference refers to a fibrous material and pith content of 65.6 % and 34.4 % respectively for the La Cofradía agave bagasse, whereas for the La Regional agave bagasse, the fibrous material and pith content were 43.7 % and 56.3 % respectively after passing the agave bagasse samples through a sieve with an opening of 1cm (wet basis analysis) (Íñiguez et al. 2010). On the other hand, Íñiguez et al. (2010) reported different contents of hemicelulose, cellulose, and acid detergent lignin (ADL) content for both bagasses before composting. For La Cofradía agave bagasse, the hemicelulose, cellulose and ADL content were 7.9, 14.2 and 32.3 % respectively, whereas La Regional agave bagasse, the hemicelulose, cellulose and ADL content was 14.8, 14.1 and 28.8 % respectively.
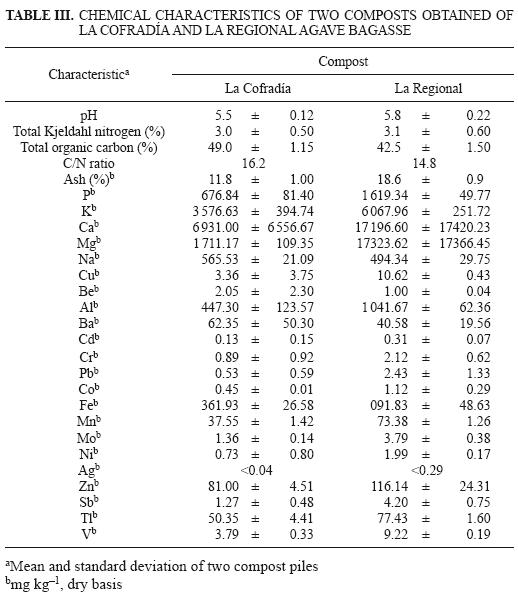
Of all potential quality standards in compost, that of heavy metals has been the focus of most attention. In the case of heavy metals (Cu, Cd, Cr, Pb, Ni, Zn) in the compost of La Cofradía and La Regional, no problems are expected in the application of these composts in agricultural soils according to heavy metals limits (mg/kg) for some European countries and the USA. For instance, the heavy metals limits in the USA for Cd, Cr, Cu, Hg, Ni, Pb and Zn are 0.7–10, 70–200, 70–600, 0.7–10, 20–200, and 70–1.000 and 210–4.000, respectively (Brinton 2000).
CONCLUSIONS
The results showed that IR spectroscopy as well as TG/DTG analysis gave complementary information on the chemical change composition of the initial and partial composted agave bagasse materials. Furthermore, both techniques have proven to be appropriate tools for assessing the degree of stabilization obtained by organic matter under aerobic treatment. Although minimal changes were observed by the FTIR analysis concerning the chemical composition of La Regional and La Cofradia bagasses samples during the composting process, environmental changes are clearly detected in early stages, such as 28 days. Moreover, in all samples the presence of some metabolic products such as aldehydes, ketones and amides during intermedia stages was observed. In addition, some of those compounds were lost in the last stages as a result of progressive biodegradation of the organic matter. On other hand, it was found that agave bagasse (without composting) had the higher mass percentage loss in TGA and these losses diminished as the composting process progressed. DTG and FTIR results agreed in that agave bagasse samples are principally composed of hemicelluloses, cellulose and lignin. In particular, evaluation of TG/DTG curves allowed for clearly distinguishing the stages of agave bagasse stabilization. In fact, the R1 parameter based on compost TG and DTG curves increased with the progress of the composting. Finally, by analyzing scanning electronic microscopy samples of agave bagasse during composting, it was found that bagasse was susceptible to being attacked by fungi which leads to the breakdown of the vascular bundle and may represent an improvement in the water retention capacity of bagasse and can be used as a substrate for the production of greenhouse vegetables.
ACKNOWLEDGMENT
The authors want to acknowledge the contribution of Roger M. Rowell Professor Emeritus of the University of Wisconsin–Madison for the assistance in the preparation and invaluable comments on the manuscript. Also thanks to Q.I Tanit Toledano–Thompson for the SEM photomicrographs and to Hilda Palacios Juárez and H. G. Richter for the help with the interpretation of the SEM images.
REFERENCES
Álvarez P., Santamaría R., Blanco C. and Granda M. (2005). Thermal degradation of lignocellulosic materials treated with several acids. J. Anal. Appl. Pyrolysis 74, 337–343. [ Links ]
Aggarwal P., Dollimore D. and Heon, K. (1997). Comparative thermal analysis study of two biopolymers, starch and cellulose. J. Therm. Anal. 50, 7–17. [ Links ]
APHA (1985). Standard Methods of the Examination of Water and Wastewater, 15th edn. American Public Health Association, Washington, D. C. [ Links ]
Barberis, R. y Nappi, P. (1996). Evaluation of compost stability. In The science of composting (De Bertoldi, M., Sequi, P., Lemmes, B. and Papi, T. (Eds.). Blackie Academic and Professional, Glasgrow, Scotland, pp. 175–184. [ Links ]
Barkakaty B.C. (1976). Some structural aspects of sisal fibers. Appl. Polym. Sci. 20, 2921–2940. [ Links ]
Bernal M.P., Paredes C., Sánchez Monedero M.A. and Cegarra J. (1998). Maturity and stability parameters of composts prepared with a wide–range of organic wastes. Bioresource Technol. 63, 91–99. [ Links ]
Blanco M.J. and Almendros G. (1994). Maturity assessment of wheat straw compost by thermogravimetric analysis. J. Agric. Food Chem. 42, 2454–2459. [ Links ]
Blanco M.J. and Almendros G. (1997). Chemical transformation, phytotoxicity and nutrient availability in progressive compost stages of wheat straw. Plant Soil 196, 15–25. [ Links ]
Bodîrlău R. and Teacá C.A. (2009). Fourier transform infrared spectroscopy and thermal analysis of lignocelluloses fillers treated with organic anhydrides. 8th International Balkan Workshop on Applied Physics, 5–7 July. Constanţa, Romania. [ Links ]
Brinton W. F. (2000). Compost quality standards and guidelines. http://compost.css.cornell.edu/Briton.pdf. 14/01/2011. [ Links ]
Butler T.A., Sikora L.J., Steinhilber P.M. and Douglass L.W. (2001). Compost age and sample storage effects on maturity indicators of biosolids compost. J. Environ. Qual. 30, 2141–2148. [ Links ]
Carballo T., Gil M.V., Gómez X., González F. and Morán A. (2008). Characterization of different compost extracts using Fourier transform infrared spectroscopy (FTIR) and thermal analysis. Biodegradation 19, 815–830. [ Links ]
Cagnon B., Py X., Guillot A., Stoeckli F. and Chambat G. (2009). Contributions of hemicelluloses, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from lignocellulosic precursors. Bioresource Technol. 100, 292–298. [ Links ]
Chen Y. and Inbar Y. (1993). Chemical and spectroscopical analyses of organic matter transformations during composting in relation to compost maturity. In: Science and engineering of composting: design, environmental, microbiological and utilization aspects (H.A.J. Hoitink and Keener H.M. Eds.). The Ohio State University, pp. 551–600. [ Links ]
Chen, Y., Inbar, Y., Chefetz, B. y Hadar, Y. (1996). Compost and recycling of organic waste. In Modern agriculture and the environment. (D Rosen, E. Tel–Or, Y. Hadar and Y. Chen (Eds). Kluwer Academic Publishers, Dordrecht, the Netherlands, pp. 341–362. [ Links ]
CWMI (1976). The science and engineering of composting. Monitoring compost pH. Cornell Waste Management Institute. http://compost.css.cornell.edu/monitor/monitorph.html. 02/08/2008. [ Links ]
Dell'Abate M.T., Canali S., Trinchera A., Benedetti A. and Sequi P. (1998). Thermal analysis in the evaluation of compost stability: A comparison with humification parameters. Nutr. Cycling Agroecosyst. 51, 217–224. [ Links ]
Dell'Abate M.T., Benedetti A. and Sequi P. (2000). Thermal methods of organic matter maturation monitoring during a composting process. J. Therm. Anal. Calorim. 61, 389–396. [ Links ]
Dell'Abate. M.T. y Tittarelli, F. (2002). Monitoring of a composting process: Thermal stability of raw materials and products. In Microbiology of composting. Insam, H.; Krammer, S. and Riddech, N. (Eds.). Springer–Verlag, Heidelberg, Germany. pp. 357–372. [ Links ]
Dresboll D.B. and Magid J. (2006). Structural changes of plant residues during decomposition in a compost environment. Bioresource Technol. 97, 973–981. [ Links ]
Drouzzi Z., D'Orazio V., Provenzano M.R. and Ouatmane, A. (2009). Study of the biodegradation and transformation of olive–mill residues during composting using FTIR spectroscopy and differential scanning calorimetry. J. Hazard. Mater. 164, 1281–1285. [ Links ]
Drouzzi Z., D'Orazio V., Hafidi M., M.R. and Ouatmane A. (2009). Elemental and spectroscopic characterization of humic–acid–like compounds during composting of olive mill by–products, J.Hazard. Mater. 163, 1281–1285. [ Links ]
Du Xian–yuan, Liu Jian–lin, Huang Guo–he and Li Yu (2010). Formation of struvite crystals in a simulated food waste aerobic composting process. Chem. Res. Chinese Universities, 26, 210–216. [ Links ]
Faix O. (1991). Classification of lignins from different botanical origins by FTIR spectroscopy. Holzforschung 45, 21–27. [ Links ]
Filip Z., Pecher W. and Berthelin J. (2000). Microbial utilization and transformation of humic acid–like substances extracted from a mixture of municipal refuse and sewage sludge disposed of in a landfill. Environ. Pollut. 109, 83–89. [ Links ]
Goluleke C.G. (1977). Biological processing: Composting and hydrolysis. In: Handbook of solid waste management (D.G. Wilson, Ed.) Van Norstrand Reinhold, New York, pp. 197–225. [ Links ]
Gómez X., Cuetos M.J., García A.I. and Morán A. (2007). An evaluation of stability by thermogravimetric analysis of digestate obtained from different biowastes, J. Hazard. Mater. 149, 97–105. [ Links ]
Grandmaison J.L., Thibault J. and Kallaguine S. (1987). Fourier transform infrared spectrometry and thermogravimetry of partially converted lignocellulosic materials. Anal. Chem. 59, 2153–2157. [ Links ]
Grube M., Zagreba E., Gromozova E. and Fomina M. (1999). Comparative investigation of the macromolecular composition of mycelia forms Thielavia terrestris by infrared spectroscopy. Vibr. Spectrosc. 19, 301–306. [ Links ]
Harada Y., Inoko A., Tadaki M. and Izawa I. (1981). Maturing process of city refuse compost during piling. Soil Sci. Pl. Nutr. 27, 357–64. [ Links ]
Hesse M., Meier, H. and Zeeh B. (1995). Spektroskopische Methoden in der organischen Chemie. Goerg Thieme Verlag: Stuttgart, New York, 364 pp. [ Links ]
Hergert H.L. (1971). Infrared spectra, In: Lignins: occurrence, formation, structure and reactions (K.V. Sarkanen, C.H. Ludwig, Eds.). Wiley, New York, pp. 267–297. [ Links ]
Hsu J.H. and Lo S.L. (1999). Chemical and spectroscopic analysis of organic matter transformation during composting of pig manure. Environ. Pollut. 104, 189–196. [ Links ]
Itävaara M., Venelampi O., Vikman M. and Kapanen A. (2002). Compost maturity—Problems associated with testing. In: Microbiology of composting (H. Insam, N. Riddech and S. Klammer, Eds.). Springer–Verlag, Heidelberg, Germany pp. 373–382. [ Links ]
Íñiguez G., Acosta N., Martínez L., Parra J. and González O. (2005). Utilización de subproductos de la industria tequilera. Parte 7. Compostaje de Bagazo de agave y vinazas tequileras. Rev. Int. Contam. Ambie. 21, 37–50. [ Links ]
Íñiguez, G., Martínez, A. G., Flores, A. P. y Virgen, G. (2011). Utilización de subproductos de la industria tequilera. Parte 9. Monitoreo de la evolución del compostaje de dos fuentes distintas de bagazo de agave para la obtención de un substrato para jitomate. Rev. Int. Contam. Ambie. 27, 47–59. [ Links ]
Jolanun B. and Towprayoon S. (2010). Novel bulking agent from clay residue for food waste composting. Bioresource Technology, 101, 4484–4490. [ Links ]
Korosec R. and Lavric B. (2009). Thermogravimetry as possible tool for determining modification degree of thermally treated norway spruce wood. J. Therm. Anal. Calorim. 98, 189–195. [ Links ]
Kumar A., Wang L., Dzenis Y., Jones D. and Hanna M.A. (2008). Thermogravimetric characterization of corn stover as gasification and pyrolysis feedstock, Biomass and Bioenergy. 460–467. [ Links ]
Li G., Zhang F., Sun Y., Wong C. and Fang M. (2001). Chemical evaluation of sewage sludge composting as a mature indicator for composting process. Water, Air Soil Poll. 132, 333–345. [ Links ]
Luangkiattikhun P., Tangsathitkulchai C. and Tangsathitkulchai M. (2008). Non–isothermal thermogravimetric analysis of oil–palm solid wastes. Bioresource Technol. 99, 986–997. [ Links ]
Machinet G.E., Bertrand I., Chabbert B., Watteau F., Villemin G. and Recous S. (2009). Soil biodegradation of maize root residues: Interaction between chemical characteristics and the presence of colonizing micro–organisms. Soil Biology and Biochemistry, 41, 1253–1261. [ Links ]
Melis P. and Castaldi P. (2004). Thermal analysis for the evaluation of the organic matter evolution during municipal solid waste aerobic composting process. Thermochim. Acta 413, 209–214. [ Links ]
Mohan D., Pittman C.U. and Steele P. (2006). Pyrolysis of wood biomass for bio–oil. A critical review. Energ. Fuel. 20, 848–889. [ Links ]
Nanny M.A. and Ratasuk N. (2002). Characterization and comparison of hydrophobic neutral and hydrophobic acid dissolved organic carbon isolated from three municipal landfill leachates. Water Res. 36, 1572–1584. [ Links ]
Naumann D., Schultz C.P. and Helm D. (1996). What can infrared spectroscopy tell us about the structure and composition of intact bactaerial cells? In: Infrared Spectroscopy of Biomolecules (H.H. Mantsch and D. Chapman, Eds.). Wiley–Liss: New York, pp 279–310. [ Links ]
Ouatmane A., Provengano M.R., Hafidi M. and Senesi, N. (2000). Compost maturity assessment using calorimetry, spectroscopy and chemical analysis. Compost Sci. Util. 8, 124–134. [ Links ]
Owen N.L. and Thomas D.W. (1989). Infrared studies of "hard" and "soft" woods. Appl. Spectroscopy 43, 451–455. [ Links ]
Ranalli, G., Bottura, G., Taddei, P., Garavani, M., Marchetti, R., Sorlini, C., (2001). Composting of solid and sludge residues from agricultural and food industries. Bioindicators of monitoring and compost maturity. Journal of Environmental Science and Health A 36 415-436. [ Links ]
Rynk R. (1992). On–farm composting handbook, Northeast Regional Agricultural Engineering Service. Publication 54, Ithaca NY, http://www.cfe.cornell.edu/compost/OnFarmHandbook/coverpg.html. 25/07/2008. [ Links ]
Sánchez Monedero M.A., Roig A., Cegarra J. and Bernal, M.J. (1999). Relationship between water soluble carbohydrate and phenol fractions and the humification indices of different organic wastes during composting. Bioresour. Technol. 72, 33–41. [ Links ]
Smidt E. (2001). Eignung der FT–IR Spektroskopie zur Charakterierung der organischen Substanz in Abfällen. PhD thesis, University of Agricultural Sciences, Inst. of Waste Management, Vienna, Austria. [ Links ]
Smidt E., Lechner P., Schwanninger M., Haberhauer G. and Gerzabek M.H. (2002). Characterization of waste organic matter by FT–IR spectroscopy — application in waste science. Appl. Spectrosc. 56, 1170–1175. [ Links ]
Smidt, E. y Meissl, K. (2007). The applicability of Fourier transform infrared (FTIR) spectroscopy in waste management. Waste Manag. 27, 268–76. [ Links ]
Smith B. (1999). Infrared spectral interpretation. CRC Press: Boca Raton, FL. 264 pp. [ Links ]
Sharma S. (1990). Analysis of the components of lignocellulose degraded by Agaricus bisporus and Pleurotus ostreatus, Thermochim. Acta 173, 241–252. [ Links ]
Socrates G. (2001). Infrared and raman characteristic group frequencies. Tables and carts, 3rd ed. John Wiley and Sons Ltd.: Chichester, 347 pp. [ Links ]
Som M.P., Lemeé L. and Amblés A. (2009). Stability and maturity of green waste and biowaste compost assessed on the basis of a molecular study using spectroscopy, thermal analysis, thermodesorption and thermochemolysis, Bioresource technol. 100, 4404–4416. [ Links ]
Spaccini R. and Piccolo A. (2007). Molecular characterization of compost at increasing stages of maturity. 1. Chemical fractionation and infrared spectroscopy. J. Agric. Fodd Chem. 55, 2293–2302. [ Links ]
Spaccini R. and Piccolo A. (2008). Molecular characteristics of humic acids extracted from compost at increasing maturity stages. Soil Biol. Biochem. 41, 1164–1172. [ Links ]
Sun R., Fang J.M., Rowlands P. and Bolton J. (1998). Physicochemical and thermal characterization of wheat straw hemicelluloses and cellulose. J. Agric. Food Chem. 46, 2804–2809. [ Links ]
Tan, K. H. (1993). Humus and humic acids. In: Principles of Soil Chemistry: Colloidal Cehmistry of Organic Soil Constituents. CRC press. M. Dekker Inc. pp. 79–127. [ Links ]
TMECC (2001). Method 05.05–A. Method 3.01–C. Method 04.12–E. Test Methods for the Examination of Composting and Compost. http://tmecc.org/tmecc 25/05/2008. [ Links ]
Tsui L. and Juang M.A. (2010). Effects of composting on sorption capacity of bagasse–based chars. Waste Manage. 30, 995–999. [ Links ]
Valadez–González, A., Cervantez–Uc J.M., Olayo R. and Herrera–Franco P.J. (1999). Chemical modification of henequén fibers with an organosilane coupling agent. Compos. Part B– Eng. 30, 321–331. [ Links ]
Wang S., Liu Q., Luo Z., Wen L. and Cen, K. (2007). Mechanism study on cellulose pyrolysis using thermogravimetric analysis coupled with infrared spectroscopy. Front. Eng. China, 1, 413–419. [ Links ]
Wielage B., Lampke Th., Marx G., Nestler K. and Starke D. (1999). Thermogravimetric and differential scanning calorimetric analysis of natural fibers and polypropylene. Thermochim. Acta, 337, 169–177. [ Links ]
Willson G.B. (1989). Combining raw materials for composting. BioCycle. 30, 82–83. [ Links ]
Wu L. and Ma L.Q. (2002). Relationship between compost stability and extractable organic carbon. J. Environ. Qual. 31, 1323–1328. [ Links ]
Wu L. and Ma L.Q. (2001). Effects on sample storage of bisolids compost stability and maturity evaluation J. Environ. Qual. 30, 222–228. [ Links ]
Wu L., Ma L.Q. and Martínez G.A. (2000). Comparison of methods for evaluating stability and maturity of biosolids compost. J. Environ. Qual. 29, 424–429. [ Links ]
Zach A. and Schwanninger M. (1999). Charakterisierung von Huminsäuren aus Abfallstoffen mittels FT–IR. Öterreichische Wasser– und Abfallwirtschaft 11, 333–340. [ Links ]
Zaccheo P., Ricca G. and Crippa L. (2002). Organic matter characterization of composts from different feedstocks. Compost. Sci. Util. 10, 29–38. [ Links ]
Zaccheo P., Cabassi G., Ricca G. and Crippa L. (2002). Decomposition of organic residues in soil: experimental technique and spectroscopy approach. Org. Geochem. 33, 327–343. [ Links ]














