Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista internacional de contaminación ambiental
versión impresa ISSN 0188-4999
Rev. Int. Contam. Ambient vol.26 no.3 Ciudad de México ago. 2010
Bioaccumulation of cadmium (Cd), lead (Pb) and arsenic (As) in Crassostrea virginica (Gmelin, 1971), from Tamiahua lagoon system, Veracruz, Mexico
Bioacumulación de cadmio (Cd), plomo (Pb) y arsénico (As) en Crassostrea virginica del sistema lagunar Tamiahua, Veracruz, México
Fabiola LANGO–REYNOSO1, Cesáreo LANDEROS–SÁNCHEZ 2 and María del Refugio CASTAÑEDA–CHÁVEZ1
1 Instituto Tecnológico de Boca del Río, km 12 Carretera Veracruz–Córdoba C.P. 94290, Boca del Río, Veracruz; México.
2 Colegio de Postgraduados, Campus Veracruz (COLPOS), Km 88.5 Carretera Federal Xalapa–Veracruz, vía Paso de Ovejas entre Paso San Juan y Puente Jula, Tepetates, Veracruz, México. C.P. 91700, Apartado Postal 421. E–mail: clandero@colpos.mx
Recibido octubre 2009
Aceptado abril 2010
ABSTRACT
The accumulation of heavy metals in oysters, C. virginica, from Tamiahua Lagoon System along the gulf coast in the state of Veracruz, México, results from inputs provided by anthropogenic activities and the physicochemical and ecophysiological processes occurring in these systems. The objective of this study was to determine concentrations of Cd, Pb and As in gonad–digestive gland (GDG) and muscle–mantle–gill (MMG) tissues in females and males of C. virginica from Tamiahua Lagoon. Two sampling sites were selected, and each sample consisted of 500 oysters of commercial size. Concentrations of Cd, Pb and As were determined using atomic absorption spectrophotometry and a graphite furnace. The highest concentrations were found in MMG tissues, whose mean values for these metals are 11.77 ± 1.32, 0.484 ± 0.08, 4.02 ± 0.56 mg kg–1. Cadmium concentrations exceeded the limits for the consumption of bivalve mollusks established by the sanitary regulations, indicating a risk to human health.
Key words: oyster, contamination, bivalve, gonad–digestive gland, muscle–mantle–gill.
RESUMEN
La acumulación de metales pesados en ostión C. virginica de las lagunas del Golfo de de México, se debe principalmente a las descargas de actividades antropópicas y a procesos fisicoquímicos y ecofisiológicos que ocurren en estos sistemas. El objetivo de este estudio fue determinar las concentraciones de Cd, Pb y As en las porciones gónada–sistema digestivo (GSD) y músculo–manto–branquias (MMB) de hembras y machos de ostiónen el sistema lagunar de Tamiahua, Veracruz. Se seleccionaron dos sitios de muestreo. Cada muestra consistió de 500 organismos de talla comercial. Las concentraciones de Cd, Pb y As se determinaron por espectrofotometría de absorción atómica y horno de grafito; el tejido MMB registró las concentraciones más altas, de estos metales con valores medios de 11.77 ± 1.32, 0.484 ± 0.08, 4.02 ± 0.56 mg kg–1. El Cd superó los límites permisibles de consumo que establecen las normas sanitarias para moluscos bivalvos y se estima que representa un riesgo para la salud humana.
Palabras clave: ostión, contaminación, bivalvo, gónada–glándula digestiva, músculo–manto–branquia.
INTRODUCTION
A large variety of native mollusks exist in México, of which many species are found in the Gulf of México. One of these, the American oyster Crassostreavirginica is highly important because it is the sixth species most produced at the national level with 51 339 tons/year. Nationally, the state of Veracruz is the main producer of this oyster species along the gulf coast, with 24 475 tons harvested in 2003 (SAGARPA 2006). However, the production of this species in the state over the last several years has been affected by biological (bacterial and viral) and chemical (heavy metals, organochlorate pesticides, and hydrocarbons) contamination and pollution (Barrera–Escorcia and Wong–Chang 2005, Albert and Benítez 2005, Guzmán–Amaya et al. 2005, Páez–Osuna 2005, Robert et al. 2008). Above thresholds of acceptance, these contaminants are considered pollutants, causing problems with food safety and health of the oysters produced (Munro and Chaibonneau 1981, Rainbow 1990, Páez–Osuna 1996, Galaviz 2003, SENASICA 2003, Castañeda–Chávez et al. 2005).
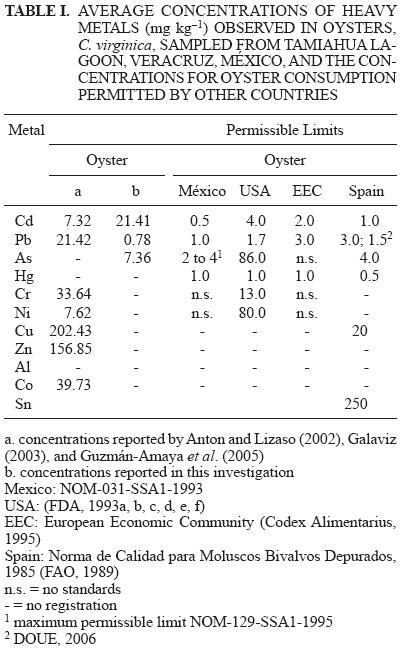
Permissible limits of heavy metals in oysters for human consumption in the European Union, United States of America, Spain, and México are listed in table I. These countries regulate overall oyster production including cultivation, wild harvesting, and marketing, and legislations exist dictating whether or not oysters can be cultivated or harvested from certified areas in order to ensure oyster quality and to avoid public health problems (SENASICA 2003). However, oyster harvesting and culture in México, and particularly in the Gulf region, is carried out in uncertified areas which are exposed to anthropogenic contamination and pollution (Wong–Chang and Barrera–Escorcia 2005).
Although in México there are few studies on heavy metal pollution of bivalve molluscs in comparison with other countries, some have reported physiological alterations in these animals. Gold et al. (1995) reported damage to the gills, digestive tract, and connective tissue in the diverticulum in C. virginica collected from the lagoons of Mecoacan, Carmen and Machona in the state of Tabasco, from Cd, oil, and associated environmental interactions. Additional studies conducted in the lagoons of Sontecomapan, La Mancha, Alvarado, Mandinga and Tamiahua in the state of Veracruz have reported the concentrations of heavy metals in C. virginica without examining the physiological effects on these animals or their impact on public health (Luna et al. 2002, Aguilar and Amador del Ángel 2003, Lango et al. 2003, Guzmán–Amaya et al. 2005, Baqueiro–Cárdenas et al. 2007a, b).
Bioaccumulation and biomagnification of heavy metals in oysters have implications for human health because they are a popular dietary item. The risks that these metals present to public health include: 1) Cd affects digestion, bones, reproduction, the central nervous and immune systems, and causes brain damage and cancer; 2) Pb can damage the brain and kidneys, interfere with the synthesis of hemoglobin, alter gastrointestinal function, harm the reproductive system, and cause acute and chronic damage to the nervous system; 3) As is deposited in the hair and nails, and can cause cancer in the skin, kidneys, bladder, scrotum, liver, lymph system, and lungs (Anton and Lizaso 2002, ATSDR 2005, 2007). The objective of this study was to determine concentrations of Cd, Pb, and As in the gonad–digestive gland (GDG) and the muscle–mantle–gill (MMG) tissues of females and males of C. virginica from Tamiahua Lagoon in the state of Veracruz, México.
MATERIALS AND METHODS
Study area and sampling method
The Tamiahua Lagoon is located along the gulf coast of México in the northern part of the state of Veracruz. It is bordered by the municipalities of Tamiahua, Ozuluama, and Tampico (between 21° 06' to 21° 20' N, and 97° 23' to 97° 46' W), and covers an area of 88 000 ha (Fig. 1).
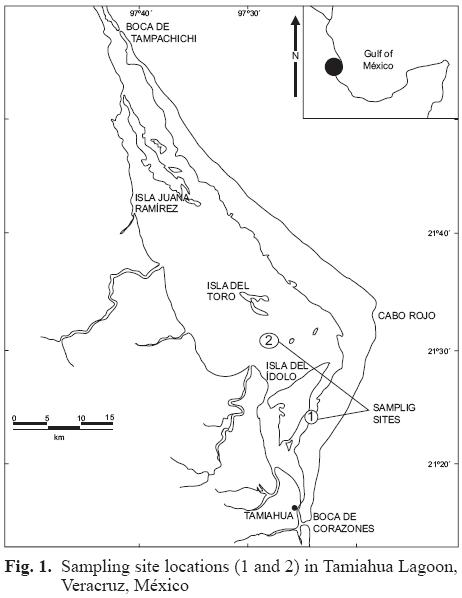
Two sites were selected for oyster sampling, site 1 in the central part of the lagoon, and site 2 in the southern portion (Fig. 1). Samples were collected once per month during January, March, April, May, June, and July 2004. Physicochemical variables were measured in situ using an aquatic probe (YSI Model 6600 multiparameter data logger – Yellow Springs, OH 45387–1107, USA), and included salinity, temperature, and pH.
Six samples were collected from each site, for a total of 12 samples collected each month. Each sample consisted of 500 oysters of harvestable size (10 ± 3 cm), which were thoroughly cleaned to remove adhering debris and algae, and then transported on ice in thermal containers according to the protocols of NOM–109–SSA1–1994 (SSA, 1994). The Condition Index (CI) (Lucas and Beninger 1985) was determined for each sample using 30 oysters from each sample. The sex ratio of each sample was determined using microscopic analyses of gonad smears, with each determination graded into four categories: male, female, hermaphrodite, or undifferentiated (Lango–Reynoso et al. 1999, 2000).
Determination of heavy metal concentrations in samples
Thirty males and 30 females were selected from every sample, and from every oyster the GDG and MMG tissues were removed and frozen at –87 °C in an ultrafreezer. The samples were dehydrated in a lyophilizer (ThermoSavant, Model OD–114, Holbrook, NY) for 72 hours at –49 °C and 36x10–3 mbar. After dehydration, the samples were milled and screened using No. 30 mesh with an opening of 595 µm, and the ground material stored in Ziplock® bags. The samples of GDG and MMG tissues were digested in a microwave oven CEM Model MARS 5 (CEM Corporation, Matthews, NC) following the Oyster Pure method (EPA 1996). Upon completion of digestion, the samples were filtered into Kitazato flasks using a FluoroporeTM membrane and a vacuum pump, and diluted to volume of 25 mL in a volumetric flask using Type II water. The diluted samples were transferred to polypropylene jars for later analysis. Every sample of tissue was analyzed using two replicates. Concentrations of Cd were determined using a Duo Varian Spectra atomic absorption spectrophotometer with FS220 Flame (containing ultra lamps) and S1PS20 Autodilutor, and an SPS5 Autosampler (Mulgrave, Victoria, Australia). Concentrations of Pb and As were determined using a 220Z graphite furnace (NOM–117–SSA1–1994) (SSA 1994). In order to assure the precision of lab results, use was made of reference standards of NIST Oyster Tissue 1566a.
Statistical analysis
Heavy metal concentrations from the GDG and MMG oyster tissues from the two sites were examined for normality using the Wilks–Shapiro test. A Manova (Statistica version 7.0) was subsequently used to assess significant effects from month, sex, and tissue, and the interactions of month–sex, sex–tissue, and month–sex–tissue.
RESULTS
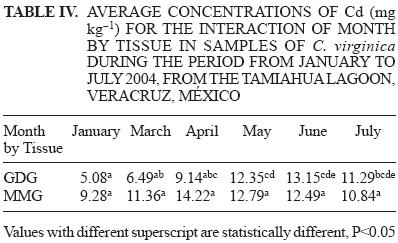
Average concentrations of Cd, Pb, and As by sampling site, tissue type (GDG or MMG), and sex in C. virginica from Tamiahua Lagoon are presented in table II. Manova results (P < 0.05) between sampling sites, sex, months, and tissue for C. virginica during the period from January to July, 2004, are presented in table III. Tukey test results for multiple comparisons of average concentrations of Cd for month by tissue interaction are shown in table IV. Average concentrations by site, based on dry weight for Cd, Pb, and As are compared with the permissible limits provided by the Norma Mexicana NOM–031–SSA1–1993, and similar limits for the United States of America, the European Economic Community, and Spain in table I.
DISCUSSION
Cadmium (Cd)
Cadmium was the predominant heavy metal in the GDG and MMG tissues of C. virginica, with an average concentration of 21.42 mg kg–1 (Table I). Botello (1994) reported an average concentration of 1.10 mg kg–1 of Cd from the lagoons of Llano, Veracruz, Galaviz (2003) reported an average concentration of 5.86 mg kg–1 of Cd from the lagoon at La Mancha, and Guzmán–Amaya et al. (2005) reported average concentrations of 2.94 and 4.61 mg kg–1 of Cd from the lagoons at Mandinga and Alvarado. Concentrations of Cd found in the coastal lagoons mentioned above, including those observed in this study, are mainly due to the contributions by runoff (Guzmán–Amaya et al. 2005). In comparison to the aforementioned lagoons, average Cd concentrations from Tamiahua lagoon were higher. Likely causes for these concentrations include: a) the discharge of untreated domestic and municipal sewage effluent containing organic material, pesticides, fertilizers, detergents, and metals (Cuevas et al. 2006); and b) the high level of agricultural activity in the areas surrounding the lagoon (the estimated spatial area for agricultural activity is 68 311.154 hectares, of which 46 645.851 hectares are integrated into 3266 production units) that practice monoculture which requires large quantities of agrochemicals (UNEP 2000, Altieri and Nicholls 2007), particularly the use of phosphate fertilizers containing Cd that are commonly applied to corn, beans, green chilies and orange crops in the region and eventually leach from the soil and are transported to the lagoons with runoff; c) the ingestion and accumulation of Cd in molluscs is a function of exposure conditions, physiological factors, reproduction, and excretion; factors that also are variable. Hence, interpretations of the mechanisms can be complicated by these and other factors involved during exposure and toxicokinesis (Rodríguez de la Rua et al. 2005).
Due to the processes described above, the concentration of Cd in water in coastal Mexican lagoons exceeds the maximum permissible limit (MPL) set forth in the NOM–001–ECOL–1996 (SEMARNAT 1996), which sets the maximum allowable limits for contaminants in wastewater discharges into water and national assets. In this and similar lagoons, which generally have brackish water, the bioavailability of cadmium is the result of continual changes of salinity which determines the concentration of this metal in sessile animals such as C. virginica (Márquez et al. 2000). In the present study, the concentrations of Cd in the oyster samples were relatively high. In comparison to other studies, the MMG tissues in the present study had higher average concentrations of Cd in both sampling sites, and in females and males, with values of 9.28 to 14.22 mg kg–1 respectively. Further, concentrations of Cd were higher in MMG tissues of males, and the values fluctuated between 10.35 and 13.54 mg kg–1. Walsh and O'Halloran (1998) reported that heavy metal accumulation could be ranked by different tissues beginning with gills, kidneys, digestive gland, mantle, and adductor muscle, and that there was a high correlation between some tissues such as digestive gland–kidney and gills–adductor. The process of incorporation of Cd into the MMG tissues of C. virginica that were analyzed in this study most likely followed the primary routes of incorporation of metals into aquatic invertebrates, in which water and food were the main transport vehicle (Badii and Garza 2005, Guzmán–García et al. 2007).
Regarding the average concentrations of Cd observed in C. virginica during January to July 2004, the GDG tissues presented the lowest concentration, with a value of 13.15 mg kg–1 in June, while the highest concentration for MMG tissues was 14.22 mg kg–1 during the month of April (Table IV). The Manova analysis showed significant differences in the concentrations of Cd for the months studied (Table III). In the interaction between month and tissue (Table IV), concentrations of Cd in MMG tissues were higher than those for GDG tissues during the months January to May. However, in the months of June and July, the concentrations of Cd in GDG tissues were higher than those for MMG. The variation in Cd concentration in the GDG tissues coincides with the breeding season of C. virginica, which takes place from May to July, and corresponds precisely with the period of gametogenesis in this species (Lango–Reynoso et al. 1999, 2000, Arias 2006, Baqueiro et al. 2007b). The concentration of Cd in these tissues also tends to increase during the dry season, due to the sedimentation of particles containing Cd from the water column and the greater exposure time of the oysters to these particles (Palomarez–García et al. 2007, 2009). Concentrations of Cd may be related in the MMG tissues the interaction of month by tissue, due to the amount of water involved in food uptake and waste disposal. In addition, these tissues have direct contact with the environment, and both water and food are the principle routes of entry for heavy metals. Consequently, the MMG tissues accumulate more heavy metals than the GDG tissues. In other bivalve molluscs, the concentration of Cd has also been reported to be higher in the gills, and the elimination of this metal likely happens via the kidney (Guzmán–García et al. 2005, Raimundo and Vale 2008).
Lead (Pb)
Studies of the rivers and coastal lagoons of the Gulf of México have reported that the state of Veracruz presents one of the highest concentrations of Pb in the water with 212.18 mg L–1, which exceeds the permissible limits for coastal waters (Guzmán–Amaya et al. 2005). However, the average concentration of Pb in oysters, C. virginica, from Tamiahua Lagoon in this study was 0.77 mg kg–1, a value lower than in the lagoons at Llano (2.22 mg kg–1) (Botello 1994), La Mancha (9.41 mg kg–1) (Galaviz 2003), Mandinga and Alvarado (13.17 and 9.05 mg kg–1, respectively) (Guzmán–Amaya et al. 2005), Tampamachoco (0.74 mg kg–1) (Rosas et al. 1983), and Tamiahua (21.42 mg kg–1) (Guzmán–Amaya et al. 2005). When compared to lagoons in the Gulf of México, the low concentration of Pb in C. virginica from the Tamiahua Lagoon in this study is most likely due to the absence of industrial activity, leading to the lower concentration of this metal in the water column and sediment (Apeti et al. 2005, Guzmán–Amaya et al. 2005). However, the earlier presence of these activities has promoted a 2000 % increase in this metal (Villanueva–Fragoso and Páez–Osuna 1996) in lagoons in Tabasco, Carmen and Términos in Campeche.
The Tamiahua Lagoon is classified as a RAMSAR site, although it has a high level of aquatic contamination due to untreated sewage, hydrocarbons, trash, solid waste, agrochemicals, and fertilizers. These inputs have resulted in elevated concentrations of copper, lead, cadmium, and chromium (Guzmán–Amaya 2005, Páez–Osuna 2005, RAMSAR 2006, 2008). However, the presence and concentration level of Pb in C. virginica depends on the bioaccumulation process which is a function of the bioavailability of the contaminant and 1) mobilization of the metals in interstitial water and their chemical speciation, 2) transformation of As, Hg, Pb, and Sn by processes such as biometilation, which affects solubility, volatility, and chemical properties, 3) distribution and abundance of sediment components (e.g. iron oxide and organic material), 4) competition between metals such as Cu, Ag, Zn, and Cd at points of entry into organisms, and 5) effects of bioturbation, salinity, the redox coefficient, and pH (Guzman–Amaya et al. 2005, Baqueiro–Cárdenas et al. 2007a).
As with Cd, there were higher concentrations of Pb in MMG than in GDG tissues for both males and females (0.49 mg kg–1 and 0.7 mg kg–1, respectively) (Table II) (e.g. Oliver et al. 2001). This pattern of concentration (MMG>GDG) has been observed in other bivalve molluscs such as the blue mussel, Mytilusedulis, where the following pattern of concentrations by tissue has been reported: gills>digestive gland>foot>gonad (Mubiana and Blust 2007).
Arsenic (As)
The presence of As in Tamiahua Lagoon is primarily from forestry activity occurring in the adjacent region which combines 739 units of rural production, of which 49 are engaged in timber production (INEGI 2009). Arsenic is used as a fungicide, insecticide, herbicide, algaecide, rodenticide, and as a preservative applied directly on the wood (EPA 2003). An additional source of As in the Tamiahua region is runoff from petroleum exploration wells which are abandoned and not sealed (RAMSAR 2006, 2008).
The average concentration of As observed in C. virginica in this study was 7.36 mg kg–1, which is less than 23 mg kg–1 reported for this species by Hernández et al. (2003) in the Bahía Cienfuegos of Cuba. Other studies on C. virginica in gulf coast waters of the United States have reported total concentrations of As to fluctuate between 4.1 and 39 mg kg–1 and which were attributed to the drainage of natural deposits of phosphates (Wilson et al. 1992). On the other hand, studies on the Japanese oyster, C. gigas, in coastal zones of southeast Taiwan and in Sonora, México, have shown As concentrations of 9.9 and 0.05 mg kg–1, respectively (García and Ramos 2001, Liu et al. 2007, 2008). C. gigas and C. virginica share similar characteristics with physiological connections such as: they are sedentary organisms, they are strong bioaccumulators and have abundant populations, they have a relatively long average life expectancy, they are manageable and easily acclimatize to experimental conditions, and both have been used as environmental indicators (Rodríguez de la Rua et al. 2005). The concentration of As in C. gigas in coastal regions of the southwest of Taiwan was higher than in this study due to wastewater discharges containing high levels of inorganic As (Liu et al. 2008).
In the present study, significant differences in As concentrations were found between sampling sites, month, and sexes (Table III). These differences are likely due to the reproductive cycle which is influenced by changes in seasonal climate and the abundance of food that occurs during this cycle (Lango–Reynoso et al. 2000). In studies of Términos Lagoon, Campeche, Aguilar and Amador del Angel (2003) found that the condition index for C. virginica was negatively correlated with heavy metal concentrations in the tissues, and that the differences were associated with tissue function and exposure time to the heavy metals. These results support those obtained for C. gigas on the southwest coast of Taiwan where As concentrations followed a seasonal pattern of spring>summer>winter (Liu et al. 2008). Changes in temperature and salinity also induce the accumulation of As in C. virginica (Valette–Silver et al. 1999, Guzman–Amaya et al. 2005).
The differences between sampling sites are likely due to their location in the lagoon (Fig. 1). Sampling site 1, located in the navigation channel, between the island of Idol and the eastern part of Tamiahua municipality, receives inputs from human settlements located on the shores of the island. In contrast, sampling site 2 is in the center of the lagoon, between the islands of Idol and del Toro, is not directly impacted by any population. However, it is located near the mouths of the rivers San Jerónimo and Tancochi, which discharge livestock, agricultural, and urban waste from the adjacent municipalities. This site also is negatively impacted by abandoned oil wells due to its proximity (Catan I, II and Acamayas).
The same pattern of As, Cd, and Pb concentrations exist in tissues (i.e., MMG>GDG) of C. virginica, females had a higher average concentration of As (4.47 mg kg–1) than males (3.64 mg kg–1). Amiard et al. (1994) found a negative correlation between individual weight and the concentration of heavy metals. These observations coincide with the data obtained in this study; the highest concentrations of As were observed in spawned females, which had experienced a decrease in body mass.
CONCLUSIONS
MMG tissues from both males and females of C. virginica had the highest concentrations of Cd and Pb during the three seasons studied, although the highest concentrations of As were observed in females. These results can be explained in terms of the physiological processes of filtration and feeding with regard to sampling site location, sources of pollution, and weather conditions prevailing during each of the sampling periods (Leah et al. 2001).
The concentrations of Cd observed in this study exceeded permissible limits for human consumption established by public health standards for bivalve molluscs in México (SSA 1993), the European Economic Community (EEC) (FAO 1989), the United States of America (USA) (EPA 2003), and Spain. Concentrations of Pb did not exceed permissible limits. The existing regulations for health specifications in México regarding "fishery products, fresh and chilled bivalve molluscs", does not provide specifications on permissible concentrations of As. However, the NOM–129–SSA1–1995 (SSA 1995) suggests that for "fishery products: dry–salted. Provisions and specifications health", the level permitted for As in oysters is 2 to 4 mg kg–1 (Table I).
In México, the average consumption of oysters/person/day along coastal zones of the Gulf of México is approximately 12, with an approximate wet weight of 84 g (Lippmann 1992). The consumption of this quantity of oysters, considering the average concentration of Cd observed in this study, results in ingestion of 1.798 mg/day of Cd, a value that exceeds the permissible limits in the NOM–031–SSA1–1993 (SSA 1993) (Table I). Consequently, this metal is a strong biotoxicant and represents a public health risk in communities having a high oyster consumption (Fermín et al. 2003).
ACKNOWLEDGEMENTS
We thank the Consejo del Sistema Nacional de Educacion Tecnológica (COSNET) for the research project "Programa Ostión clave COSNET 1252.01". We also thank Consejo Nacional de Ciencia y Tecnologia (CONACyT) for providing assistance to Dr. Fabiola Lango Reynoso during Estancia Sabática Vinculada al Fortalecimiento de la Calidad del Posgrado Nacional 2008 in the Colegio de Postgraduados, Campus Veracruz. We also thank the Instituto Tecnológico de Boca del Río and the Colegio de Postgraduados Campus Veracruz, for their help during the preparation of this article within the academic collaboration agreement among these institutions since October 2006.
REFERENCES
Aguilar C.A. and Amador del Ángel L.E. (2003). Metales pesados en el ostión (Crassostrea virginica) de la laguna de Términos, Campeche, México. [on line]. http://www.geocities.com/leamador/Marcuba2003.pdf>. 06/08/2009. [ Links ]
Albert L.A. and Benítez J.A. (2005). Impacto ambiental de los plaguicidas en los ecosistemas costeros. In: Golfo de México. Contaminación e Impacto Ambiental: Diagnostico y Tendencias (A.V. Botello, J. Rendón–von Osten, G. Gold–Bouchot and C. Agraz–Hernández, Eds). 2nd Ed. Universidad Autónoma de Campeche, Universidad Nacional Autónoma de México, Instituto Nacional de Ecología. pp. 157–176. [ Links ]
Altieri M.A. and Nicholls C.I. (2007). Conversión agroecológica de sistemas convencionales de producción: teoría, estrategias y evaluación. Ecosistemas 16, 3–12. [ Links ]
Amaiard J.C., Métayer C., Baud J.P. and Ribeyre F. (1994). Influence de facteurs écologiques et biologiques sur la bioaccumulation d'éléments métalliques chez de jeunes huîtres (Crassostrea gigas Thunberg) au cours du prégrossissement en nourricerie. Water Res. 28, 219–23. [ Links ]
Antón A. and Lizaso J. (2002). Los metales pesados en la alimentación. Fundación Ibérica para la seguridad alimentaría [on line]. http://www.fundisa.org/articulod/fmetales.pdf> 06/08/2009. [ Links ]
Apeti D.A., Robinson L. and Johnson E. (2005). Relationships between heavy metal concentration in the American oyster Crassostrea virginica and metal level in the water column and sediment in Apalachicola Bay, Florida. Am. J. Environ. Sci. 1, 179–186. [ Links ]
Arias de León C. (2006). Gametogenesis del ostión Crassostrea virginica (G.) en los sistemas lagunares de Tamiahua y Vega de Alatorre, Veracruz, México. Ms. Sc. Thesis. Instituto Tecnológico de Boca del Río. Boca del Río, Veracruz, México, 101 p. [ Links ]
ATSDR (2005). Reseña toxicológica del arsénico. Agencia para Sustancias Tóxicas y el Registro de Enfermedades. Manual. Atlanta, Georgia, pp. 1–11. [ Links ]
ATSDR (2007). Toxicological profile for arsenic. Agency for Toxic Substances and Disease Registry. Departament of Health and Human Services, Public Health Service. Atlanta, Georgia. [ Links ]
Badii M.N. and Garza A. (2005). Monitoreo Biológico como herramienta esencial en la evaluación de riesgo ecológico y el impacto ambiental. CULCyT 2, 17–26. [ Links ]
Baqueiro–Cárdenas E. R., Borabe L., Goldaracena–Islas C. G. and Rodríguez–Navarro J. (2007a). Los moluscos y la contaminación. Una revisión. Rev. Mex. Biodiv. 78, 1–7. [ Links ]
Baqueiro–Cárdenas E. R., Aldana–Aranda D., Sevilla M. L. and Rodríguez–Espinosa P. F. (2007b). Variations in the reproductive cycle of the oyster Crassostrea virginica (Gmelin, 1791), Pueblo Viejo Lagoon, Veracruz, México. Transit. Waters Bull. 2, 37–46. [ Links ]
Barrera–Escorcia G. and Wong–Chang I. (2005). Diagnostico de la contaminación microbiológica en el Golfo de México. En:Golfo de México. Contaminación e Impacto Ambiental: Diagnostico y Tendencias (A.V. Botello, J. Rendón–von Osten, G. Gold–Bouchot y C. Agraz–Hernández, Eds.). 2nd Ed. Universidad Autónoma de Campeche, Universidad Nacional Autónoma de México, Instituto Nacional de Ecología. México, pp. 515–524. [ Links ]
Botello A.V. (1994). Evaluación ambiental de las lagunas costeras de Pueblo Viejo, Tamiahua y Tampamachoco, Veracruz, para el aprovechamiento y conservación de su biodiversidad. Reporte final del proyecto multidisciplinario. Div. CBS, UAM–I, México, 98 p. [ Links ]
Castañeda–Chávez M. R., Orantia B. E., Pardio–Sedas V. and Lango–Reynoso F. (2005). Influence of water temperature and salinity on seasonal occurences of Vibrio cholerae and enteric bacteria in oyster–produccing areas of Veracruz, México. Mar. Pollut. Bull. 50, 1641–1648. [ Links ]
Codex (1995). Norma General del Codex para los Contaminantes y las Toxinas Presentes en los Alimentos, Codex Stan. FAO/OMS, 193 p. [ Links ]
Cuevas B.J., Seguel S.O. and Ellies Sch A. (2006). Efectos de las enmiendas orgánicas sobre las propiedades físicas del suelo con especial referencias a la adición de lodos urbanos. R.C. Suelo Nutr. Veg. 6, 1–12. [ Links ]
DOUE (2006). Contenido máximo de determinados contaminantes en los productos alimenticios. Diario Oficial de la Unión Europea. Reglamento (CE) No. 1881/2006. Comisión de 19 de diciembre de 2006. [ Links ]
EPA (2003). Responses to requests to Cancel certain chromated copper arsenate (CCA) wood preservative products and amendments to terminate certain uses of other CCA products. United States Environmental Protection Agency,Federal Register, FRL–7301–2. (68),14 pp. [ Links ]
FAO (1989). Report of the workshop and study tour on mollusc sanitation and marketing, regional seafarming development and demonstration project RAS/86/024 15–28 October [on line]. http://www.fao.org/docrep/field/003/AB710E/AB710E24.htm 10/08/2009 [ Links ]
FDA (1993a). Guidance Document for arsenic in shellfish. US Department of Health and Human Services, Public Health Service, Office of Seafood (HFS–416). Food and Drug Administration. Washington, DC. 44 p. [ Links ]
FDA (1993b). Guidance document for cadmium in shellfish. US Department of Health and Human Services, Public Health Service, Office of Seafood (HFS–416). Food and Drug Administration. Washington, DC. 44 p. [ Links ]
FDA (1993c).Guidance document for chromium in shellfish. US Department of Health and Human Services, Public Health Service, Office of Seafood (HFS–416). Food and Drug Administration. Washington, DC. 40 p. [ Links ]
FDA (1993e). Guidance document for lead in shellfish. US Department of Health and Human Services, Public Health Service, Office of Seafood (HFS–416). Food and Drug Administration. Washington, DC. 45 p. [ Links ]
FDA (1993f). Guidance Document for Nickel in Shellfish. U.S. Department of Health and Human Services, Public Health Service, Office of Seafood (HFS–416), Food and Drug Administration. Washington, DC. 39 pp. [ Links ]
Fermín I., Senior W., López F. and Martínez G. (2003). Contenido total de cobre, cadmio, zinc, plomo, hierro, cromo y níquel en sedimentos superficiales de la laguna de Unare, Estado Anzoátegui, Venezuela. Dpto. Oceanografía; Instituto Oceanográfico de Venezuela, Venezuela. 16 p. [ Links ]
Galaviz V.I. (2003). Estudio de la calidad sanitaria del ostión Crassostrea virginica (Gmelin, 1791) de lo sistemas lagunares de Alvarado y La Mancha, Veracruz; mediante el método de análisis de riesgos, identificación y control de puntos críticos (ARICPC o HACCP). Ms. Sc. Thesis. Instituto Tecnológico del Mar 01. Boca del Río, Veracruz, México. 101 pp. [ Links ]
García R.L. and Ramos R.R. (2001). Determination of total metals in cultivated oyster Crassostrea gigas from the northwest coast of México by microwave digestion and atomic absorption spectrometry. J. Aoac. Int. 84, 1909–1913. [ Links ]
Gold–Bouchot G., Noreña–Barroso E. and Zapata–Pérez O. (1995). Hydrocarbon concentration in the American oyster Crassostrea virginica, in laguna de Términos, Campeche, México. Bull. Environ. Contam. Toxicol. 54, 222–227. [ Links ]
Guzmán–Amaya P.S., Villanueva F. A. and Botello A.V. (2005). Metales pesados en tres lagunas costeras del estado de Veracruz. In:Golfo de México. Contaminación e impacto ambiental: Diagnostico y tendencias (A.V. Botello, J. Rendón–von Osten, G. Gold–Bouchot y C. Agraz–Hernández, Eds). 2nd Ed. Universidad Autónoma de Campeche, Universidad Nacional Autónoma de México, Instituto Nacional de Ecología. pp. 361–372. [ Links ]
Guzmán–García X., Martínez–López A., Rodríguez–Medina L., González–Márquez H. and Vázquez–Botello A. (2007). Cambios tisulares en el ostión Crassostrea virginica por exposición y depuración al cadmio. Hidrobiol. 17, 41–48. [ Links ]
Hernández A., Gómez B. M., Muñoz C.A., Pérez S.S., Díaz A. M., Estévez A. J., Pupo G. I. and Alberro M.N. (2003). Total arsenic in marine organisms from Cienfuegos Bay, Cuba. Centro de Estudios Ambientales de Cienfuegos. Ciudad Nuclear, Cienfuegos, Cuba, 7 p. [ Links ]
INEGI (2009). Información sobre agricultura y vegetación. [on line]. http://mapserver.inegi.org.mx/geografia/espanol/estados/ver/agri.cfm?c=444 & e=14 13/07/2009. [ Links ]
Lango–Reynoso F., Devauchelle N., Le Pennec M. and Hatt P.J. (1999). Elements of reproductive strategy in oysters, Crassostrea gigas, from the "Rade de Brest", France. Invertebr. Reprod. Dev. 36, 141–144. [ Links ]
Lango–Reynoso F.J., Chávez–Villalba J., Cochard J.C., and Le Pennec M. (2000). Oocyte size, a means to evaluate the gametogenic development of the Pacific oyster, Crassostrea gigas (Thunberg). Aquaculture 190,183–199. [ Links ]
Lango–Reynoso F., Leyva R.M. and Castañeda C.M. (2003). Presencia de metales pesados en ostión (Crassostrea virginica) en las lagunas de Alvarado y La Mancha, Ver. Memorias del Congreso de Ciencias del Mar, Marcuba 2003. Comité Oceanográfico Nacional. Cuba December 1–5, 2003. 10 p. [ Links ]
Leah M.O., Fisher W.S., Winstead J.T., Hemmer B.L. and Long E.R. (2001). Relationships between tissue contaminants and defense–related characteristics of oyster (Crassostrea virginica) from five Florida bays. Aquat. Toxicol. 55, 203–222. [ Links ]
Lippmann M. (1992). Enviromental toxicants: human exposures and their health effects. Van Nostrand Reinhold, New York, 699 p. [ Links ]
Liu C.W., Liang C.P., Lin K.H., Jang Ch.S., Wang Sh.W., Huang Y.K. and Hsueh Y.M. (2007). Bioaccumulation of arsenic compounds in aquacultural clams (Meretrix lusoria) and assessment of potential carcinogenic risks to human health by ingestion. Chemosphere 69, 128–134. [ Links ]
Liu C.W., Huang Y.K., Hsueh K.H., Lin C.S. and Jang Huang L.P. (2008). Spatiotemporal distribution of asernic species of oyster Crassostrea gigas in the coastal area of southwestern Taiwan. Environ. Monit. Assess. 138, 181–190. [ Links ]
Lucas A. and Beninger P.G. (1985). The use of physiological condition indices in marine bivalve aquaculture. Aquaculture 44, 187–200. [ Links ]
Luna J.M., Rendón–von J.O. and Alpuche L. (2002). Presencia de plomo en agua y ostión en las lagunas de Alvarado y La Mancha. In: La pesca en Veracruz y sus perspectivas de desarrollo. Instituto Nacional de la Pesca y Universidad Veracruzana. pp. 145–154. [ Links ]
Márquez A., Senior W. and Martínez G. (2000). Concentraciones y comportamiento de metales pesados en una zona estuarina de Venezuela. Interciencia 25, 284–291. [ Links ]
Mubiana V.K. and Blust R. (2007). Effects of temperature on scope for growth and accumulation of Cd, Co, Cu and Pb by the marine bivalve Mytilus edulis. Mar. Environ. Res. 63, 219–235. [ Links ]
Munro I.C. and Chaibonneau S. (1981). Contenido de mercurio y arsénico en atún y sardinas enlatadas mexicanas In: Food Safety (H.R. Roberts Ed.) Wiley New York. pp. 175–160. [ Links ]
Oliver L., Fisher W., Winstead J., Hemmer B. and Long E. (2001). Relationships between tissue contaminants and defense–related characteristics of oysters (Crassostrea virginica) from five Florida bays. Aquatic Toxicol. 55, 202–222. [ Links ]
Páez–Osuna F. (1996). Efectos de los metales. In:Golfo de México. Contaminación e impacto ambiental: Diagnostico y tendencias (A.V. Botello, G.J. Rojas, Benítez, J.A. and L.D. Zarate, Eds.). Universidad Autónoma de Campeche, EPOMEX. pp. 349–361. [ Links ]
Páez–Osuna F. (2005). Fuentes de metales en la zona costera marina. In: Golfo de México. Contaminación e impacto Ambiental: Diagnostico y tendencias, (A. V. Botello J. Rendon–von Osten, G. Gold–Bouchot and C. Agraz–Hernández, Eds) 2da Edición. Universidad Autónoma de Campeche, Universidad Nacional Autónoma de México, Instituto de Ecología. 696 p. [ Links ]
Palomarez–García J. M, Castañeda–Chávez M. del R., Lango–Reynoso F. and Landeros–Sánchez C. (2009). Niveles de metales pesados en camarón café Fartantepenaeus aztecas de la laguna de Tamiahua, Veracruz, México. Invest. Marinas 30, 63–69. [ Links ]
Palomarez–García J. M, Castañeda–Chávez M. del R. and Lango–Reynoso F. (2007). Niveles de metales pesados en camarón café Farfentepenaues aztecus de la laguna de Tamiahua. In:Avances en la Investigación agrícola, pecuaria, forestal y acuícola trópico mexicano. Libro Científico No. 4. Veracruz, México, Veracruz. Instituto de Investigaciones Forestales, Agrícolas y Pecuarias, Universidad Veracruzana, Colegio de Posgraduados, Universidad Autónoma Chapingo, Instituto Tecnológico de Ursulo Galván, Instituto Tecnológico de Boca del Río, Universidad Nacional Autónoma de México pp. 33–40. [ Links ]
Raimundo J. and Vale C. (2008). Partitioning of Fe, Cu, Zn, Cd and Pb concentrations among eleven tissues of Octopus vulgaris from the portugueses coast. Ciencias Marinas 34, 297–305. [ Links ]
Rainbow P.S., Phillips D.J. and Depledge M.H. (1990). The significance of trace metal concentrations in marine invertebrates. Mar. Pollut. Bull. 7, 321–324. [ Links ]
RAMSAR (2006). The Ramsar Convention Manual. A Guide to the Convention on Wetlands (Ramsar, Iran, 1971). 4th Ed. Gland, Switzerland: Ramsar Convention Secretariat, 118 p. [ Links ]
RAMSAR (2008). The Ramsar List. Established in response to Article 2.1 of the Convention on Wetlands (Ramsar, Iran, 1971). The Secretariat of the Convention on Wetlands, Switzerland, 41 p. [ Links ]
Robert D.A., Johnston E.L. and Poore A.G.B. (2008). Contamination of marine biogenic habitats and effects upon associated. Mar. Pollut. Bull. 56,1057–1065. [ Links ]
Rodríguez de la Rua A., González de Canales, M. L., Blasco, J. and Sarasquete C. (2005). Accumulation of cupper and histopathological alterations in the oyster Crassostrea angulata. Ciencias Marinas 31, 455–466. [ Links ]
Rosas I., Báez A. and Belmont R. (1983). Oyster Crassostrea virginica as indicator of heavy metal pollution in some lagoons of the Gulf of México. Water Air Soil Poll. 20, 127–135. [ Links ]
SAGARPA (2006). Carta Nacional Pesquera. México. Diario Oficial de la Federación, 25 de agosto, 149 pp. [ Links ]
SEMARNAT (1996). Norma Oficial Mexicana NOM–001–ECOL–1996. Límites máximos permisibles de contaminantes en las descargas de aguas residuales en aguas y bienes nacionales. Aclaración Diario Oficial de la Federación 30 abril de 1997. [ Links ]
SENASICA (2003). Manual de buenas prácticas de producción acuícola de moluscos bivalvos para la inocuidad alimentaria. Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación de México. México, 83 p. [ Links ]
SSA (1993). Norma Oficial Mexicana NOM–031–SSA1–1993. Limites máximos permisibles de contaminantes para productos de la pesca como moluscos bivalvos frescos y refrigerados; además de la metodología para la determinación de Vibrio cholerae. Secretaría de Salud, Diario Oficial de la Federación, 6 de Marzo de 1995. [ Links ]
SSA (1994). Norma Oficial Mexicana Bienes y Servicios NOM–117–SSA1–1994, Método de prueba para la determinación de cadmio, arsénico, plomo, estaño, cobre, fierro, zinc y mercurio en alimentos, agua potable y agua purificada por espectrometría de absorción atómica. Secretaría de Salud, Diario Oficial de la Federación. 29 de junio de 1995. [ Links ]
SSA (1994). Norma Oficial Mexicana NOM–109–SSA1–1994, Bienes y servicios. Procedimientos para la toma, manejo y transporte de muestras de alimentos para su análisis microbiológico. Secretaría de Salud, Aclaración Diario Oficial de la Federación 26 de mayo de 1994. [ Links ]
SSA (1995). Norma Oficial Mexicana Bienes y Servicios NOM–129–SSA1–1995. Productos de la pesca: secos–salados, ahumados, moluscos cefalópodos y gasterópodos frescos–refrigerados y congelados. Disposiciones y especificaciones sanitarias. Secretaría de Salud, Diario Oficial de la Federación. 29 de enero de 1996. [ Links ]
UNEP (2000). Regional seas reports and studies No. 174: Overview on land–based pollutant sources and activities affecting the marine, coastal, and freshwater environment in the Pacific islands region. United Nations Environment Programme. Nairobi, Kenya, 43 p. [ Links ]
Valette–Silver N.J., Riedel G.F., Crecelius E.A., Windom H., Smith R.G. and Dolvin S.S. (1999). Elevated arsenic concentration in bivalves from the southeast coasts of the USA. Mar. Environ. Res. 48, 311–333. [ Links ]
Villanueva–Fragoso S. and Páez–Osuna F. (1996). Niveles de metales en el Golfo de México: agua, sedimentos y organismos. En: Contaminación e impacto ambiental: diagnóstico y tendencias, Golfo de México. (A.V. Botello, J.L. Rojas–Galavíz, J. Benítez and D. Zárate–Lomelí, Eds.). Universidad Autónoma de Campeche, EPOMEX, pp. 309–347. [ Links ]
Walsh A.R. and O'Halloran J. (1998). Accumulations of chromium by population of mussels (Mytilus edulis L.) exposed to leather tannery effluent. Environ. Toxicol. Chem. 17, 1429–1438. [ Links ]
Wilson E.A., Powell E.N., Wade T.L., Taylor R.J., Presley B.J. and Brooks J.M. (1992). Spatial and temporal distributions of contaminant body burden and disease in Gulf of México oyster populations: The role of local and large–scale climatic controls. Helgoländer Meeresunter. 46, 201–235. [ Links ]
Wong–Chang I. and Barrera–Escorcia G. (2005). Estado actual de la contaminación microbiológicas en el Golfo de México. In: Golfo de México. Contaminación e impacto Ambiental. Diagnostico y Tendencias, (A. V. Botello, J. Rendón–von Osten, G. Gold–Bouchot and C. Agraz–Hernández, Eds.), 2nd. Ed. Universidad Autónoma de Campeche, Universidad. Nacional Autónoma de México, Instituto Nacional de Ecología. 696 p. [ Links ]














