Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista internacional de contaminación ambiental
versão impressa ISSN 0188-4999
Rev. Int. Contam. Ambient vol.26 no.1 Ciudad de México Fev. 2010
Artículos
Genotoxicity in lymphocytes of smokers living in México City
Genotoxicidad en linfocitos de fumadores habitantes de la Ciudad de México
Carmen CALDERÓN–EZQUERRO*1, César GUERRERO–GUERRA1, Raúl SANSORES–MARTÍNEZ2, María Elena CALDERÓN–SEGURA1, Rafael VILLALOBOS–PIETRINI1, Omar AMADOR–MUÑOZ1 and Sandra GÓMEZ–ARROYO1
1 Centro de Ciencias de la Atmósfera, Universidad Nacional Autónoma de México (UNAM). Circuito Exterior, Ciudad Universitaria, C.P. 04510, México D.F.*Autor responsable: mclce@atmosfera.unam.mx
2 Instituto Nacional de Enfermedades Respiratorias. Calzada de Tlalpan 4502, Col. Sección XVI, Delegación de Tlalpan. Ciudad de México. C.P. 14710
Recibido octubre 2008
Aceptado julio 2009
ABSTRACT
The genotoxic damage that tobacco smoke produces in active smokers was evaluated using cytokinesis–blocked micronucleus assay. The effect of tobacco smoke on the cellular cycle was analyzed by means of nuclear division index (NDI) and cytokinesis proliferation block index (CPBI). The results indicated a significantly lower frequency of micronuclei (MN) in the smoker group than in the control group. The nuclear division and cytokinesis proliferation block indexes indicated a delay in the cellular cycle of smokers and controls. The delay was greater in the controls (non–smokers) compared to smokers. Nicotine and cotinine contents in the urine samples of the subjects of both groups were also measured using gas chromatography/mass spectrometry; significantly higher levels were found in smokers, while values for controls could not be established accurately due to the fact that they fell below the limits of resolution accepted by the mass spectrometer. In general, no association was established between evaluated cytogenetic variables —binucleated (BN) cells with MN, total MN, NDI and CPBI— and nicotine and cotinine contents in smokers. However, when the information was analyzed according to subgroups —light, moderate and heavy—, an increase in correlation coefficients was found. The same strategy was used to analyze the rest of the cytogenetic variables and nicotine and cotinine. The results indicated that only the light–smoker subgroup exhibited a significant correlation coefficient between nicotine and the number of BN cells with MN.
Key words: micronuclei, human lymphocytes, nuclear division index (NDI), cytokinesis proliferation block index (CPBI), nicotine, air pollution.
RESUMEN
Se evaluó el daño genotóxico que produce el humo de tabaco en fumadores activos, a través de la prueba de micronúcleos por bloqueo de la citocinesis. El efecto del humo de tabaco sobre el ciclo celular también fue analizado mediante el empleo de los índices de división nuclear (IDN) y de bloqueo de proliferación de la citocinesis (IBPC). Los resultados mostraron una frecuencia significativamente menor de micronúcleos (MN) en el grupo de fumadores, con respecto al grupo testigo. Los valores de los índices IDN e IBPC mostraron que hubo retraso en el ciclo celular de los fumadores y de los testigos, aunque este retraso fue mayor en los testigos. También se midieron los contenidos de nicotina y cotinina en la orina de los sujetos fumadores y testigos evaluados, mediante cromatografía de gases/espectrometría de masas; en los fumadores se registraron los niveles significativamente más altos de estos compuestos. Los valores de los testigos no pudieron establecerse con exactitud debido a que cayeron por debajo de los límites de resolución aceptados por el espectrómetro de masas. En general, entre las variables citogenéticas evaluadas no se estableció ninguna asociación —células binucleadas con mincronúcleos, micronúcleos totales, índice de división nuclear, e índice de bloqueo de proliferación de la citocinesis— y el contenido de nicotina y cotinina en fumadores. Sin embargo, cuando la información fue analizada de acuerdo a subgrupos —ligeros, moderados e intensos—, se encontró un incremento en los coeficientes de correlación. En consecuencia, la misma estrategia se aplicó para el análisis del resto de las variables citogenéticas y los niveles de nicotina y cotinina. Como resultado, sólo el subgrupo de fumadores ligeros mostró un coeficiente de correlación signifcativo entre la nicotina y el número de células binucleadas.
Palabras clave: micronúcleos, linfocitos humanos, índice de división nuclear (IDN), índice de bloqueo de proliferación de citocinesis (IBPC), nicotina, contaminación del aire.
INTRODUCTION
Humans require clean environments to protect themselves from diseases. However, such environments are frequently pervaded by different substances noxious to health (Bardana and Montanaro 1997). Mexico City's inhabitants are exposed to an atmosphere containing a large variety of gases and contaminant particles such as ozone, carbon monoxide and dioxide, aromatic hydrocarbons, alcohols, inorganic gases, volatile organic compounds, aldehydes, metals, nitrogen oxide, particulate pollutants, pesticides, biologic contaminants and tobacco smoke, among others (Raga et al. 2001, Bravo et al. 2002).
It is well known that tobacco smoke is the cause of various diseases and is responsible for numerous deaths worldwide. Different types of cancer have been associated with smoking. Moreover, it has been shown that persons involuntarily exposed to environmental tobacco smoke (ETS) are more susceptible to these illnesses than unexposed persons (ASH 2006).
Noxious effects of tobacco smoke have been assessed for a long time by various bioassays that show alterations in the genetic material (Albertini et al. 2000). As a consequence, a damaging effect is caused to the integrity of the genetic material, mitotic spindle, or centromere. This kind of damage may be responsible for cellular death or the onset of neoplastic processes (Fenech et al. 1999). The cytokinesis–blocked micronucleus assay has been employed to evaluate possible alterations in chromosomes during interphase.
Genotoxicity studies on smokers have mainly been based on the utilization of peripheral blood lymphocytes to search for micronuclei (MN). These cells come into contact with constitutive elements of tobacco smoke as well as their by–products while entering the body. MN assessment enables the establishment of DNA damage and determines the possible affectation of the cellular cycle. Besides, it is an easier and faster method than the conventional test for chromosomal aberrations (Pardell 1996, Fenech et al. 1999, Fenech 2000).
The relation between MN appearance and tobacco smoking has been studied since mid–1980's, not only to evaluate the direct effect but also to discard a confusing effect in studies with other genotoxic agents. However, results have been contradictory and controversial.
Among the studies assessing the effect of tobacco smoke on MN induction in healthy subjects using the cytokinesis proliferation block technique is the research carried out by Au et al. (1991), Tomanin et al. (1991) and Holmen et al. (1995). These authors reported a significant increment in MN, even though the last study describes that this enhancement was restricted to the lymphocyte T8 cellular subpopulation.
On the other hand, Cheng et al. (1996) and Duffaud et al. (1999) described a confusing effect for tobacco smoke and showed a positive effect. Both studies were focused on persons with cancer (pulmonary and neck, respectively). Their results show a significant MN increment in sick patients that smoked (first case) and in healthy subjects that smoked (second case). Zhao et al. (1998) described the effect of atmospheric contamination, and Lohani et al. (2002) that of asbestos. Both studies reported a higher MN frequency, regardless of whether the subjetcs had been exposed to contaminants or asbestos or not.
Conversely, studies reporting no difference between MN frequency in smokers and nonsmokers are more numerous. Among the researches assessing the possible genotoxic effect of tobacco is the work by Surrallés et al. (1997) and Barale et al. (1998). The former found no significant differences, whereas the latter reported a significant decrement.
Other studies evaluating the genotoxic effect caused by various agents also included the possible confusing effect of tobacco smoke. Among them are Buckvic et al. (1998), Calvert et al. (1998), Burgaz et al. (1999), Pitarque et al. (1999), Lucero et al. (2000), Maluf et al. (2000) and Palus et al. (2003). The results of their researches conclude that study subjects exposed to tobacco smoke (smokers) showed no significant differences in MN frequency of lymphocytes when compared with their respective controls. Finally, Falck et al. (1999), working with pesticides, found that the control group that smoked exhibited a decrement in MN frequency regarding nonsmoker controls, even though the former also showed an increase in the frequency of chromosomal aberrations originating MN.
A possible explanation for these contradictory results comes from Bonassi et al. (2003). They evaluated and re–analyzed (meta–analysis) the results relating MN frequency to lymphocytes in smoking habits. Overall, these studies recruited 5710 persons, of whom 1409 were active smokers and 800 ex–smokers. The main finding of this meta–analysis was that the only subpopulation expressing a significant increase in MN frequency was that of heavy smokers (30 cigarettes or more daily), but only when these persons had not been previously exposed to genotoxic agents. Otherwise this increment was not found.
Studies evaluating nuclear division or cytokinesis proliferation block index are scarce. Palus et al. (2003), using the nuclear division index (NDI), reported no differences between a smoker group and its control group. Pitarque et al. (1999) employed the cytokinesis proliferation block index (CPBI) to find a slight non–significant decrement in the smoker group. These authors also evaluated the smoking effect in conjunction with the exposure to other toxic agents (cadmium, lead, and contamination produced in airports). They reported no differences between the considered groups and their respective controls, whereas Pastor et al. (2002) did find a CPBI decrement caused by tobacco.
The joint analysis of MN in lymphocytes from peripheral blood and nicotine determination has not been undertaken yet, though some preliminary results are available. Lee et al. (1990) studied the genotoxic effect of tobacco smoke in rats exposed for 90 days. They measured nicotine levels in plasma to verify smoke ingestion. No genotoxic effect was found in bone marrow cells (micronuclei, sister chromatid exchanges or chromosomal aberrations), so they did not attempt to correlate both values.
Nersessian and Arutyunyan (1994) carried out a similar study in mice; they analyzed genotoxic effects of ten different types of cigarettes from Eastern Europe. They found a strong increase in MN in polychromatic erythrocytes from bone marrow. Their results indicated not only that American cigarettes are less clastogenic, but also that there is a strong correlation between nicotine and tar contents in blood plasma and MN frequency.
The following evidence has been determined regarding to whether this correlation could be due to a genotoxic effect of nicotine per se or is a consequence of the amount of tobacco smoke one has been exposed to.
Badr et al. (1997) found that nicotine values ranging from 80 to 500 mg (per kilogram of body weight) administered orally increase MN frequency in bone marrow cells in mice infected with Schistosoma mansoni as well as in uninfected mice. Likewise, in ovary cells of the Chinese hamster (quantities similar to those ingested with nicotine chewing gum), high nicotine concentration caused an increment in the frequency of both sister chromatid exchange and chromosomal aberrations (Trivedi 1993). Lower nicotine doses (1–2 mg per kilogram of body weight) were not clastogenic as indicated by the MN test in lymphocytes (Adler and Attia 2003). Doolittle et al. (1995) evaluated genotoxic activity of nicotine and cotinine using Ames and SCE assays in Chinese hamster cells, with negative results.
In México, studies on tobacco smoking are mainly epidemiologic. The results indicate that health damage due to tobacco is clearly associated with an increase in morbidity and mortality (SSA 2002). There have been few studies assessing DNA damage in smokers and the applied method has been alkaline single cell gel electrophoresis (comet assay) (Rojas et al. 1996). More recently, two methods have been employed for the same purpose: the determination of cell proliferation kinetics and genotoxicity in lymphocytes (Calderón–Ezquerro et al. 2007).
Therefore, the main objective of this study is to assess the possible genotoxic effect in smokers living in México City by micronucleus determination in peripheral blood lymphocytes, using the proliferation cytokinesis block assay, as well as its correlation with exposure markers: nicotine and cotinine.
MATERIALS AND METHODS
Subjects
Sixty four subjects participated in this study; they were divided into two groups. The first was made of 32 smokers recruited from the Anti–Tobacco Clinic in the Instituto Nacional de Enfermedades Respiratorias (INER, National Institute of Respiratory Diseases) in México City while they still smoked. Each volunteer attended the clinic in search of treatment to quit smoking. The second group was formed by 32 healthy nonsmokers (control group) and was matched in age and gender to the smoker group.
The smoker group consisted of 18 women and 14 men. Smoker age was 47.38 ± 12.97 years old (mean ± standard deviation), with an interval from 23 to 74 years of age. Number of cigarettes smoked per day was 21.97 ± 10.73, ranging from 5 to 60. The mean number of years smoking was 27.91 ± 13.10, with a range from 4 to 60 years. In order to participate in the study, smokers were required to have had a daily minimum consumption of five cigarettes for more than a year.
The control group included 18 women and 14 men. Mean age was 47.68 ± 13.19 years old (mean ± standard deviation), in an age range from 17 to 75 years. Participants in this group were nonsmokers, unexposed to tobacco smoke (ETS)(Albertini 2000) at home or in their workplace. The age and gender of the control group were matched to the smoking group. In addition, it was verified that none of the participants were on medication, were alcoholics, or regularly exposed to genotoxic substances.
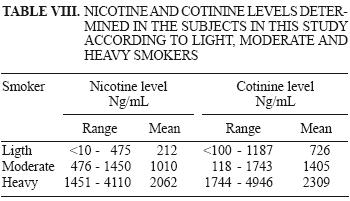
The classification of subjects within the smoking group was defined according to the number of cigarettes smoked per day: light smokers (<19 cigarettes a day), moderate smokers (20 to 29 cigarettes), and heavy smokers (> 30 cigarettes).
Samples of peripheral blood and urine were taken from all participants.
Cytokinesis–blocked micronucleus (CBMN) assay Venous blood samples from all volunteers of both groups were drawn with heparinized vacutainer tubes and transferred to the laboratory within a few hours of taking the sample. Blood (400 μL) from each sample was added to 4.5 mL RPMI medium 1640 containing L–glutamine (Gibco) plus 0.2 mL phytohemagglutinin (4 % or 5 μg/mL) (Gibco) previously sterilized using a 0.22 μm nitrocellulose filter. This procedure was done by triplicate for each of the subjects in the study.
Subsequently, cultures were incubated at 37 °C. After 44–h incubation, cytochalasin B (Sigma) previously dissolved in DMSO (6 μg/mL final concentration) was added to the cultures and stored at –20 °C for an additional 28 hours; total culture time was 72 hours. Afterwards, cells from each culture were harvested by centrifugation, given a hypotonic shock (0.075 M KCl) at 4 °C for 3 minutes followed by a prefixed procedure that slowly added four drops of a methanol–acetic acid mixture (3:1) at 4 °C. Each tube was gently shaken and let stand for 10 minutes at room temperature.
After a 10 minute centrifugation (1500 rpm), a fixation step was carried out by adding 10 mL methanol–acetic acid mixture (3:1) at 4 °C. Tubes were again centrifuged and the former procedure was carried out until the pellet looked clean.
Cells were smeared on microscope slides and air–dried. Slides were stained with the Fulgen reaction technique described by Stich and Rosin (1984) and Stich (1987) and modified as follows (Gómez–Arroyo et al. 2000): smeared cells were pretreated in 1N HCl for 10 minutes at room temperature, then placed in 1N HCl at 60 °C for another 10 minutes, rinsed in distilled water, placed in Schiff's reagent for 90 minutes and washed with running tap water. Afterwards, slides were immersed in a 1 % fast green solution for 30 seconds, then submitted to four consecutive washes in ethanol to eliminate excess dye, and finally air–dried. In order to avoid bias, all slides were coded (smoker/nonsmoker) before counting micronuclei (MN).
Using a light microscope (Olympus BX51 model), MN were scored for every 1000 binucleated cells counted per individual in accordance with accepted criteria (Fenech et al. 2003).
Micronuclei were reported in two different manners: total micronuclei (TMN) and binucleated cells with micronuclei (BN cells with MN). To obtain total MN, the number of all MN was counted on 1000 cells. For binucleated cells with micronuclei, every binucleated cell was counted as one unit, no matter the number of micronuclei inside it.
The quantification of TMN and BN cells with MN was carried out following both procedures so that a comparison of the results with those of other studies could be established, since not all reports employ the same way of counting micronuclei.
In addition, 500 more cells per individual were analyzed to count the number of cells containing 1, 2, 3 and 4 nuclei, respectively. This was carried out to derive the nuclear division and cytokinesis proliferation block indexes with the following formulas:
• Nuclear division index, NDI (provides the average number of nuclei per cell):
NDI = [M1+2(M2) +3(M3) + 4(M4)/ total number of cells]
• Cytokinesis proliferation block index, CPBI (provides the average number of complete cellular divisions per cell):
CPBI = [M1+2(M2) + 3(M3+M4)/ total number of cells]
where M1 is the number of mononuclear cells, M2 binuclear, M3 trinuclear, and M4 tetranuclear (Kirsch–Volders et al. 2003).
The value of these indexes in healthy subjects, unexposed to toxic substances, is very near to 2 or slightly higher. Values less than 2 indicate a delay in the cellular cycle; values higher than 2 mean its acceleration (Surallés et al. 1995, Albertini 2000).
Detection and quantification of nicotine and its metabolite cotinine in urine samples from the subjects evaluated
For each individual, urine samples were collected in the morning. From each sample, nine aliquots were drawn, placed in 1.5 mL Eppendorf tubes, and kept at –70 °C until needed to perform nicotine and cotinine analyses.
Nicotine and cotinine in urine were quantified using gas chromatography–mass spectrometry. The method of Hutchinson et al. (1998) was followed taking into account their respective deuterated internal standards. Analyses were isolated by liquid–liquid extraction coupled to centrifugation and evaporation. The sensitivity test indicated 10 and 100 ng/mL nicotine and cotinine, respectively. Calibration curves were made ranging from 1 to 3000 and from 1 to 10,000 ng/mL for nicotine and cotinine, respectively. All data were corrected for recovery efficiencies.
Statistical analysis
Results did not follow a normal distribution function; therefore, the non–parametric Mann–Whitney U test and Kruskal–Wallis test were used to determine significant differences. Pearson's correlation coefficient analysis was calculated to establish possible relationships between TMN frequency, BN cells with MN, NDI and CPBI parameters and nicotine and cotinine levels. Data were analyzed with the MINITAB statistics program, version 13.2.
RESULTS
Intergroup variations in MN frequency and cellular cycle alterations
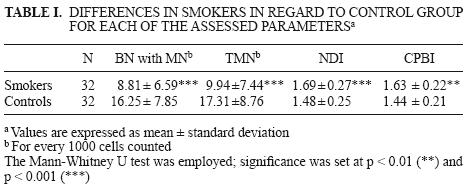
The genotoxic effect in smokers represented by the number of binuclear cells with micronuclei (BN cells with MN) and total micronuclei (TMN), as well as the alteration of the cellular cycle measured with nuclear division (NDI) and cytokinesis proliferation block (CPBI) indexes, is shown in Table I. Smokers exhibited mean values for BN cells with MN and TMN significantly smaller when compared with the control group, as well as a faster cellular cycle expressed as an increment in NDI and CPBI. Control values showed a delay in cellular cycle regarding smokers as well as in relation to the value equal to 2 reported for both indexes in healthy persons unex–posed to genotoxic agents (Surallés 1995).
Possible DNA damage in smokers was analyzed in the subgroups according to age and gender. Results are shown in Table II. Male and female smokers exhibited lower frequencies in BN cells with MN and TMN compared to the control group. Regarding the cellular cycle, female smokers showed higher mean values and significant differences for both indexes, NDI and CPBI, than controls. On the other hand, male active smokers showed no significant differences for these indexes when compared with their controls.
Considering that the age interval in the smoker group was wide (23 to 74 years old), it was divided into three subgroups. Individuals from 23 to 40 years of age (smokers and controls) showed no significant differences among them in any of the assessed parameters. Smokers from 41 to 57 years old showed significant differences in the number of BN cells with MN and TMN, as well as significant increases in NDI and CPBI with regard to the controls, whereas significant differences were only found between smokers and controls in both indexes in the 58 to 73 year–old subgroup.
Table III shows the cytogenetic analysis for smokers according to nicotine and cotinine concentrations found in urine samples. Light and moderate smokers exhibited significantly smaller values in the number of BN cells with MN and TMN, unlike heavy smokers. Only moderate smokers showed a significant difference in CPBI in regard to controls, though in the three smokers subgroups a higher mean value was observed in NDI and CPBI variables when compared with the corresponding controls.

Likewise, NDI and CPBI were correlated to BN cells with MN and TMN. For heavy smokers a correlation coefficient equal to –0.758 (p = 0.049) was found between NDI and BN cells with MN; similarly, a correlation coefficient equal to –0.764 (p = 0.046) was found between NDI and TMN. For the second index (CPBI), correlation coefficients equal to –0.764 (p = 0.046) and –0.799 (p = 0.031) were found between this index and BN cells with MN and TMN, respectively. For light and heavy smokers the correlation coefficients between the indexes and MN frequencies were not significant.
Intragroup variations in MN frequency and cellular cycle alteration in smokers
The frequency of BN cells with MN and TMN, as well as cellular cycle alterations evaluated by the indexes (NDI and CPBI), was also assessed in an intragroup manner. That is, the possible influence of gender, age, number of consumed cigarettes (according to nicotine levels) and time of exposure to tobacco smoke was evaluated among the subjects in each group (smokers and controls).
The outcome of the smoker group (Table IV) showed that the only statistical difference encountered according to gender was that women presented a significant (p < 0.05; Kruskal–Wallis test) increase in the frequency of total MN cells (12.17 ± 8.72) in regard to men (7.07 ± 4.10).
Control groups in this study were classified by age and gender. The analysis of these variables showed no significant differences in any of the evaluated parameters.
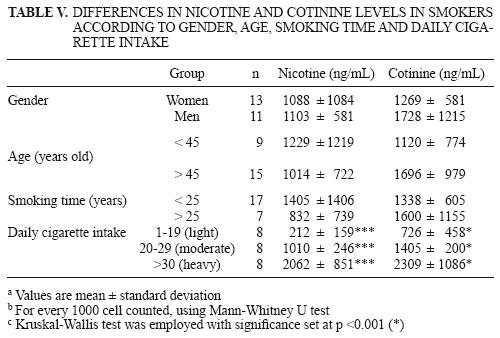
Smokers were also divided into groups according to gender, age, time with smoking habit and daily cigarette intake to determine the differences in nicotine and cotinine levels in light, moderate and heavy smokers (Table V). No significant differences were found between genders, subjects younger or older than 45 years old, and time of smoking habit (more or less than 25 years). However, when smokers were divided into light, moderate and heavy, significant differences (p < 0.001) were found, considering that recorded concentrations of these exposure biomarkers reflected the real number of cigarettes smoked daily.
Due to the small size of the sample and the resolution limits of the test employed, the analysis in control group could not be undertaken.
Table VI show the Pearson's correlation coefficients and significance values for nicotine and cotinine levels in smokers regarding other assessed parameters. No relationship was established between any of the assessed variables.
Likewise, in separating the group of smokers in light, moderate and intense it was found that Pearson's correlation coefficients obtained comparing nicotine and cotinine levels regarding the parameters assessed (BN cells with MN, TMN, NDI, CPBI) and some general characteristics (age, years of smoking habit, daily cigarette intake), no relationship was established between any of the assessed variables.
Nicotine and cotinine levels in evaluated subjects
From the 32 smokers participating in the study, only 24 urine samples were collected. These samples were used for the nicotine and cotinine quantification. Five urine samples were collected from controls. Results are shown in Table VII.
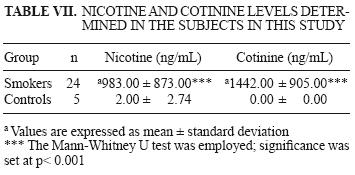
Nicotine and cotinine values for controls were below the mass spectrometer limits of resolution (10 ng/mL and 100 ng/mL for nicotine and cotinine, respectively). Hence, the exact minimum value could not be determined for either of them. Significant differences were found (p < 0.05) between smokers and nonsmokers. Smokers exhibited high concentrations of both exposure biomarkers.
Nicotine and cotinine levels in urine of smokers are shown in Table VIII.
DISCUSSION
MN frequency and cellular cycle alterations
Binuclear cells with micronuclei and total micro–nuclei. The amount of MN found in smokers (Table I) was significantly smaller than that in controls. Moreover, this difference became more specific when smokers were divided into ranges according to age and gender, particularly when comparisons were made between subjects 41–57 years old, unlike comparisons involving smokers older than 58 years of age (Table III). These results agree with Barale et al. (1998) and Bonassi et al. (2003) in the sense that tobacco smoking exerts a decreasing effect on MN frequency; this study's result is close to the historic mean value of binuclear cells with micronuclei 7.8 ± 5.2 for every 1000 cells counted (Surallés and Natarajan 1997). However, this marked reduction (almost 50 %) has not been previously reported. Barale et al. (1998) and Bonassi et al. (2003) found 16 % and from 3 to 10 % reductions, respectively.
By assessing genotoxicity in smokers through nicotine in urine (Table I) and classifying them as light, moderate and heavy smokers, significant differences were found in the case of BN cells with MN and TMN for light and moderate smokers, but not for heavy smokers (Table IV). The general trend was a lower frequency in these kind of cells regarding controls. These results are partly in accordance with the findings by Bonassi et al. (2003) in which smokers with a daily intake lower than 20 cigarettes showed a decrease in MN frequency, whereas a non–significant increase for subjects smoking between 20 and 29 cigarettes was reported. In smokers beyond 30 cigarettes, a significant increase was found in BN cells with MN and TMN, as long as these subjects were not exposed to mutagenic or carcinogenic agents; when exposed to such agents this increment was not present.
All subjects in this study live in México City, where they are exposed to an atmosphere containing a large variety of gaseous and particulate pollutants (Calderón–Garcidueñas et al. 1999, Raga et al. 2001, Bravo et al. 2002). Thus, smokers living here are not only affected by their own tobacco smoke, but also by environmental tobacco smoke (ETS) and a mixture of air–suspended chemical compounds. Several authors have demonstrated various genotoxic effects and health damage. For example, Rubio et al. (1990) suggest a possible synergistic effect between tobacco smoke and atmospheric pollution resulting in a process harmful to respiratory function. Studies of the effects of air pollutants on Drosophila melanogaster in México City have demonstrated the genotoxicity of polycyclic aromatic hydrocarbons (PAHs) and their nitro derivatives (Delgado–Rodríguez et al. 1995), as well as that of organic extracts of airborne particles (Delgado–Rodríguez et al. 1999). Similarly, Villalobos–Pietrini et al. (2000),in a genetic monitoring study of airborne particles using the Ames test (Salmonella typhimurium), demonstrated that compounds such as PAHs contained in urban atmospheres exhibit mutagenic activity. Another study about the genotoxic, cytokinetic and cytotoxic effects of extracts from airborne particles collected in México City suggested that the extent of the changes caused by pollutants was partly dependent on the seasonal weather. The highest genotoxicity rates (SCE) were found in November (cool and dry); at this time of the year, all 15 polycyclic aromatic hydrocarbons studied were present in the organic material extracted from air samples (Calderón–Segura et al. 2004). Recently, a study by Roubicek et al. (2007) investigated the ability of chemically–characterized water and PM10 organic/soluble extracts from two areas of México City to induce micronuclei in a human epithelial cell line; the association between PM chemical characteristics and genotoxicity was also evaluated. The authors reported that both industrial and residential extracts induce a significant concentration–related increase in micronucleus frequency. Comparative analyses between micronucleus induction and chemical compounds showed that cadmium and PAHs exhibit a significant correlation with micronucleus induction. Effects observed in this study emphasize the risk of PM exposure in México City.
On the other hand, Calderón–Ezquerro et al. (2007) assessed the genotoxicity in tobacco smokers living in México City and found a delay in cell proliferation kinetics (CPK), as well as a decrease in the replication index (RI). Cytokinetic effects were mainly detected in heavy and moderate smokers. These results, common in all "healthy" smokers in this study, are similar to those of other authors who studied diverse xenobiotic agents and concluded that synergistic and/or potential effects may be involved in producing cytokinetic and genotoxic effects (Grandjean et al. 1983, Hedner et al. 1983, McGowen et al. 2000, Duydu et al. 2001, Palus et al. 2003). These findings suggest that smokers living in places with high atmospheric contamination levels are subject to a greater risk from smoking due to genotoxic, mutagenic and/or carcinogenic agents.
Hence, smokers evaluated in this study were not only exposed to inhaled tobacco smoke, but also to a complex mixture of chemical compounds. According to Bonassi et al. (2003), the association between the increase in MN frequency and heavy smoking in subjects unexposed to carcinogenic or mutagenic environments may account for the outcome of lower MN frequency in smokers than in controls. It could also explain why heavy smokers exposed to various pollutants were incapable of expressing a higher MN frequency compared with controls.
Studies in several countries reported lower frequencies of MN in the control groups than those reported in this study (Barale et al. 1998, Karahalil et al. 1998, Zao et al. 1998, Burgaz et al. 1999, Pitarque et al. 1999, Baier et al. 2002, Palus et al. 2003).
Bonassi et al. (2003) state that "exposure to genotoxins may stimulate the expression of DNA repair genes or detoxification mechanisms that are also important in attenuating the genotoxic effects of chemicals in cigarette smoke." Similarly, the results obtained in light and heavy smokers compared with those of nonsmokers, in whom a decrease in MN frequency was found, coincide with other reports. These studies consider that a few cigarettes per day may stimulate an adaptive response (cell protective) causing a decrease in MN frequency, whereas continued exposure to mutagens/carcinogens may induce resistance to further DNA damage (Benner et al. 1992, Gourabi and Mozdarani 1998, Rothfub et al. 1998, Bonassi et al 2003).
Control subjects exhibited no differences regarding gender or age when compared with smokers. However, they showed higher frequencies in BN cells with MN and TMN; in some cases twice the values (16.25 ± 7.85 and 17.31 ± 8.76, respectively) of smokers (8.81 ± 6.59 and 9.94 ± 7.44, respectively).
It has been reported that factors such as gender and age may account for 32 % (male) to 48 % (female) variations in MN frequency (Fenech 1998a). However, this is not the case in this study for age and gender distributions were identical for both study groups. By looking at the results summarized in Tables II and IV subgroups of older ages did not present a significant increase in MN frequency, which may reflect the small sample size. The comparison of this study's results with those of other reports (Barale et al. 1998, Karahalil et al. 1998, Zhao et al. 1998, Pitarque et al. 1999, Baier et al. 2002, Bonassi et al. 2003, Palus et al. 2003) indicates that MN values found in this study are only surpassed by those in Lohani et al. (2002) for the nonsmoker group (62.48 ± 5.96).
Similarly, when comparing MN frequencies in this study with those reported in research on tobacco smoking associated with genotoxic agents such as PAHs (Karahalil et al. 1998), contaminants from road traffic (Zhao et al. 1998), airports (Pitarque et al. 1999), gas stations (Buckvic et al. 1998), leather factories (Somorovska et al. 1999), greenhouses (Lucero et al. 2000) and contaminated cities (Romanova and Bezdrobna 2001),it was found that MN frequency in controls was similar to that in nonsmokers exposed to diverse genotoxic agents. The same results were obtained in studies where subjects had been exposed to asbestos (Lohani et al. 2002), cadmium and lead (Palus et al. 2003), epichlorhidrine (Hindsǿlandin et al. 1997), radiation (Chang et al. 1997) and low frequency magnetic fields (Scarf et al. 1997). In cases where individuals were subjected to intense exercise (Schiff et al. 1997)or suffered diseases such as cancer (Duffaud et al. 1999), leprosy (Kalaiselvi et al 2002) or alcoholism (Maffei et al. 2002), it was also found that MN frequency in controls was similar to that in nonsmokers exposed to genotoxic agents.
The increase in MN frequency in controls, which coincides more with nonsmokers exposed to genotoxic agents than with those living in clean environments —with a minimum amount of pollutants—, suggests a possible genotoxic effect, given that a large amount of these pollutants have known oxidative properties which may induce stress in cells in contact with them. Effects of oxidative stress on cells are varied; these include induction of sister chromatid exchange (SCE), chromosomal aberrations and reduction of cellular proliferation (Lioi et al. 1998, Burgaz et al. 2002). Furthermore, oxidative stress causes decrease in the lymphocyte response to mitogenic agents (Bechoua et al. 1999), rupture of a strand of DNA (Chen et al. 2003), formation of MN cells in the buccal mucosa in children (Lahiri et al. 2000) and splenocysts and fbroblasts in mice (Dreosti et al. 1999). In addition, it has been found that elder people are more sensitive to oxidative stress than young persons (López–Hellin et al. 1998).
Several studies have shown that exposure to environmental pollutants present in urban environments contribute to the genotoxicity and carcinogenicity in humans mainly exposed to PAHs. There is evidence linking PAH metabolism with the generation of reactive oxygen species (ROS) following oxidative stress, leading to DNA damage (Flowers et al. 1997, Bolton et al. 2000, Singh et al. 2007). Likewise, exposure to breathable particulate matter can result in the influx of alveolar macrophages, which in turn can generate free radicals leading to oxidative stress (Li et al. 1997). Studies carried in México have shown that environmental pollution causes oxidative stress (Sánchez et al. 2004) leading to genotoxic damage in the nasal mucosa of children (Calderón–Garcidueñas et al. 1999) which in turn may reduce cellular proliferation (Calderón–Segura et al. 2004) and enzymatic activity associated with combating oxidative stress. This is the case with superoxide dismutase which is reduced to half its normal concentration after just 16 weeks of exposure (Medina–Navarro et al. 1997). The former suggests that living in contaminated cities can lead to an elevated frequency of micronuclei, as happens in Kiev, where Romanova et al. (2001) reported a value of 10.5 in TMN frequency in a nonsmoker sample with an average age of 42 years. Similarly, Roubicek (2007) reported that in México City exposition to particle extracts (PM10) present in the air induced a significant increase in micronucleus frequency in a human epithelial cell line.
On the other hand, and in spite the fact that smokers in the latter study live in México City and are exposed to contamination, their low MN frequency may be due to their erythrocyte protective properties. These cells have elevated concentrations of reduced glutathione (GSH) and catalase (Sierra et al. 2004). Erythrocytes possess large amounts of antioxidizing agents; it has been found that these kind of cells present more reduced glutathione and catalase in smokers than in nonsmokers. Considering that these cells are constantly exposed to oxidative stress (caused by tobacco smoke) and that cells are endowed with adaptive mechanisms, a higher yield of these protective enzymes is produced as a consequence (Sierra et al. 2004).
This adaptive process has been previously described by Rothfub et al. (1998). They discovered that hyperbaric exposure with 100 % oxygen during three 20–minute periods induces MN formation in lymphocytes that is gradually reduced to levels lower than baseline values at the beginning of the treatment. There is evidence that enzymes such as 8–oxo–7,8–dihydrodeoxyguanosine are involved in this process and capable of repairing DNA adducts originated by oxidizing agents. These enzymes have been found in mammals and their function is to remove oxidized bases in DNA. The amount and effciency of these enzymes are enhanced after exposure to reactive oxygen species (Klaunig and Kamendulis 2004). Besides, exposure to tobacco smoke increases reduced glutathione levels in the bloodstream (Oesch et al. 1994) and even though tobacco smoke has oxidative properties, it causes no reduction in the concentrations of the main enzymes (superoxide dismutase, catalase and glutathione peroxidase) involved in the protection against this type of stress; however, a reduction is present with age (Bolzan et al. 1997).
Some components in tobacco smoke, such as hydroquinone, are capable of reducing MN formation. This compound, found in large quantities in tobacco smoke, reduces MN frequency significantly in bone marrow of mice when they are exposed to a direct antioxidizing effect (O'Donoghue et al. 1999).
Yildiz (2004) pointed out that a low nicotine concentration can prevent oxidative stress, but high concentrations induce it. An adequate adaptive response in erythrocytes of smokers combined with the presence in tobacco smoke of compounds capable of reducing MN frequency makes viable the hypothesis that tobacco smoke can in fact reduce MN frequency in light and moderate smokers.
In addition, MN frequency is markedly sensitive to other factors such as diet. Inclusion of vitamins C and E, β–carotenes and zinc in food may significantly reduce MN frequency (Konopacka and Rzeszowska–Wolny 2001); even amounts as small as 700 μg of folic acid and 2.5 μg of vitamin B12 may reduce MN frequency in 25 % (Fenech 1998b). Polymorphisms in gene GSTT1 are another variable affecting MN frequency. Individuals lacking this gene or having altered versions are more susceptible to have higher MN frequencies, whether they have been exposed to genotoxic agents or not; this is due to a defcient production of glutathione–S–transferase (Vlachodimi–tropoulos et al. 1997). These factors are difficult to control; hence their effect in this study is unknown, though it is highly improbable that they would have preferentially acted on a particular group. Moreover, the participants in this study have diverse occupational activities, educational levels and socioeconomic backgrounds; the only common characteristic that enabled their classification into groups —smokers and subjects in contact with tobacco smoke only occasionally— is that all are inhabitants of México City.
In summary, results related to MN induction indicate that subject controls have been exposed to the action of one or more MN inducing agents that elicit high MN frequencies. Tobacco smoke has the capacity of: a) avoiding an increment in MN frequency in active smokers (light and moderate) by the activation of an adaptive response in erythrocytes that enhances their antioxidant properties, and b) inducing genotoxic and cytotoxic responses which reduce the dividing rate of lymphocytes and therefore hinder MN expression and observation.
Nuclear division and cytokinesis proliferation block indexes
According to Table I, results associated with the progression of the cellular cycle showed significant differences between smokers and controls considering NDI and CPBI. The corresponding control group (N = 32) for smokers presented the following values: 1.48 ± 0.25 and 1.44 ± 0.21 for NDI and CPBI, respectively. These results indicate that the cellular cycle was faster for smokers than their controls.
These results suggest that tobacco smoke exerts an accelerating effect on the cellular cycle in smokers. This finding agrees with that of Woggner and Wong (1994) and Argentin and Cicchetti (2004), who report that nicotine stimulates cellular proliferation in some cases (gingival fbroblasts and cervical epithelium, respectively). However, other studies support the opposite hypothesis, that is, tobacco smoke does not accelerate the cellular cycle, but can even cause a delay. Pastor et al. (2002) specifically report a decrease in CPBI associated with tobacco smoking. Pitarque et al. (1999) and Palus et al. (2003) show NDI and CPBI values close to 2 in smokers as well as in controls; no significant differences resulted in any of the cases. As mentioned before, a value equal to 2 occurs when there are no external factors capable of altering the cellular cycle. Scarf et al. (1997), Lucero et al. (2000) and Pastor et al. (2002), when evaluating genotoxic agents, found CPBI values for controls higher than those found in this study (1.79 ± 0.15, 1.82 ± 0.15 and 1.52 ± 0.02, respectively). On the other hand, Maffei et al. (2002) obtained a lower mean value (1.37 ± 0.05), which might show a cellular delay in the control group of that study.
There is evidence demonstrating that exposure to atmospheric pollutants decreases the lymphocyte response to phytohemagglutinin (Tomei et al. 2004) and that tobacco smoking as well as chronic exposure to nicotine reduce cellular proliferation (Sopori et al. 1993, Sánchez 2004, Yildiz 2004, Calderón–Ezquerro 2007). These results suggest the existence of a delay in the cellular cycle of smokers and controls; the latter might occur due to the environmental pollution in México City.
Subjects in this study were exposed to high levels of environmental pollution. Therefore, the finding that NDI and CPBI values are significantly lower for control groups when compared with those of smokers indicates that tobacco smoke may interact with pollutants or agents responsible for the cellular delay in such a way that its effect is reduced in smokers. The mechanism of action is not known but perhaps among the more than 400 chemical compounds comprising tobacco smoke, one or more exhibit antagonistic effects (Villalobos–Pietrini et al. 2007) toward components borne in the atmosphere and/or may have genotoxic effects, delay the cellular cycle, or even establish a competition among components from both sources once they are inside the body. Moreover, there is a possibility that components coming from tobacco smoke present a higher affinity toward cells, thus rendering less harmful. This hypothesis is supported by the strong negative correlation between both indexes (NDI and CPBI) and MN frequency. The correlation coefficient between NDI and BN cells with MN was –0.758 (p = 0.049), whereas for NDI and TMN it was –0.764 (p = 0.046). As for the CPBI, it showed a correlation coefficient of –0.764 (p = 0.046) with BN cells with MN and –0.799 (p = 0.031) with TMN. These values were derived from the heavy smoking group only. The former suggests that subjects consuming more than 30 cigarettes a day present a higher frequency of MN, and concurrently a higher cellular delay. Although, a possible reason for the lower MN frequency in smokers could be that heavy damaged cells might have failed to divide or died during their ex–vivo culture, so it was not possible the induction of MN.
This situation may not be provoked by tobacco smoke, since no correlation was established among nicotine and cotinine concentrations with BN cells with MN and TMN in this type of smokers. In light and moderate smokers, correlation coefficients between indexes and MN frequencies were not significant.
Nicotine and cotinine
Quantifcation of nicotine and cotinine was similar to that reported by the Environmental Protection Agency (EPA 1997), who issued values of 1749 and 1391 ng/mL for nicotine and cotinine in urine of active smokers. In this study the corresponding values are 983 and 1442 ng/mL, respectively (Table I).
Our results indicate that gender, age and time of smoking (Table VI) are not factors modifying nicotine and cotinine concentrations. This is not in agreement with Prather et al. (1993), who report higher excretion levels of nicotine and cotinine for women; Swan et al. (1993), who specify a positive correlation of cotinine levels with age, and Pardell (1996), who establishes a higher nicotine excretion in chronic smokers.
There is evidence showing that nicotine metabolism is affected by various factors such as food regimes, gender, ethnic group, tar content, type of cigarette and even genetic polymorphisms (Patterson et al. 2003). These aspects were not assessed in this study and may participate in the outcome of this research. Similarly, analyses were carried out with urine samples collected early in the morning; this means that nicotine and cotinine concentrations were indicative of the number of cigarettes smoked before bedtime rather than the number smoked throughout the entire day.
Nevertheless, the daily consumption of cigarettes is not a good indicator of the nicotine content entering the body. Factors such as type of cigarette, number, quality of inhalations, presence of additives (menthol favors deeper inhalations, for example), tar content, whole or partial consumption of cigarettes, all contribute significantly to the amount of absorbed nicotine (Patterson et al. 2003). In a study undertaken in Germany in 1998 (Heinrich et al. 2004), the contents of nicotine and cotinine were assayed in 5000 participants. The authors concluded that the number of cigarettes consumed accounted for only 42 % of the differences found for nicotine and 51 % for cotinine. Therefore, other factors are involved in nicotine metabolism.
In this study, correlation values (Table VII) between the number of cigarettes per day and nicotine and cotinine concentrations were low and non significant (0.90 and –0.44 for nicotine and cotinine, respectively). This could mean that there is no correlation between the previously mentioned variables. On the other hand, several reports show the following correlation values for cotinine in urine: 0.24 (Wall et al. 1988), 0.54 (Suter et al. 1995), 0.62 (Jacob et al. 1988) and 0.67 (Fried et al. 1995). However, Swan et al. (1993) found that cotinine values in the saliva of smokers followed a non–linear behavior, mainly in persons with moderate cigarette consumption. As a result, the experimental population in this study was divided in subgroups —light, moderate and heavy— to render a better correlation coefficient for light and heavy smokers; for heavy smokers this coefficient was reduced. The same scheme was followed in this study for nicotine and cotinine values in urine.
The lack of a significant correlation between daily cigarette consumption and nicotine and cotinine levels may be due to unreliable information provided by smokers, with the possibility of being underestimated in some cases and overestimated in others.
In general, no association was established among evaluated cytogenetic variables (BN cells with MN, TMN, NDI and CPBI) and nicotine and cotinine contents in smokers. However, when the information was analyzed according to subgroups —light, moderate and heavy—, an increase in correlation coefficients was found.
ACKNOWLEDGEMENTS
The authors thank the Anti–tobacco Clinic of the Instituto Nacional de Enfermedades Respiratorias for their collaboration in allowing us to work with smoker patients. We are also grateful to Miguel Ángel Meneses for his technical assistance, and Marcela Sánchez for revising the English version of this manuscript.
REFERENCES
Adler D. and Attia S. (2003). Nicotine is not clastogenic at doses of 1 or 2 mg/kg body weight given orally to male mice. Mutat. Res. 542, 139–142. [ Links ]
Albertini R., Anderson D., Douglas G., Hagmar L., Hemminki K., Merlo F., Natarajan A., Norppa H., Shuker D., Tice R., Waters M. and Antero A. (2000). IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutat. Res. 463, 111–172. [ Links ]
Argentin G. and Cicchetti R. (2004). Genotoxic and antiapoptotic effect of nicotine on human gingival fbroblasts. Toxicol. Sci. 79, 75–81. [ Links ]
ASH (2006). Research report: second hand smoke. Action on Smoking and Health [on–line]. http://www.ash.org.uk/fles/documents/ASH_597.pdf 13/08/09. [ Links ]
Au W., Walker D., Ward J., Whorton E., Legator M. and Singh V. (1991). Factors contributing to chromosome damage in lymphocytes of cigarette smokers. Mutat. Res. 260, 137–144. [ Links ]
Badr F., Abdel–Halim H., Imam S. and Shalaby I. (1997). Chromosomal damage in experimental schistosomiasis treated with nicotine and praziquantel. Mutat. Res. 379, S118. [ Links ]
Baier G., Stopper H., Kopp C., Winkler V. and Zwirner–Baier I. (2002). Respiratory diseases and genotoxicity in tobacco smoke exposed children. Laryngorhinootologie 81, 217–225. [ Links ]
Barale R., Chelotti L., Davini T., Del S., Andreassi M., Ballardin M., Bulleri M., He J., Baldacci S., Di Pede F., Gemignani F. and Landi S. (1998). Sister chromatid exchange and micronucleus frequency in human lymphocytes of 1650 subjects in an Italian population: II. Contribution of sex, age, and lifestyle. Environ. Mol. Mutagen. 31, 228–242. [ Links ]
Bardana E. and Montanaro A. (1997). Indoor air pollution and health. Marcel Dekker. New York, USA. 508 pp. [ Links ]
Bechoua S., Dubois M., Dominguez Z., Goncalves A., Némoz G., Lagarde M. and Prigent A. (1999). Protective effect of docosahexaenoic acid against hydrogen peroxide–induced oxidative stress in human lymphocytes. Biochem. Pharmacol. 57, 1021–1030. [ Links ]
Benner S.E., Lippman S.M., Wargowich M.J., Velasco M., Peters E.J., Morice R.C. and Hong W.K. (1992). Micronuclei in bronchial biopsy specimens from heavy smokers: characterization of an intermediate marker of lung carcinogenesis. Int. J. Cancer 52, 44–47. [ Links ]
Bolton J.L., Trush M.A., Penning T.M., Dryhurst G.and Monks T.J. (2000). Role of quinones in toxicology. Chem. Res. Toxicol. 13, 135–160. [ Links ]
Bolzan A., Bianchi M. and Bianchi N. (1997). Superoxide dismutase, catalase and glutathione peroxidase activities in human blood: influence of sex, age and cigarette smoking. Clinic. Biochem. 30, 449–454. [ Links ]
Bonassi S. Neri M., Lando C., Ceppi M., Lin Y. , Chang W., Holland N., Kirsch–Volders M., Zeiger E. and Fenech M. (2003). Effect of smoking habit on the frequency of micronuclei in human lymphocytes: results from the Human MicroNucleus project. Mutat. Res. 543, 155–166. [ Links ]
Bravo H., Sosa R., Sánchez P., Bueno E. and González L. (2002). Concentrations of benzene and toluene in the atmosphere of southwestern area at the México City Metropolitan Zone. Atmos. Environ. 36, 3843–3849. [ Links ]
Buckvick N., Bavaro P., Elia G., Cassano F., Fanelli M. and Guante G. (1998). Sister chromatid exchange (SCE) and micronucleus (MN) frequencies in lymphocyte of gasoline station attendants. Mutat. Res. 415, 25–33. [ Links ]
Burgaz S., Karahalil B., Bayrak P., Taskin L., Yavuzaslan F., Bökesoy I., Anzion R., Bos R. and Platin N. (1999). Urinary cyclophosphamide excretion and micronuclei frequencies in peripheral lymphocytes and in exfoliated buccal epithelial cells of nurses handling antineoplastics. Mutat. Res. 439, 97–104. [ Links ]
Burgaz S., Demircigil G., Karahalil B. and Karakaya A. (2002). Chromosomal damage in peripheral blood lymphocytes of traffic policemen and taxi drivers exposed to urban air pollution. Chemosphere 47, 57–64. [ Links ]
Calderón–Ezquerro C., Sánchez–Reyes A., Sansores R.H., Villalobos–Pietrini R., Amador–Muñoz O., Guerrero–Guerra C., Calderón–Segura M.E., Uribe–Hernández R. and Gómez–Arroyo S. (2007). Cell proliferation kinetic and genotoxicity in lymphocytes of smokers living in México City. Hum. Exp. Toxicol. 26, 715–722. [ Links ]
Calderón–Garcidueñas L., Wen–Wang L., Zhang Y., Rodríguez–Alcaraz A., Osnaya N., Villarreal–Calderón A. and Santella R. (1999). 8–Hydroxy–2'–deoxyguano–sine, a major mutagenic oxidative DNA lesion, and DNA strand breaks in nasal respiratory epithelium of children exposed to urban pollution. Environ. Health Perspect. 107, 469–474. [ Links ]
Calderón–Segura M., Gómez–Arroyo S., Villalobos–Pietrini R., Butterworth F. and Amador–Muñoz O. (2004). The effects of seasonal weather on the genotoxicity, cytokinetic properties, cytotoxicity and organochemical content of extracts of airborne particulates in México City. Mutat. Res. 558, 7–17. [ Links ]
Calvert G., Talaska G., Mueller C., Ammenheuser M., Au W., Fajen J., Fleming L., Briggle T. and Ward E. (1998). Genotoxicity in workers exposed to methyl bromide. Mutat. Res. 417, 115–128. [ Links ]
Chang W., Hwang BF., Wang D. and Wang J. (1997). Cytogenetic effect of chronic low–dose, low–dose–rate gamma–radiation in residents of irradiated buildings. Lancet 350, 330–333. [ Links ]
Chen C., Wang Y., Huang W. and Huang Y. (2003). Nickel induces oxidative stress and genotoxicity in human lymphocytes. Toxicol. Appl. Pharmacol. 189, 153–159. [ Links ]
Cheng T., Christiani D., Xu X., Wain J., Wiencke J. and Kelsey K. (1996). Increased micronucleus frequency in lymphocytes from smokers with lung cancer. Mutat. Res. 349, 43–50. [ Links ]
Delgado–Rodríguez A., Ortiz–Marttelo R., Graf U., Villalobos–Pietrini R. and Gómez–Arroyo S. (1995). Geno–toxic activity of environmentally important polycyclic aromatic hydrocarbons and their nitro derivatives in the wing spot test of Drosophila melanogaster. Mutat. Res. 341, 235–247. [ Links ]
Delgado–Rodríguez A., Ortiz–Marttelo R., Villalobos–Pietrini R., Gómez–Arroyo S. and Graf U. (1999). Genotoxicity of organic extracts of airborne particles in somatic cells of Drosophila melanogaster. Chemosphere 39, 33–43. [ Links ]
Doolittle D., Winegar R., Lee C., Caldwell W., Hayes A.J. and de Bethizy D. (1995). The genotoxic potential of nicotine and its major metabolites. Mutat. Res. 344, 95–102. [ Links ]
Dreosti I., Baghurst P., Partick E. and Turner J. (1999). Induction of micronuclei in cultured murine splenocytes exposed to elevated levels of ferrous ions, hydrogen peroxide and ultraviolet irradiation. Mutat. Res. 244, 337–343. [ Links ]
Duffaud F., Orsicre T., Digue L., Villani P., Volot F., Favre R. and Botta A. (1999). Micronucleated lymphocyte rates from head–and–neck cancer patients. Mutat. Res. 439, 259–266. [ Links ]
Duydu Y., Suzen H.S., Aydin A, Cander O., Uylsa H., Isimer N. and Vural N. (2001). Correlation between lead exposure indicators and sister chromatid exchange (SCE) frequencies in lymphocytes from inorganic lead exposed workers. Arch. Environ. Contam. Toxicol. 41, 241–6. [ Links ]
EPA. (1997). Health effects of exposure to environmental tobacco smoke. United States Environmental Protection Agency [on–line]. http://www.oehha.org/air/environmental_tobacco/finalets.html 12/03/2003. [ Links ]
Falck G., Hirvonen A., Scarpato R., Saarikoski S., Migliore L. and Norppa H. (1999). Micronuclei in blood lymphocytes and genetic polymorphism for GSTM1, GSTT1 and NAT2 in pesticide–exposed greenhouse workers. Mutat. Res. 441, 225–237. [ Links ]
Fenech M. (1998a). Important variables that influence base–line micronucleus frequency in cytokinesis–blocked lymphocytes, a biomarkers for DNA damage in human populations. Mutat. Res. 404, 155–165. [ Links ]
Fenech M. (1998b). Chromosomal damage rate, aging, and diet. Ann. NY Acad. Sci. 854, 23–36. [ Links ]
Fenech M. (2000). The in vitro micronucleus technique. Mutat. Res. 455, 81–95. [ Links ]
Fenech M., Holland N., Wushou P., Zeiger E. and Bonassi S. (1999). The Human MicroNucleus Project: an international collaborative study on the use of the micronucleus technique for measuring DNA damage in humans. Mutat. Res. 428, 271–283. [ Links ]
Fenech M., Change W.P., Kirsch–Volders M., Holland N., Bonassi S. and Zeiger E. (2003). HUMN project: detailed description of the scoring criteria for the cytokinesis–block micronucleus assay using isolated human lymphocyte cultures. Mutat. Res. 534, 65–75. [ Links ]
Flowers L., Ohnishi S.T. and Penning T.M. (1997). DNA strand scission by polycyclic aromatic hydrocarbon o–quinones: role of reactive oxygen species, Cu(II)/ Cu(I) redox cycling and o–semiquinone anion radicals. Biochemistry 36, 8640–8648. [ Links ]
Fried P., Perkins S., Watkinson B. and McCartney J. (1995). Association between creatinine–adjusted and unadjusted urine cotinine values in children and the mother's report of exposure to environmental tobacco smoke. Clin. Biochem. 28, 415–420. [ Links ]
Gómez–Arroyo S., Díaz–Sánchez Y., Villalobos–Pietrini R. and De León–Rodríguez J. (2000). Cytogenetic biomonitoring in a Mexican foriculture worker group exposed to pesticides. Mutat. Res. 466, 117–124. [ Links ]
Gourabi H. and Mozdarani A. (1998). Acytokinesis–blocked micronucleous study of the radioadaptative response of lymphocytes of individuals occupationally exposed to chronic doses of radiation. Mutagenesis 13, 475– 480. [ Links ]
Grandjean P, Wulf H. and Niebuhr E. (1983). Sister chro–matid exchange in response to variations in occupational exposure. Environ. Res. 32, 199–204. [ Links ]
Hedner K., Högstedt B., Kolnig A.M., Mark–Vendel E., Ström–Baeck B. and Mitelman F. (1983). Sister chromatid exchanges and structural chromosome aberrations in relation to smoking in 91 individuals. Hereditas 98, 77–87. [ Links ]
Heinrich J., Hölscher B., Seiwert M., Carty C., Merkel G. and Schulz C. (2004). Nicotine and cotinine in adults urine:the German Environmental Survey 1998. J. Exp. Anal. Environ. Epidem. 15, 74–80. [ Links ]
Hindsølandin H., Grummt T., Laurent C. and Tates A. (1997). Monitoring of occupational exposure to epichlorhydrin by genetic effects and hemoglobin adducts. Mutat. Res. 381, 217–226. [ Links ]
Holmén A., Karlsson A., Bratt I. and Högstedt B. (1995). Increased frequencies of micronuclei in T8 lymphocytes of smokers. Mutat. Res. 334, 205–208. [ Links ]
Hutchinson J., Tizabi Y. and Taylor R. (1998). Rapid method for the simultaneous measurement of nicotine and cotinine in urine and serum by gas chromatography–mass espectometry. J. Chromatogr. B 708, 87–93. [ Links ]
Jacob P., Benowitz N. and Shulgin A. (1988). Recent studies of nicotine metabolism in humans. Pharmacol. Biochem. Behav. 30, 249–253. [ Links ]
Kalaiselvi K., Rajaguru P., Palanivel M., Usharani M. and Ramu G. (2002). Chromosomal aberration, micronucleus and comet assays on peripheral blood lymphocytes of leprosy patients undergoing multidrug treatment. Mutagenesis 17, 309–312. [ Links ]
Karahalil B., Burgaz S., Fisek G. and Karakaya A. (1998). Biological monitoring of young workers exposed to polycyclic aromatic hydrocarbons in engine repair workshops. Mutat. Res. 412, 261–269. [ Links ]
Kirsch–Volders M., Sofuni T., Aardema M., Albertini S., Eastmond D., Fenech M., Ishidate M., Kirchner S., Lorge E., Morita T., Norppa H., Surrallés J., Vanhauwaert A. and Wakata A. (2003). Report from the in vitro micronucleus assay working group. Mutat. Res. 540, 153–163. [ Links ]
Klaunig J., Kamendulis L. (2004). The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 44, 239–267. [ Links ]
Konopacka M. and Rzeszowska–Wolny J. (2001). Antioxidant vitamins C, E and beta–carotene reduce DNA damage before as well as after gamma–ray irradiation of human lymphocytes in vitro. Mutat. Res. 491, 1–7. [ Links ]
Lahiri T., Roy S., Basu C., Ganguly S., Ray M. and Lahiri P. (2000). Air pollution in Calcutta elicits adverse pulmonary reaction in children. Indian J. Med. Res.112, 21–26. [ Links ]
Lee C., Brown B., Reed E., Lowe G. McKarns S., Fulp C., Coggins C., Ayres P. and Doolittle D. (1990). Analysis of cytogenetic effects in bone–marrow cells of rats subchronically exposed to smoke from cigarettes which burn or only heat tobacco. Mutat. Res. 240, 251–257. [ Links ]
Li X.Y., Gilmour P.S., Donaldson K. and MacNee W. (1997). In vivo and in vitro proinfamatory effects of particulate air pollution (PM10). Environ. Health Perspect. 105, 1279– 1283. [ Links ]
Lioi M., Scarfi M., Santero A., Barbieri R., Zeni O., Di–Berardino D. and Ursini M. (1998). Genotoxicity and oxidative stress induced by pesticide exposure in bovine lymphocyte cultures in vitro. Mutat. Res. 403, 13–20. [ Links ]
Lohani M., Dopp E., Becker H., Seth K., Schiffmannc D. and Rahman Q. (2002). Smoking enhances asbestos–induced genotoxicity, relative involvement of chromosome 1: a study using multicolor FISH with tandem labeling. Toxicol. Lett. 136, 55–63. [ Links ]
López–Hellin J., García–Arumi E. and Schwartz S. (1998). Oxidative stress induces age–dependent changes in lymphocyte protein synthesis and second messenger levels. Life Sci. 63, 13–21. [ Links ]
Lucero L., Pastor S., Suárez S., Durbán R., Gómez C., Parrón T., Creus A. and Marcos R. (2000). Cytogenetic biomonitoring of Spanish greenhouse workers exposed to pesticides: micronuclei analysis in peripheral blood lymphocytes and buccal epithelial cells. Mutat. Res. 464, 255–262. [ Links ]
Maffei F., Forti G., Castelli E., Stefanini G., Mattioli S. and Hrelia P. (2002). Biomarkers to assess the genetic damage induced by alcohol abuse in human lymphocytes. Mutat. Res. 514, 49–58. [ Links ]
Maluf S. and Erdtmannb B. (2000). Follow–up study of the genetic damage in lymphocytes of pharmacists and nurses handling antineoplastic drugs evaluated by cytokinesis–block micronuclei analysis and single cell gel electrophoresis assay. Mutat. Res. 471, 21–27. [ Links ]
McGowan R.M., Freeman D.C. and Butterworth F.M. (2000). A new way to view complex mixture. Measurement of genotoxic effects of mixture of a polychlorinated biphenyl, a polyaromatic hydrocarbon, and arsenic. In: Biomonitors and biomarkers as indicators of environmental change 2: a handbook (F.M. Butterworth, A. Gunatilaka, M.E. Gonsebatt, Ed.). Environmental Science Research, Vol. 56. Kluwer Academic/Plenum Publishers. New York, USA. 239–255. [ Links ]
Medina–Navarro R., Lifshitz A., Wacher N. and Hicks J. (1997). Changes in human serum antioxidant capacity and peroxidation after four months of exposure to air pollutants. Arch. Med. Res. 28, 205–208. [ Links ]
Nersessian A. and Arutyunyan R. (1994). The comparative clastogenic activity of mainstream tobacco smoke from cigarettes widely consumed in Armenia. Mutat. Res. 321, 89–92. [ Links ]
O'Donoghue J., Barber E., Hill T., Aebi J. and Fiorica L. (1999). Hydroquinone: genotoxicity and prevention of genotoxicity following ingestion. Food Chem. Toxicol. 37, 931–936. [ Links ]
Oesch F., Hengstler J. and Fuchs J. (1994). Cigarette smoking protects mononuclear blood cells of carcinogen exposed workers from additional work exposure–induced DNA single strand breaks. Mutat. Res. 321, 175–185. [ Links ]
Palus J., Rydzynski K., Dziubaltowska E., Wyszynska K., Natarajan A. and Nilsson R. (2003). Genotoxic effects of occupational exposure to lead and cadmium. Mutat. Res. 540, 19–28. [ Links ]
Pardell H. (1996). Manual de diagnóstico y tratamiento del tabaquismo. Panamericana. Madrid, España. 196 pp. [ Links ]
Pastor S., Creus A., Xamena N., Siffel C. and Marcos R. (2002). Occupational exposure to pesticides and cytogenetic damage: results of a hungarian population study using the micronucleus assay in lymphocytes and buccal cells. Environ. Mol. Mutag. 40, 101–109. [ Links ]
Patterson F., Benowitz N., Shields V. , Jepson C., Wileyto P., Kucharski S. and Lerman C. (2003). Individual differences in nicotine intake per cigarette. Cancer Epid. Biomar. Prev. 12, 468–471. [ Links ]
Pitarque M., Creus A., Marcos R., Hughes J. and Anderson D. (1999). Examination of various biomarkers measuring genotoxic endpoints from Barcelona airport personnel. Mutat. Res. 440, 195–204. [ Links ]
Prather R., Tu T., Rolf C. and Gorsline J. (1993). Nicotine pharmacokinetics of Nicoderm (nicotine transdermal system) in women and obese men compared with normal sized men. J. Clin. Pharmacol. 33, 644–649. [ Links ]
Raga G.B., Baumgardner D., Castro T., Martínez–Arroyo A. and Navarro–González R. (2001). México City air quality: a qualitative review of gas and aerosol measurements (1960–2000). Atmos. Environ. 35, 4041–4058. [ Links ]
Rojas E., Valverde M., Sordo M. and Ostrosky–Wegman P. (1996). DNA damage in exfoliated buccal cells of smokers assessed by single cell gel electrophoresis assay. Mutat. Res. 370, 115–120. [ Links ]
Romanova O. and Bezdrobna L. (2001). Spontaneous frequency of micronuclei in peripheral blood lymphocytes of Kiev residents. Tsitologiia i Genetika 35, 56–58. [ Links ]
Rothfub A., Dennog C. and Speit G. (1998). Adaptative protection against the induction of oxidative DNA damage after hypebaric oxygen treatment. Carcinogenesis 19, 1913–1917. [ Links ]
Roubicek A.D., Gutiérrez–Castillo M.E., Sordo M., Cebrián–García M.E. and Ostrosky–Wegman P. (2007). Micronuclei induced by airborne particulate matter from México City. Mutat. Res. 631, 9–15. [ Links ]
Rubio M., Lezama M., Pérez–Neria J., Hernández E. and Selman M. (1990). Determinación de la función respiratoria en fumadores residentes en la ciudad de México y en provincia: posible efecto sinérgico de la contaminación atmosférica. Rev. Inst. Nal. Enf. Resp. Méx. 2, S–9. [ Links ]
Sánchez R. (2004). Evaluación de daño genotóxico en un grupo de mujeres fumadoras que asisten a la clínica Anti–tabaco del Instituto Nacional de Enferemedades Respiratorias (INER). Thesis. Facultad de Ciencias, Universidad Nacional Autónoma de México. México City, México. [ Links ]
Scarfi M., Lioi M., Della–Noce M., Zeni O., Franceschi C., Monti D., Castellani G. and Bersani F. (1997). Exposure to 100 Hz pulsed magnetic fields increases micronucleus frequency and cell proliferation in human lymphocytes. Biolectrochem. Bioenerg. 43, 77–81. [ Links ]
Schiff C., Zierres C. and Zanki H. (1997). Exhaustive physical exercise increases frequency of micronuclei. Mutat. Res. 38, 243–246. [ Links ]
Sierra M., Guzmán–Grenfell A., Olivares–Corichi I., Torres Y. and Hicks J. (2004). Participación de las especies reactivas del oxígeno en las enferemedades pulmonares. Rev. Inst. Nal. Enf. Resp. Méx. 17, 135–148. [ Links ]
Singh R., Kaur B., Popov A.T., Georgieva T., Garte S., Binkova B., Sram J.R., Taioli E. and Farmer B.P. (2007). Effects of environmental air pollution on endogenous oxidative DNA damage in humans. Muta. Res. 620, 71–82. [ Links ]
Somorovska M., Szabova E., Vodicka P., Tulinska J., Barancokova M., Fabry R., Liskova A., Riegerova Z., Petrovska H., Kubova J., Rausova K., Dusinska M. and Collins A. (1999). Biomonitoring of genotoxic risk in workers in a rubber factory: comparison of the comet assay with cytogenetic methods and immunology. Mutat. Res. 445, 181–192. [ Links ]
Sopori M., Savage S., Christner R., Geng Y. and Donaldson L. (1993). Cigarette smoke and the immune response: mechanism of nicotine–induced immunosuppression. Adv. Biosci. 86, 663–672. [ Links ]
SSA (2002). México contra el tabaquismo. Secretaría de Salud [on–line]. http://www.conadic.salud.gob.mx/pie/mexicocontratabaquismo.html 12/05/09. [ Links ]
Stich H.F. and Rosin M.P. (1984). Micronuclei in exfoliated human cells as a tool for studies in cancer risk and cancer intervention. Cancer Lett. 22, 241–253. [ Links ]
Stich H.F. (1987). Micronucleated exfoliated cells as indicators for genotoxic damage and as markers in che–moprevention trials. J. Nutrit. Growth Cancer 4, 9–18. [ Links ]
Surrallés J., Xamena N., Creus A., Catalán J., Norppa H. and Marcos R. (1995). Induction of micronuclei by fve pyrethroid insecticides in whole–blood and isolated human lymphocyte cultures. Mutat. Res. 341, 169–184. [ Links ]
Surrallés J. and Natarajan A. (1997). Human lymphocytes micronucleus assay in Europe. An international survey. Mutat. Res. 392, 165–174. [ Links ]
Suter P., Spitzbarth A., Gautschi K., Erdmenger L., Vonderschmitt D. and Vetter W. (1995). Cotinine: a useful biomarker for tobacco use. Scweiz. Rundsch. Med. Prax. 84, 821–825. [ Links ]
Swan G., Habina K., Means B., Jobe J. and Esposito J. (1993). Saliva cotinine and recent smoking–evidence for a nonlinear relationship. Pub. Health Rep 108, 779–783. [ Links ]
Tomanin R., Ballarin C., Nardini B., Mastrangelo G. and Sarto F. (1991). Infuence of smoking habit on the frequency of micronuclei in human lymphocytes by the cytokinesis block method. Mutagenesis 6, 123–126. [ Links ]
Tomei F., Rosati M., Baccolo T., Bernardini A., Ciarrocca M., Caciari T. and Tomao E. (2004). Response of lympho–monocytes to phytohemagglutinin in urban workers. Environ. Toxicol. Pharmacol. 17, 13–18. [ Links ]
Trivedi A., Dave B. and Adhvaryu S. (1993). Genotoxic effects of nicotine in combination with arecoline on CHO cells. Cancer Lett. 74, 105–110. [ Links ]
Villalobos–Pietrini R., Gómez–Arroyo S. and Amador–Muñoz O. (2000). Genetic monitoring of airborne particles. In: Biomonitors and biomarkers as indicators of environmental change 2: a handbook (F.M. Butterworth, A. Gunatilaka, M.E. Gonsebatt, Ed.). Environmental Science Research, Vol. 56. Kluwer Academic/ Plenum Publishers. New York, USA. 457–485 [ Links ]
Villalobos–Pietrini R., Hernández–Mena L., Amador–Muñoz O., Munive–Colín Z., Bravo–Cabrera J.J., Gómez–Arroyo S., Frías–Villegas A. and Ortiz–Muñiz R. (2007). Biodirected mutagenic chemical assay of PM10 extractable organic matter in Southwest México City. Mutat. Res. 634, 192–204. [ Links ]
Vlachodimitropoulos D., Norppa H., Autio K., Catalan J., Hirvonen A., Tasa G., Uuskuela M., Demopoulos N. and Sorsa M. (1997). GSTT1–dependent induction of centromere–negative and –positive micronuclei by 1,2:3,4–diepoxybutane in cultured human lymphocytes. Mutagenesis 12, 397–403. [ Links ]
Wall M., Johnson J., Jacob P. and Benowitz N. (1988). Cotinine in the serum, saliva, and urine of nonsmokers, passive smokers, and active smokers. Am. J. Public Health 78, 699–701. [ Links ]
Woggner S. and Wong X. (1994). Effects of nicotine on proliferation of normal, malignant, and human papillomavirus–transformed human cervical cells. Gynecol. Oncol. 55, 91–95. [ Links ]
Yildiz D. (2004). Nicotine, its metabolism and overview of its biological effects. Toxicon. 43, 619–632. [ Links ]
Zhao X., Niu J., Wang Y., Yan C., Wang X. and Wang J. (1998). Genotoxicity and chronic health effects of automobile exhaust: a study on the traffic policemen in the city of Lanzhou. Mutat. Res. 415, 185–190. [ Links ]














