Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista internacional de contaminación ambiental
versión impresa ISSN 0188-4999
Rev. Int. Contam. Ambient vol.26 no.1 Ciudad de México feb. 2010
Artículos
Biodegradation of the organophosphate pesticide tetrachlorvinphos by bacteria isolated from agricultural soils in México
Biodegradación del plaguicida organofosforado tetraclorvinfos por bacterias aisladas de suelos agrícolas en México
Ma. Laura ORTIZ–HERNÁNDEZ and Enrique SÁNCHEZ–SALINAS
Laboratorio de Investigaciones Ambientales, Centro de Investigación en Biotecnología, Universidad Autónoma del Estado de Morelos. Av. Universidad 1001, Col. Chamilpa; C.P. 62210, Cuernavaca, Mor. México.Tel. +52 777 329 7057, Fax. +52 777 329 7030; E–mail ortizhl@uaem.mx
Recibido agosto 2008
Aceptado junio 2009
ABSTRACT
A bacterial consortium which degrades tetrachlorvinphos (phosphoric acid, 2–chloro–1–(2,4,5–trichlorophenyl) ethenyl dimethyl ester) was isolated from agricultural soil. This consortium was composed of six pure strains which were characterized based on their morphological and biochemical characteristics. The strains were presumptively identified as Stenotrophomonas malthophilia, Proteus vulgaris, Vibrio metschinkouii, Serratia ficaria, Serratia spp. and Yersinia enterocolitica. The consortium and the six bacteria were assessed in order to discover their ability to degrade tetrachlorvinphos (TCV) in mineral medium and in rich medium. Growth curve experiments showed that the bacterial consortium was able to grow in mineral medium containing TCV as the only carbon source. However, only one pure strain was able to remove TCV in mineral medium, while all of them removed it in rich medium. Hydrolysis products were detected and identified by gas chromatography–mass spectrometry. These data indicate that the isolated strains can be used for waste biodegradation or bioremediation of TCV–contaminated soil or water.
Key words: bacteria, biodegradation, pesticides, tetrachlorvinphos.
RESUMEN
Se aisló un consorcio bacteriano de suelos agrícolas, capaz de degradar tetraclorvinfos (ácido fosfórico, 2–cloro–1–(2,4,5–triclorofenil) etenil dimetil ester). Este consorcio estuvo formado por seis cepas puras que fueron caracterizadas con base en sus características bioquímicas y morfológicas. Las cepas fueron presumiblemente identificadas como Stenotrophomonas malthophilia, Proteus vulgaris, Vibrio metschinkouii, Serratia ficaria, Serratia spp. and Yersinia enterocolitica. El consorcio y las seis cepas puras fueron cultivados en medio mineral y en medio rico, para evaluar su capacidad para degradar TCV. Los resultados de las curvas de crecimiento mostraron que el consorcio es capaz de crecer en presencia de TCV como única fuente de carbono. Sin embargo, sólo una cepa pura removió el TCV del medio de cultivo, pero todas las cepas removieron este plaguicida en medio rico. Los productos de la hidrólisis fueron detectados por cromatografía de gases acoplada a espectrometría de masas. Estos datos indican que las cepas aisladas pueden ser utilizadas para la biodegradación de residuos o para la biorremediación de suelos o aguas contaminadas con este plaguicida.
Palabras clave: plaguicidas, agricultura, residuos, biodegradación.
INTRODUCTION
Pesticides are organic compounds manufactured and used for pest control. When pesticides are dispersed in the environment, they become pollutants, with ecological effects that require remediation. Environmental pollution is caused by both excessive and continuous use of pesticides, and begins when these compounds enter the environment by various means (accidental spills, direct application, residues from cleaning of containers, state of equipment used and methods used to apply the products). The quality of soils, ground water, inland and coastal waters, and air are all affected by pesticide contamination (Chapalamadugu and Chaudry 1992).
Organophosphate pesticides (OP) constitute a group of widely used, very heterogeneous compounds that share a phosphoric acid derivative chemical structure. There are currently 140 OP compounds being used as pesticides and as plant growth regulators around the world. These compounds are components of more than 100 types of commercially available pesticides (such as Paraoxon, Parathion, Coumaphos and Diazinon), and it has been estimated that over 1500 different OP have been synthesized during the past century (Kang et al. 2006). In the United States of America alone, 60,000 tons/year of these types of compounds are produced (Chapalamadugu and Chaudry 1992). Although they have very useful properties, their intensive and indiscriminate use has caused short and long term environmental hazards and health problems (Ortiz–Hernández et al. 1997).
Most synthetic OP compounds are highly toxic and are powerful inhibitors of acetylcholinesterase, a vital enzyme involved in neurotransmission, in the form of acetylcholine substitutes (Donarski et al. 1989, Sultatos 1994, Grimsley et al. 1998, Bakry et al. 2006). Organophosphates may also cause delayed neurotoxic effects which are not due to acetylcholinesterase inhibition. The function of other esterases found in animal organisms is not well understood. In the presence of OP, these enzymes are phosphorylated and inactivated. Once 80 % of the enzyme is inactivated, usually within four days of exposure, potentially lethal symptoms can be observed, including neck muscle weakness, diarrhea and respiratory depression (Grimsley et al. 1998).
Serious contamination issues arise at waste disposal sites close to agricultural fields and at OP production facilities, due to inappropriate handling and improper storage (Ortiz–Hernández et al. 1997). Another problem is buildup of wastes. In undeveloped countries (Africa, Latin America, Asia and East Europe), there are about a hundred thousand metric tons of obsolete pesticides that are no longer usable. These pesticides have simply expired; their storage conditions are very poor with inadequate safety measures, resulting in improper containment, leaks, filtration into soil and water bodies, and accidental spills. Environmental hazards and health risks caused by obsolete pesticides could therefore potentially affect many countries (Martínez 2004). Another current problem is the 400,000 liters of pesticide waste on the México–United States border, which contains approximately 1,500 mg/L OP that has been used for parasite control in livestock (Mulbry et al. 1996).
Pesticides in soil and water can be degraded by biotic and abiotic pathways, however biodegradation by microorganisms is the primary mechanism of pesticide breakdown and detoxification in many soils. Thus microbes may have a major effect on the persistence of most pesticides in soil (Surekha et al. 2008).
Isolation of indigenous bacteria capable of metabolizing OP compounds has received considerable attention because these bacteria provide an environmentally friendly method of in situ detoxification (Richins et al. 1997, Mulchaldani et al. 1999). In some contaminated environments, autochthonous microbial populations have evolved over time to adapt to these contaminants (Pahm and Alexander 1993). These sites are therefore the most appropriate ecological niches to find and isolate strains capable of degrading OP compounds (Ramos and Rojo 1990, Oshiro et al. 1996, Ortiz–Hernández et al. 2001, Horne et al. 2002). One of the most important enzymes is phosphotriesterase (PTE), first found in Pseudomonas diminuta MG and Flavobacterium sp. ATCC 27551, which is able to hydrolyze a considerable number of synthesized OP (Mulbry et al. 1986, Serdar et al. 1989, Mulbry 2000). This enzyme is called organophosphorus hydrolase (OPH) (Mansee et al. 2005) and exhibits high catalytic activity, hydrolyzing a broad range of organophosphates through cleavage of P–O and P–S bonds in these OP (Ang et al. 2005).
The most significant step in OP compound detoxification is hydrolysis, since it makes compounds more vulnerable to further biodegradation (Kumar et al. 1996). The mechanism of hydrolysis and its kinetic characteristics are well known (Brown 1980, Lewis et al. 1988, Mulbry and Karns 1989, Dumas et al. 1989, Dumas and Rauschel 1990, Ortiz–Hernández et al. 2003). PTE has potential for use for cleaning up OP–contaminated environments. However, in previous laboratory trials using cultures in aqueous medium, it was observed that this enzyme does not have any effect on some OP, which suggests a specificity in its activity that depends on the type of phosphoric acid substitutes used to form the OP (Ortiz–Hernández et al. 2001, 2002 and 2003).
Biodegradation is a common method for the removal of organic pollutants because of its low cost and low collateral destruction of indigenous animal and plant organisms (Liu et al. 2007). Studies of microbial biodegradation are useful in the development of strategies for detoxification of pesticides by microorganisms (Qiu et al. 2006).
In previous studies, ten OP pesticides were tested with PTE from Flavobacterium sp. ATCC 27551. The results showed that the enzyme does not have activity towards some chemical structures of OP. In particular, PTE does not have activity towards TCV (Ortiz–Hernández et al. 2003). In this paper, we describe the isolation and characterization of a TCV–degrading bacterial consortium from agricultural soils with potential use in bioremediation. Metabolite analysis was also carried out.
MATERIALS AND METHODS
Pesticide description
TCV is an organophosphate pesticide used in México for external parasites of livestock and poultry; for pests of fruit, vegetable, ornamental and forest plants; and in recreational areas and on agricultural equipment. It is also added to soil before crop cultivation and is useful for control of flies in livestock manure (CICOPLAFEST 2004). It is moderately toxic, affecting the human respiratory system, and is quickly incorporated into the body through the skin. Exposure to this OP causes acetylcolinesterase inhibition, and it is also responsible for carcinogenic problems, liver damage (cancer), and damage to thyroid cells. The chemical structure of TCV and its general characteristics are shown in table I.
Reagents and materials
Analytical grade tetrachlorvinphos (97.0 % pure) was purchased from Ultra Scientific (Analytical Standards). Solvents for gas chromatography (GC) and gas chromatography–mass spectra (GC–MS) were purchased from Mallinckrodt ChromAR® HPLC. Tripticasein soy agar (TS) was obtained from Bioxon; potassium monobasic phosphate, potassium dibasic phosphate, ammonium sulphate, magnesium sulphate, sodium chloride, calcium chloride, iron sulphate, sodium molybdate and manganese sulphate and Tris buffer were purchased from J. T. Baker.
Isolation of bacteria that degrade TCV
The soil used for isolation of microorganisms was collected from a commercial cornfield in central México which had been treated with organophosphate pesticides twice a year for the previous fifteen years. The soil was a vertisol type and was collected at 10 cm below the soil surface at the selected sites. The characteristics of the soil were 35 % moisture; pH 7.52; electric conductivity 730 mS/cm; 2.88 % organic matter; 0.12 % total nitrogen; 35.49 mg/kg available phosphorus; and cation exchange capacity 18.85 cmol/kg. The soil analysis was performed according to Ortiz–Hernández et al. (1993a and 1993b).
Sterile Petri dishes were used to store and transport samples at 4 °C until isolation of the bacteria (Van–Elsas and Smalla 1997). One gram of soil was suspended in 5 mL of sterile mineral medium (MM) and this suspension was considered the inoculum. The MM had the following composition (per liter): 0.2 g KH2PO4; 0.5 g K2HPO4 (sterilized separately at 125 °C for 25 min to prevent precipitation and later aseptically added to the rest of the salts); 1 g (NH4)2SO4; 0.2 g MgSO4•7H2O; 0.2 g NaCl; 0.05 g CaCl2•2H2O; 0.025 g FeSO4•7H2O; 0.005 g Na2MoO4; 0.0005 g MnSO4 (pH 7.0 ± 0.3).
Flasks (125 mL) were supplemented with TCV as the only carbon source, which was added as a filtersterilized methanol solution (Millipore membrane, pore size 0.25 mm). The methanol was evaporated to dryness before the addition of MM, and 20 mL of MM and 0.2 mL of the inoculum (about 2 × 107 CFU/ mL of bacteria) were then transferred to the fasks and thoroughly homogenized. The final concentrations of TCV were 10, 15 and 25 mg/L. In this acclimation period, bacteria that had grown in the presence of 10 mg/L of TCV were sequentially cultured first in 15 mg/L and then in 25 mg/L of TCV. The culture was incubated at room temperature (about 25 °C), without contact with the ambient atmosphere, and shaken continuously (120 rpm, Lab Line shaker) for seven days. All cultures were made in triplicate. The consortium bacteria isolated at 25 mg/L of TCV was selected for colony purification and for further evaluation of the capacity of isolated bacteria to hydrolyze TCV
Several different colonies were chosen from the isolated consortium. They were streaked separately on TS agar plates containing 25 mg/L of the pesticide. Those colonies considered as visually different were plated on TS agar with pesticide and incubated at 37 °C. This procedure was repeated several times to ensure the purity of the isolated colonies.
In order to characterize and identify the isolated bacteria, a BBL crystal system (Becton Dickinson) was used. Additional biochemical tests were also performed. The shape and morphology of bacterial cells were determined by light microscopy (Granados and Villaverde 1996, 1997). Identification was made following Bergey's Manual of Systematic Bacteriology (Krieg and Holt 1984).
Evaluation of the capacity of isolated bacteria to hydrolyze TCV in MM
We carried out the growth and degradation experiments with the consortia and pure colonies isolated in 25 mg/L TCV. The cells were seeded in MM and maintained in agitation as described above. One milliliter sub–samples for TCV analysis were taken every 12 h from each fask and were placed in glass tubes. These portions of the cultures were extracted twice with equal volumes of ethyl acetate as the extracting reagent. The mixture was centrifuged at 3000 rpm for ten minutes. The ethyl acetate with residual TCV was filtered and dried with anhydrous sodium sulfate followed by filtration through glass–fiber paper (Whatman GF/B). This operation was conducted sequentially and the filtrates were mixed. The filtrate was evaporated to dryness and resolved in 50 μL HPLC grade dichloromethane for analysis. The amount of pesticide was quantified on a Hewlett–Packard 6890 gas chromatograph equipped with a nitrogen–phosphorus detector (NPD) on a cross–linked 5 % phenylmethylsilicone capillary column (30 m by 0.2 mm inner diameter). The operating conditions were: injector temperature 240 °C; detector temperature 280 °C; oven temperature 100 to 250 °C at 12 °C/min; and carrier flow 2 ml/min. Calibration curves from 0 to 25 mg/L were made for the TCV. The experiments were set for 72 h in triplicate. Systems of mineral medium and TCV without bacteria, and mineral medium with inoculum without TCV were run as controls.
Evaluation of the capacity of isolated bacteria to hydrolyze TCV in a rich medium
In order to measure bacterial growth and TCV degradation in a rich culture, an experiment was performed using tripticasein soy broth (Bioxon, Becton Dickinson de México). Nutrient broth was prepared according to the manufacturer's instructions. The experiment was made under the same experimental conditions as those described above.
Bacterial growth
Cell growth was measured spectrophotometrically by measuring OD600 in a Spectronic 601 Milton Roy spectrophotometer fitted with deuterium and tungsten lamps.
Metabolite analysis
Each isolated strain was cultured under the conditions described above. We extracted the culture using ethyl acetate as described above. Each of these extractions was later analyzed by gas chromatography (Trace GC) coupled to a mass spectrometer (Thermo Finnigan Polaris Q) under the following conditions: Equity–5 column 30m × 0.25 mm ID, 0.25 μm, oven at 120 °C (3 min) and at 270 °C at 5 °C/min, 250 °C injector, MSD detector, scan range 45–450 amu, 325 °C transfer line, 30cm/sec at 120 °C helium flow, 1.0 μL, splitless (0.3 min) injection, splitless liner, double taper.
RESULTS
Isolation of bacteria using TCV as a carbon source
Six bacterial strains capable of utilizing TCV as the sole carbon source for growth were isolated from the agricultural soil sample in the presence of TCV. They were presumptively identified according to their physiological and biochemical characteristics. The YS strain was identified as Stenotrophomonas malthophilia; A1 as Proteus vulgaris; A5 as Vibrio metschinkouii; A3 and C2 as Serratia ficaria and Serratia spp. respectively and A2 as Yersinia enterocolitica. Table II shows the various pheno–typical characteristics of the selected TCV–degrading bacteria strains. Additionally, the pattern resulting from the 30 reactions in the BBL crystal system was considered, which converted into a ten digit profile number was used as the basis for identification.
TCV degradation by isolated bacteria in MM
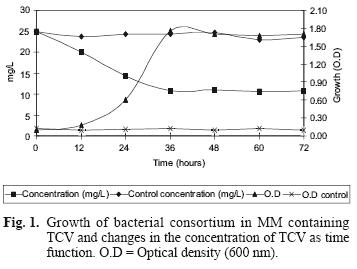
Figure 1 shows growth and TCV degradation by the isolated consortium. This consortium was able to decrease TCV concentration from 25 mg/L to 10.70 mg/L (57 %) in MM within the first 36 hours. No degradation of TCV was observed in un–inoculated controls.
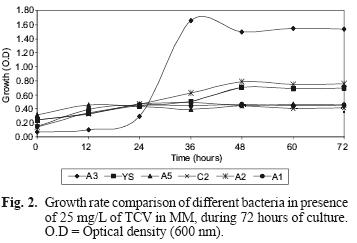
TCV degradation with the pure strain separated from the isolated consortium was analyzed. Figure 2 shows the results obtained from measuring the optical density of the bacterial culture medium (MM) of each type of bacteria from the consortium, in the presence of TCV, during a 72–h period. It can be seen that the A3 strain showed the most growth in comparison to the other strains. YS, A1, A5, C2, A2 strains showed some growth without significant differences between them.
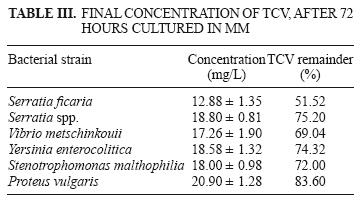
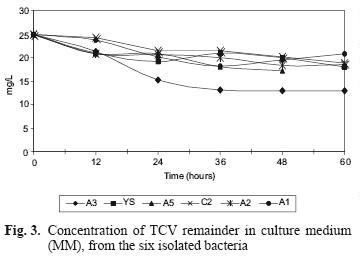
The final TCV concentration and the percentage of TCV remaining in solution are displayed in table III. Growth of the A3 strain increased during 72 h of culture, and therefore was the most efficient strain for removing TCV (48.48 %). Figure 3 shows TCV concentration as a function of time.
Results of pesticide concentration in TS broth
The ability of the consortium to degrade TCV in TS broth was measured. The results indicate that 100 % of the pesticide was removed from the rich medium. Additionally, figure 4 shows growth and TCV degradation in the TS–enriched medium. It can be observed that the concentration of pesticide decreased by almost 100 % in the culture of all isolated strains. The strain that showed the lowest removal rate was C2 (81.20 %). These results suggest a co–metabolic process, since they differ greatly from those obtained in mineral medium.
In this study, the A3 strain showed a 49 % TCV decrease in MM; this measurement was obtained without the addition of an additional carbon source. When the bacteria were cultured in presence of a rich medium, including a carbon source, the percentage of TCV removed from the medium increased considerably (98 %). This suggests a cometabolism process. For the other bacteria isolated from the sample, the TCV removal percentage was lower in the mineral medium, but when they were cultured in a medium rich in carbon and nutrients, TCV removal capacity equaled that of the A3 strain. Under these conditions, any of the strains isolated could be effective for treating wastes or restoring contaminated environments.
In order to evaluate the effect of pH on TCV hydrolysis, 50 mg TCV in 10 mL of 1:1 ethanol:water was exposed to pH conditions in a range from 7 to 13 modified with 1 M NaOH without bacteria for a period of 6 h. Chemical hydrolysis occurred at pH>12 only (Ortiz–Hernández et al. 2003). This suggests that TCV removal from the culture medium was due to bacterial activity.
A3 has not previously been reported as having a gene that codes for OP pesticide hydrolysis. This strain is interesting since it has rarely been isolated from clinical samples, and therefore its pathogenicity is low. It would be useful to test this strain with other OP pesticides in order to find catalytic activity that might make it a recommendable treatment of wastes or polluted environments, with a low potential effect on public health.
Metabolite identification
Figure 5 shows retention times of the different components of the A3 strain extract cultured for 72 h in MM. Gas chromatography revealed four peaks (retention times 11.22, 13.05, 14.19 and 17.54 minutes).
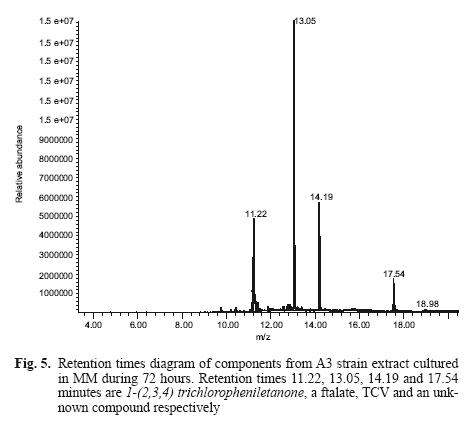
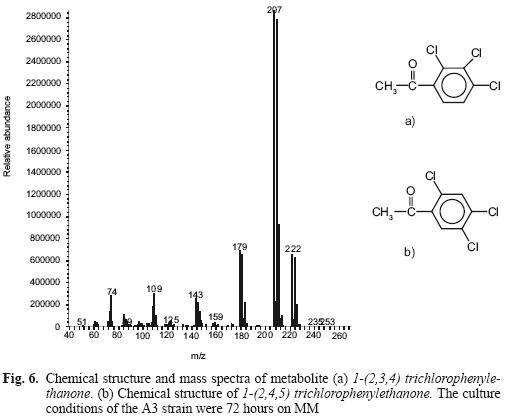
Figure 6 shows mass spectra and the scheme of a metabolite that occurs at minute 11.22, identified as 1–(2,3,4) trichlorophenylethanone (Fig. 6a). This fragment is equal to 1–(2,4,5) trichlorophenyl–ethanone (Fig. 6b), which belongs to a metabolite resulting from TCV hydrolysis. The peak at 14.19 minutes is the substrate (TCV), a phthalate (retention time 13.05 min). We failed to determine the peak at 17.54 min. This procedure was also carried out for the controls. The pesticide molecule was found, but not the metabolite.
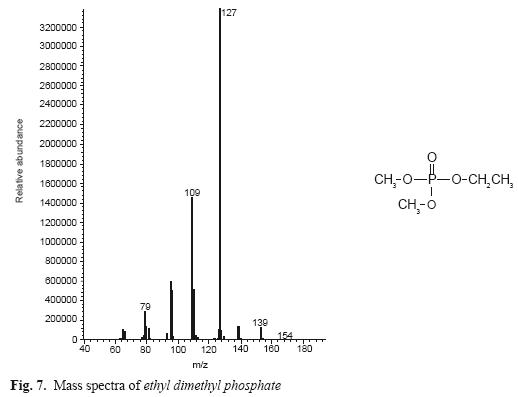
In addition, in enzymatic hydrolysis, TCV chemistry hydrolysis was assessed using 0.5 M NaOH at pH 12 in thin layer chromatography with eluent ethyl acetate:ethanol 60:40. The result of this reaction was extracted and analyzed in GC–Mass as described above. In this chromatogram, we found the same metabolite as in enzymatic hydrolysis, but the other fraction of TCV (phosphoric acid) was found too, with an ethyl group (ethyl dimethyl phosphate) probably arising from ethanol used in chemical reaction (Fig. 7).
DISCUSSION
The bacterial consortium isolated from the sample was made up of a group of strains whose action was reflected in significant pesticide depletion. Unfortunately laboratory methods are only capable of isolating 1 to 10 % of all bacteria growing in soil, so several of the bacteria interfering in the degradation processes in natural environments cannot be obtained in a laboratory. However, isolation of bacteria or bacterial consortia that might be used in the cleaning of wastes or polluted environments is very important.
From the results of this study, we cannot be sure whether this hydrolysis subsequently results in complete mineralization or if the resulting metabolites remain in the medium. Other studies (Chapalamadugu and Chaudry 1992) have shown that the advantage of an isolated consortium with pesticide as the sole carbon source is that a bacterial strain may first carry out the hydrolysis, and other strains are then able to use the resulting compounds as phosphorus and carbon sources. Therefore it is advisable to conduct further studies to evaluate which bacteria of the consortium carries out the initial hydrolysis and whether there are others that produce complete mineralization.
Otherwise it is the case that when bacteria isolated from soil are in contact with OP pesticides, they generate new enzymes (such as the phosphotriesterase of Flavobacterium sp. ATCC 27551), resulting in new metabolic pathways for pesticide degradation. Environmental conditions, soil pH, agricultural management and the amount of pesticide added are important factors for bacterial use of xenobiotic compounds (such as pesticides) as a growth substrate.
Evolution can occur very quickly, resulting in microorganisms with new genetic properties which enable them to degrade compounds. Although further studies are still necessary, there is evidence to show that genes for many catabolic functions are located in plasmids; that an exchange of genes occurs in the environment; and that the best and most usual degraders come from environmental samples highly impacted by pollutant compounds. It is also possible that the gene that codes for these enzymes was initially present in a non–expressed form. It has also been observed that shortly after repeated applications of a degradable pesticide, the soil becomes richer in bacterial populations which are capable of degrading it, which dramatically reduces the effectiveness of subsequent pesticide applications (LaGrega et al. 1996, Madigan et al. 2004).
Proof of this is the isolation of bacteria from agricultural soils where repeated applications of OP pesticides have been reported. Although this isolation of bacteria was preceded by a stage of adaptation to the laboratory conditions where they were maintained with TCV as the only source of carbon, the bacteria showed different levels of efficacy in removing the pesticide from the culture medium.
The physiological base for co–metabolism is not well known, but the most accepted hypothesis is related to the specificity of enzymes. Many of the enzymes which are present in microbial cells catalyze reactions on substrates that are different but chemically related. If the products of any of these activities are not an adequate substrate for any other of the activities, this compound will be accumulated, even if the original enzyme converts its natural substrate into products that provide energy and a source of carbon to the active species.
There are several explanations of cometabolism, which are supported by experimental evidence (Alexander 1994):
a) The initial enzymes convert the substrate into an organic product that is not subsequently converted by other enzymes in the same microorganism into metabolic intermediates that would eventually be used for biosynthesis and energy production.
b) The initial substrate is transformed into products that inhibit the mineralization activity of later enzymes or that suppress the growth of organisms.
c) The organism needs a second substrate to carry out a particular reaction.
According to Alexander (1994), the first explanation is the most common one and is based on the fact that many enzymes act on structurally related substrates. The chemical transformation of a xenobiotic compound by co–metabolism is interesting from the environmental point of view for two reasons: Firstly, since the size of the microbial population that acts on an organic compound in the environment is very low, the compound degrades slowly and the proportion converted does not increase with time. Secondly, many organic products accumulate as the result of co–metabolism and these products tend to persist because one species cannot continue metabolizing the product.
Similar observations have been made in previous studies, where it has been shown that complete degradation of methyl parathion takes place more efficiently in mixed cultures of bacteria than in pure cultures (Munnecke and Hsiem 1976, Chaudry et al. 1988). However, Pahm and Alexander (1993) report that bacteria cultivated in pure culture are useful for determining and evaluating the degradation capacity of each species separately. Soil bacteria in their natural environment have high degradation activity, however it is diffcult to grow them in the laboratory.
The reason why any particular species cannot use a xenobiotic compound is because it is possible that in natural environments, the species takes part in the metabolism in a very specific way. This can be due to a cooperative process between microorganisms, which allows the existence of reactions in one metabolic pathway. Many metabolic pathways depend on enzymes which are codified in a plasmid; the ability of the microorganisms to degrade organic compounds then depends on the stability of the plasmids or the microorganism's genes.
The metabolites found in the A3 culture in both culture media suggest that this species hydrolyzes the pesticide in both media, using it as source of carbon and energy. It also suggests that the mechanism of TCV hydrolysis is similar to that of PTE hydrolysis with other OP; namely through a nucleophilic attack (Fig. 8).
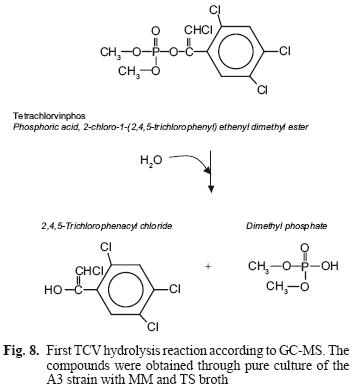
After this first reaction, with two resulting metabolites, dimethyl phosphate is more soluble in water and therefore more available for attack by other microorganisms, being able to use them as a source of phosphorous (Maloney et al. 1988). However, the other metabolite is a chloride compound which can become recalcitrant and remain in the environment for a longer time.
The enzymatic hydrolysis results did not detect dimethyl phosphate, because this metabolite is more soluble in water and was not extracted by the solvent used. Another possible reason is that it was used by the cultured bacteria. This fraction of the pesticide was detected in the chemical hydrolysis extracts.
The present study reports isolation of a bacterial consortium which is capable of utilizing TCV as a source of carbon. Utilization of xenobiotic compounds by soil microorganisms is a crucial phenomenon by which these compounds are removed from the environment, thus preventing environmental pollution. The results of the present study suggest that the bacteria which were isolated are able to grow in medium in the presence of added pesticide and may therefore be used for bioremediation of pesticide–contaminated soil.
REFERENCES
Alexander M. (1994). Biodegradation and bioremediation. Academic Press, San Diego, CA. 292 pp. [ Links ]
Ang E. L., Zhao H. M. and Obbard J. P. (2005). Recent advances in the bioremediation of persistent organic pollutants via molecular engineering. Enz. Microb. Technol. 37, 487–496. [ Links ]
Bakry N. M., El–Rashidy A. H., Eldefrawi A. T. and Eldefrawi M. E. (2006). Direct actions of organo–phosphate anticholinesterases on nicotinic and muscarinic acetylcholinic receptors. J. Biochem. Toxicol. 3, 235–259. [ Links ]
Brown K. (1980). The phosphotriesterase of Flavobacterium sp. Soil Biol. Biochem. 12, 105–112. [ Links ]
Chapalamadugu S. and Chaudhry G.R. (1992). Microbial and biotechnological aspects of metabolism of carbamates and organophosphates. Crit. Rev. Biotechnol. 12, 357–389. [ Links ]
Chaudry R.G., Ali N.A. and Wheeler B.W. (1988). Isolation of a methyl–parathion degrading Pseudomonas sp. that possesses DNA homologous to the opd gene from Flavobacterium sp. Appl. Environ. Microbiol. 54, 289–293. [ Links ]
CICOPLAFEST (2004). Catalogo oficial de plaguicidas. Comisión Intersecretarial para el Control del Proceso y Uso de Plaguicidas, Fertilizantes y Sustancias Tóxicas. México, 250 pp. [ Links ]
Donarski W. J., Dumas D. P., Heitmeyer D. P., Lewis V. E. and Raushel F. M. (1989). Structure–activity relationships in the hydrolysis of substrates by the phos–photriesterase from Pseudomonas diminuta. Biochem. 28, 4650–4655. [ Links ]
Dumas D. P., Caldell S. R., Wild J. R. and Rauschel M. F. (1989). Purification and properties of the phosphotriesterase from Pseudomonas diminuta. J. Biol. Chem. 269, 19659–19665. [ Links ]
Dumas D. P. and Rauschel M. F. (1990). Chemical and kinetic evidence for essential histidine in the phos–photriesterase from Pseudomonas diminuta. J. Biol. Chem. 265, 21498–21503. [ Links ]
Granados P.R. and Villaverde P.C. (1996). Microbiología. Bacteriología: características y clasificación bacteriana. Paraninfo, Barcelona. España. 298 pp. [ Links ]
Granados P.R. and Villaverde P.C. (1997). Microbiología. Bacteriología: medios de cultivo y pruebas bioquímicas. Paraninfo, Barcelona. España. 365 pp. [ Links ]
Grimsley J., Rastogi V. and Wild J. (1998). Biological detoxification of organophosphorus neurotoxins. In: Bioremediation: principles and practice– Biodegradation technology developments. (S. Sikdar and R. Irvine, Eds.). Technomic Pub., New York, Vol. 2, pp. 557–613. [ Links ]
Horne I., Harcourt R. L., Sutherland T. D., Russel R. J. and Oakeshott J. G. (2002). Isolation of a Pseudomonas monteili strain whit a novel phosphotroiesterase. FEMS Microbiol. Lett. 206, 51–55. [ Links ]
Kang D. G., Choi S. S. and Cha H. J. (2006). Enhanced biodegradation of toxic organophosphate compounds using recombinant Escherichia coli with sec pathway–driven periplasmic secretion of organophosphorus hydrolase. Biotechnol. Prog. 22, 406–410. [ Links ]
Krieg N. R. and Holt J. G. (1984). Bergey's manual of systematic bacteriology. Williams & Wilkins, USA, 943 p. [ Links ]
Kumar S., Mukerji K.G. and Lal R. (1996). Molecular aspects of pesticides biodegradation by microorganisms. Crit. Rev. Microbiol. 22, 1–26. [ Links ]
LaGreca M. D., Buckingham P. L. and Evans J. C. (1996). Gestión de residuos tóxicos. Tratamiento, eliminación y recuperación de suelos. McGraw–Hill. Madrid, 1317 pp. [ Links ]
Lewis V., Donarski W., Wild J. and Raushel F. (1988). Mechanism and stereochemical course at phosphorus of the reaction catalized by a bacterial phosphotriesterase. Biochem. 27, 1591–1597. [ Links ]
Liu F., Hong M., Liu D. and Li Y. (2007). Biodegradation of methyl parathion by Acinetobacter radioresistens USTB–04. J. Environ. Sci. 19, 1257–1260. [ Links ]
Madigan M. T., Martinko J. M. and Parker J. (2004). Biología de los micoorganismos. Pearson Prentice Hall. Madrid, 1011 pp. [ Links ]
Maloney S.E., Maule A. and Smith, A.R.W. (1988). Microbial transformation of the pyrethroid insecticides: permethrin, deltamethrin, Fastac, fenvalerate, and fuvalinate. Appl. Environ. Microbiol. 54, 2874–2876. [ Links ]
Mansee A. H., Chen W. and Mulchandani A. (2005). Detoxification of the organophosphate nerve agent coumaphos using organophosphorus hydrolase immobilized on cellulose materials. J. Ind. Microbiol. Biotechnol. 32, 554–560. [ Links ]
Martínez J. (2004). Guía práctica sobre la gestión ambientalmente adecuada de plaguicidas obsoletos en los países de América Latina y el Caribe. Centro Coordinador del Convenio de Basilea para América Latina y el Caribe. Montevideo, 66 pp. [ Links ]
Mulbry W. W., Karns J. S., Kearney P. C., Nelson J. O., McDaniel C. S. and Wild J. R. (1986). Identification of a plasmid–borned parathion hydrolase gene from Flavobacterium sp. by southern hybridization with opd from Pseudomonas diminuta. Appl. Environ. Microbiol. 51, 926–930. [ Links ]
Mulbry W. W. and Karns S.J. (1989) Parathion hidrolase specified by the Flavobacterium opd gene: Relationship between the gene and protein. J. Bacteriol. 171, 6740–6746. [ Links ]
Mulbry W.W., Del Valle P.L. and Karns J.S. (1996). Biodegradation of organophosphate insecticide coumaphos in highly contaminated soil and in liquid wastes. Pestic. Sci. 48, 149–155. [ Links ]
Mulbry W. (2000). Characterization of a novel organo–phosphorus hydrolase from Nocardiodes simplex. NRRL B–24074. Microbiol. Res. 154, 285–288. [ Links ]
Mulchandani A., Kaneva I. and Chen W. (1999). Detoxification of organophosphate pesticides by immobilized Escherichia coli expressing organophosphorus hydrolase on cell surface. Biotechnol Bioeng. 63, 216–223. [ Links ]
Munnecke D.M and Hsieh D.P.H. (1976). Pathways of microbial metabolism of parathion. Appl. Environ. Microbiol. 31, 63–69. [ Links ]
Ortiz–Hernández M. L., Sánchez–Salinas E. and Gutiérrez–Ruiz M. E. (1993a). Análisis de suelos: fundamentos y técnicas. Parte I. Universidad Autónoma del Estado de Morelos. Cuernavaca, Morelos, México. 104 pp. [ Links ]
Ortiz–Hernández M. L., Sánchez–Salinas E. and Gutiérrez–Ruiz M. E. (1993b). Análisis de suelos: fundamentos y técnicas. Parte II. Universidad Autónoma del Estado de Morelos. Cuernavaca, Morelos, México. 142 pp. [ Links ]
Ortiz–Hernández M. L., Sánchez–Salinas E., Vázquez R. and Quintero R. (1997) Plaguicidas organofosforados y ambiente. Biotech. 2, 129–151. [ Links ]
Ortiz–Hernández M. L., Monterrosas–Brisson M., Yañez–Ocampo G. and Sánchez–Salinas, E. (2001). Biodegra–dation of methyl–parathion by bacteria isolated of agricultural soil. Rev. Int. Contam. Ambie. 17, 147–155. [ Links ]
Ortiz–Hernández M. L. (2002). Biodegradación de plaguicidas organofosforados por nuevas bacterias aisladas del suelo. Ph. D. Thesis. Centro de Investigación en Biotecnología, Universidad Autónoma del Estado de Morelos. Cuernavaca Morelos, México, 92 pp. [ Links ]
Ortiz–Hernández M. L., Quintero–Ramírez R., Nava–Ocampo C and Bello–Ramírez M. (2003). Study of the mechanism of Flavobacterium sp. for hydrolyzing organophosphate pesticides. Fund. Clin. Pharmacol. 17, 717–723 [ Links ]
Oshiro K., Kakuta T., Sakai T., Hirota H., Hoshino T. and Uchiyama T. (1996). Biodegradation of organophos–phorus insecticides by bacteria isolated from turf green soil. J. Ferment. Bioeng. 82, 299–305. [ Links ]
Pahm M. and Alexander M. (1993). Selecting inocula for the biodegradation of organic compounds at low concentration. M. Microb. Ecol. 25, 275–286. [ Links ]
Qiu X. H., Bai W. Q., Zhong Q. Z., Li M., He F. Q. and Li B. T. (2006). Isolation and characterization of a bacterial strain of the genus Achrobactrum with methyl parathion mineralizing activity. J. Appl. Microbiol. 101, 986–994. [ Links ]
Ramos J. and Rojo F. (1990). Biodegradación e ingeniería genética. Investigación y Ciencia 164, 72–79. [ Links ]
Richins D., Kaneva I., Mulchandani A. and Chen W. (1997). Biodegradation of organophosphorus pesticides by surface–expresed organophosphorus hydrolase. Nature Biotechnol. 15, 984–987. [ Links ]
Serdar C., Murdock D. and Rohde M. (1989). Parathion hidrolase gene from Pseudomonas diminuta MG: sub–cloning, complete nucleotide sequence and expression of the mature portion of the enzime in Escherichia coli. Nat. Biotechnol.. 7, 1151–1155. [ Links ]
Sultatos L. (1994). Mammalian toxicology of organo–phosphorus pesticides. J. Toxicol. Environ. Health. 43, 271–289. [ Links ]
Surekha R. M., Lakshmi P. K. L., Suvarnalatha D., Jaya M., Aruna S., Jyothi K., Narasimha G. and Venkateswarlu K. (2008). Isolation and characterization of a chlorpyrifos degrading bacterium from agricultural soil and its growth response. Afr. J. Microbiol. Res. 2, 026–031. [ Links ]
Van–Elsas J. D. and Smalla K. (1997). Methods for sampling soils microbes. In: Manual of Environmental Microbiology (Ch. Hurst, G. Knudsen, M. McInerney, L. Stetzenbach and M. Walter, Eds.). American Society of Microbiology. Washington, D.C. pp. 383–390. [ Links ]














