Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista internacional de contaminación ambiental
versión impresa ISSN 0188-4999
Rev. Int. Contam. Ambient vol.24 no.1 Ciudad de México ene./mar. 2008
BIOTECHNOLOGICAL TREATMENT FOR COLORLESS DENIM AND TEXTIL WASTEWATER
TREATMENT WITH LACCASE AND ABTS
Tratamiento biotecnológico para la decoloración de la mezclilla y efluentes textiles empleando lacasa y ABTS
Myrna SOLÍS–OBA1, Javier ALMENDÁRIZ2, Gustavo VINIEGRA–GONZÁLEZ2
1 Centro de Investigación en Biotecnología Aplicada, Instituto Politécnico Nacional, carretera estatal Santa Inés Tecuexcomac–Tepetitla, km 1.5, Tepetitla de Lardizábal, Tlaxcala, México, C.P. 90700, msolis@ipn.mx
2 Departamento de Biotecnología, Universidad Autónoma Metropolitana, Unidad Iztapalapa, Av. San Rafael Atlixco 186, Col. Vicentina 09340, México D.F.
(Recibido mayo 2007, aceptado octubre 2007)
ABSTRACT
The ABTS (2,2'–[3–etil benzotiazolin–6–sulphonic]acid)–Laccase system was applied for discoloration of both commercial denim fabric and a synthetic indigo solution. After treatment, the denim fabric looked like an aged one, although fibers did not exhibit any damage and the synthetic indigo solution was bleached. Additionally, an analysis of both the denim discoloration residual water and the synthetic indigo solution biodegradation, showed that in the two cases the residual water was easily biodegraded in either an aerobic or anaerobic mode, while the control indigo solution required 5 days to be biodegraded in an aerobic mode, and it became unaltered in anaerobic mode. The oxidized ABTS or the laccase could not bleach denim or the synthetic indigo solution in the same experimental conditions as when using the system ABTS–laccase. This is explained considering that, during this process, as when using the mediator recycles several times between the laccase and the indigo, in such way that in a 30 min period, one molecule of ABTS oxidized 800 indigo molecules; also, the observed discoloration rate was two orders of magnitude higher than the rates observed when either the oxidized mediator or the enzyme by itself were applied.
Key words: denim, discoloration, ABTS, indigo, laccase enzyme, dye oxidation, recycling
RESUMEN
El sistema ABTS (ácido 2,2'–[3–etil benzotiazolin–6–sulfónico])–lacasa se empleó para decolorar tanto tela de mezclilla como una solución preparada de índigo. Después del tratamiento, la mezclilla adquirió un aspecto de usada, sin que las fibras mostraran evidencia de daño, mientras que la solución de índigo se decoloró. Adicionalmente, el análisis de la biodegradación de los desechos acuosos de la decoloración de la mezclilla y la solución decolorada control de índigo mostraron que, en ambos casos el agua residual fue fácilmente biodegradada tanto aeróbica como anaeróbicamente, mientras que la solución control de índigo requirió de 5 días para biodegradarse aeróbicamente y no sufrió ningún cambio en el sistema anaeróbico. El ABTS oxidado ó la lacasa por sí solos no decoloraron la mezclilla ni la solución de índigo bajo las mismas condiciones experimentales en que se empleó la combinación de ambos ABTS–lacasa. Esto se explica porque, durante la reacción de decoloración, el mediador se recicla varias veces entre la enzima y el colorante, de tal forma que en 30 minutos, una molécula de ABTS oxida 800 moléculas de índigo, además la velocidad de decoloración fue dos órdenes de magnitud mayor que la velocidad cuando se empleó el ABTS ó la enzima en forma individual.
Palabras clave: denim, decoloración, ABTS, oxidación, pigmentos, reciclaje
INTRODUCTION
Synthetic colors are widely used in industry, it is estimated that color products in the market are more than 10,000 species; and the worldwide industrial production is about 7x105 metric tons (Tan et al. 1999). Also, according to Balan and Monteiro (2001) 5–10 % of the used color in industrial process goes unaltered to residual wastewaters.
The most frequently used colors correspond to azo, anthraquinone and indigo types; these compounds are classified like hazardous wastes because in aerobic conditions they are recalcitrant to degradation, and in anaerobic conditions they evolve into highly toxic amines (Podgornik et al. 2001). Treatment of wastewater polluted with color can be approached by physical or chemical procedures; although any of those options usually turns out to be expensive, inefficient, and creates secondary products which can barely be disposed of. Biological processes have shown to be less expensive and more efficient in wastewater treatment (Cheng et al. 2003). Enzymes produced by white fungi like the lignine peroxidase or laccase, have been successfully applied in discoloration of some colors (Nyanhongo et al. 2002). Campos et al. (2001) have reported that the indigo molecule becomes oxidized by laccase enzyme producing isatin, an easily hydrolysable compound that evolves up to anthranilic acid, which is a toxic compound for aquatic organisms, and so far to the environment (Instituto Nacional de Seguridad Higiene en el Trabajo 2003). Bourbonnais and Paice (1990) proposed that laccase substrate ranges could be augmented by including a mediator; the last is a compound of low molecular weight willing to be oxidized by the enzyme, and once oxidized it becomes able of interact with the final substrate. The ABTS molecule is the most studied mediator for laccase, since it exhibits high stability in ionic state, also its redox chemistry is well known; the only minus is that the mechanism of interactions between ABTS and lacease is not well defined (Collins et al. 1998). Potthast et al. in 1995 have found evidence suggesting that ABTS acts as an activator or co–oxidant of the enzyme; they explain that the ABTS transfers one electron to the enzyme to activate it, working as a co–oxidant agent and not like an electronic mediator of the substrate (Potthast et al. 1995).
Denim market has a high proportion of "look like aged products", this appearance is reached through several process, one of the most popular is the stone wash in which the fabric receives attrition by calcareous stones in a bathtub machine; this process produces damage for both the fabric and the machine, also the removal of fine stones retained by the fabric increase process expenses (Olson et al. 1993). An alternative method is applying cellulose enzyme in the bath, but usually the enzyme produces the back staining of indigo dye (Cavaco–Paulo 1998). Another popular option is applying bleach for denim discoloration, but bleach is a strong oxidant which usually damage fibers and to control the discoloration process becomes troublesome (Tzanov et al. 2003) plus a high possibility of generating toxic halogenated compounds that are unwanted side products.
This work was based on using both ABTS and lacease enzyme to setup an efficient treatment system for discoloration of both denim and indigo solutions, with the advantage of lowering costs, improve process control and lower environmental threats, since the ABTS and laccase produce biodegradable residual wastewater products. The mechanism followed between laccase and ABTS during the indigo oxidation is also explained. The process of discoloration described here is in process to be patented.
MATERIALS AND METHODS
Commercial formula for laccase enzyme
The laccase enzyme used in this study was a commercial one named Deni Lite II S, donated by Novozymes México S.A. de C.V., the distributor of the product. This item is a laccase (EC 1.10.3.2) produced by submerged fermentation of a genetically modified Aspergillus microorganism. It is a light grey powder product available in standard strength 120 laccase units/g (Novo Nordisk 1999). Its formulae includes laccase enzyme, 10 phenothiazolin, propionic acid as mediator and a non–ionic surfactant as buffer. Deni Lite II chemical composition was characterized by Greca et al. (2001).
Laccase purification
The enzyme was purified as follows: mixing one g of Deni Lite II and 10 cc of distilled water, the mixture was shaken for 30 min at room temperature; after that, it was centrifuged at 5000 rpm for 10 min. The solid phase was discarded and the liquid one was slowly added to a flask containing acetone at 4 °C, this mixture was placed in a cooled bath for 1 h. Later the mixture was centrifuged at 10,000 rpm for 15 min, the solid obtained in this stage was the enzyme fraction, which should be washed out with cold acetone several times, before placing it onto a glass dish and letting the solvent to evaporate. Finally, the purified enzyme was dissolved into an acetate buffer solution at pH=5 and stored at 8 °C.
Enzymatic activity determination
The laccase activity was measured at λ= 420 nm in a spectrophotometer UV–Visible Beckman DU 640 using ABTS as a carrier, absorbance increment was measured considering a coefficient ε=36000 mM–1 cm–1 (Bourbonnais et al. 1998). A laccase unit is considered equivalent to the amount of enzyme required to oxidize 1 µmol of ABTS in a 1 min period.
Laccase immobilization
This stage was accomplished following the procedure reported by Ho and Liao (1983).
Oxidized ABTS preparation
ABTS was oxidized by adding 40 cc of 5 mM ABTS solution to 10 g of immobilized laccase, left at rest for 7 days at room temperature. After this period, the solution was filtered and stored at 4 °C for later use.
Denim discoloration using laccase and ABTS
A sample of commercial denim fabric was cut in small pieces, 7 of these were placed in each of 3 flasks named series 1, 2, and 3. The first was added with 40 µmol of oxidized ABTS; the second was added with 40 laccase units; and the third one was added with 40 µmols of oxidized ABTS plus 40 laccase units. The final volume in all flasks was set up to 200 cc, and then incubated at room temperature. A piece of fabric was taken out from each flask at the following times: 0, 2, 6, 12, 30, 36, and 48 h. Each fabric piece was analyzed with a Hunter Lab colorimeter D25–PC2, taking the direct measurement of the following parameters Lightness (L), Hue (H) and Chrome (C). The parameter L has values from 0 to 100, where 100 corresponds to white color and 0 to black color. The parameter H is measured as an angle over a circle divided in 100 parts; while the C parameter is a measure of fabric opacity.
Denim discoloration wastewater treatment
Residual wastewater biochemical oxygen demand (BOD) was analyzed in order to estimate its potential biodegradation in aerobic conditions; the analysis was done following the BOD Hach standard procedure technique. At this stage 3 samples were prepared as follows: the first one was filled with wastewater from denim discoloration bath; the second was filled with indigo solution; and the third one was filled with a mixture of ABTS and laccase. The concentration of the solutions was adjusted to getting a chemical oxygen demand (COD) of 1 g l–1. Each bottle was inoculated with aerobic microorganisms and incubated for 5 days at 25 °C. BOD was analyzed every day during five days.
Biodegradability in anaerobic regimen was measured using 3 samples as follows: sample 1 was discoloration denim wastewater, sample 2 was an indigo solution, and sample 3 was a positive control solution prepared with sodium acetate; the last was chosen because the experimental microorganisms were grown in that media. The concentration of each solution was adjusted up to COD equal to 1 g L–1. Initial dissolved oxygen was washed out by a nitrogen gas flow. During 72 h, a 3 % sodium hydroxide solution was used in order to wash out and quantify the methane production.
Indigo discoloration rate in presence of oxidized ABTS and laccase enzyme
Three reaction mixtures were prepared as follows:
a) 0.1 µMol indigo and 10 mMol of oxidized ABTS.
b) 0.1 µMol indigo and 2 laccase units.
c) 0.1 µMol indigo, 1µMol oxidized ABTS and 2 laccase units.
The indigo discoloration rate was estimated by measuring variations in absorbance, taking as reference λ=610 nm (indigo maximum response) and λ=420 nm (oxidized ABTS maximum response).
Cycling of ABT
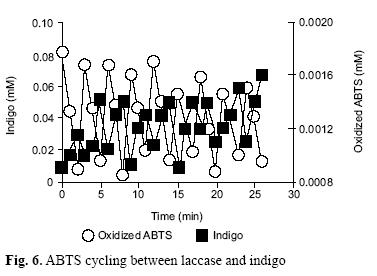
A 2 ml sample was prepared in the spectrophotometer cell by adding 1 laccase enzyme unit, and 2.5 µmol of oxidized ABTS; also, every three minutes a fixed concentration of 200 µmols indigo was added to the cell, until 10 indigo additions. Absorbance lectures at λ=420 and λ=610 were taken, and from a plot of these lectures versus time, an estimation of the amount of oxidized ABTS as well as indigo concentration was obtained, before and after each addition.
RESULTS
Laccase enzyme
The purified enzyme had a specific activity of 0.5 U mg–1, and a Michaelis–Menten constant of 9.83 x 10 –5 mM a 25 °C (KM), it is the concentration of substrate in which the initial velocity is the half of the maxima velocity (Lehninger 1978). The immobilized laccase produced a mixture of ABTS and ABTS+1 (Solís–Oba et al. 2005), since the reduced form of ABTS did not react with indigo, it was not necessary to separate the chemical species.
Oxidized ABTS and laccase enzyme for denim discoloration
Figure 1 shows a photography of denim pieces after incubation with either oxidized ABTS, laccase and a mixture of ABTS and laccase (see procedure in Denim discoloration using laccase ABTS). A careful analysis of each piece revealed that the piece treated with the mixture was the only one exhibiting discoloration, and its appearance was like an aged piece, without any damage of the fibers, and fabrics were not opaque with this treatment. During the test, discoloration was fairly gradual and uniform (no color spots), so far it can be affirmed that denim discoloration treatment can be done without problems and that in this way the process control was easy.
Colorimetric testing for discolored denim
Each denim treatment was evaluated by three parameters: Lightness (L), Hue (H), and Chrome (C). The results are resumed in figure 2 and figure 3 showing the parameters L and H, respectively. Only denim treated with ABTS and laccase changed in the parameters L and H because denim was bleached with those reagents. There were not changes in the C parameter with any of the three processes.
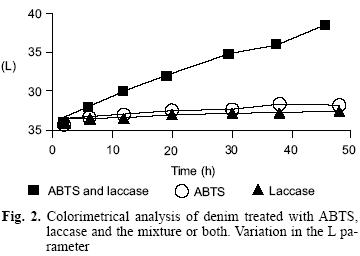
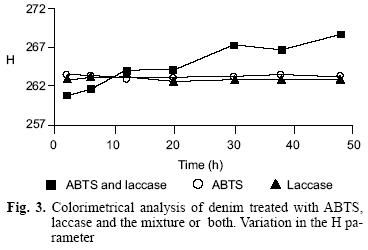
Biodegradability test for residual denim discoloration wastewater.
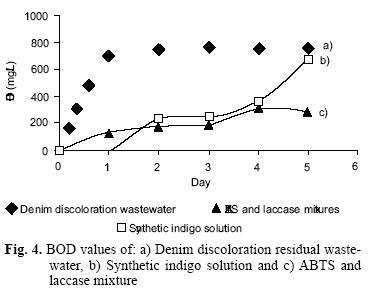
Figure 4 plots the analytical values of BOD from:
a) Denim discoloration residual wastewater. For this sample BOD/COD was 0.696 in the first day, and at the 5th day the ratio BOD/COD was 0.764, this result indicates that the sample was easily biodegraded aerobically.
b) Synthetic indigo solution. This sample was unaltered the first day, but started to change during the second day. The ratio BOD/COD at the 5th day was 0.67, and even though this value could indicate biodegradability, dark blue color in the sample was unaltered.
c) ABTS and laccase mixture. With this option the ratio BOD/COD reached at the 5th day a value of 0.31, so the observed BOD/COD in a) was attributed to the easy biodegradation of indigo oxidation products not to the ABTS or the laccase.
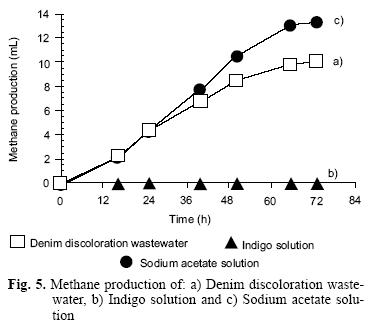
The 72 hrs methane production was analyzed considering the following experiments (Fig. 5):
a) Denim discoloration wastewater. – Methane production reached 75 % of the theoretical value. This result indicates that the denim wastewater was susceptible of biodegradation under anaerobic conditions
b) Indigo solution.– Methane production was null, this condition is attributed to toxic compounds production, as it is referred by some authors (Tan et al. 1999,Wong and Jang, 1999)
c) Sodium acetate solution reached a 90 % of the theoretic value.
Indigo oxidation rate
The oxidation rates for indigo considering the following treatments were:
a) Using oxidized ABTS. The calculated indigo oxidation rate was k=4x10–6 mol min–1.
b) With laccase. The oxidation rate was estimated as k= 8x10–5 mol min–1. This value is 20 times the one obtained with the mediator, which means that the enzyme by itself provokes a faster oxidation for indigo.
c) Using the mixture of oxidized ABTS and laccase. This procedure reaches an oxidation rate of 6x10–4 mol min–1, so far this process is actually faster compared to the previous ones; even when it was used a [Indigo]/ [oxidized ABTS]= 100.
ABTS cycling quantification
Figure 6 shows the set of results obtained by adding 10 indigo dosages to the spectrophotometric cell; after each indigo addition, a fast indigo solution discoloration was observed. In this process the oxidized ABTS was rapidly reduced, and in absence of indigo, it exhibits accumulation up by the laccase, until the next indigo addition; this explanation was deducted from the periodic oscillations present in the plot of oxidized ABTS, in which the valleys match the times of indigo addition. It is estimated that in a 30 min period, 1 ABTS µmol was capable of oxidizing an amount of 800 indigo µmols having laccase in the reaction medium.
DISCUSSION
The results found in this work are: a) denim tested with ABTS and laccase was the only one discolored, b) the indigo oxidation rate was higher using ABTS and laccase than using the oxidized ABTS or laccase alone, and c) 1 ABTS umol oxidized 800 indigo umols in the presence of laccase, and give enough evidence that the ABTS was cycling between laccase enzyme and indigo, working as an electronic mediator of the substrate.
The cycling process followed is explained in the next scheme:

It was demonstrated that the ABTS mediator accomplishes a cycling between laccase enzyme and indigo color; then the required amount of mediator is very small compared to the amount of indigo which can be oxidized by the mediator. With this argument it can be viable to include expensive mediators like ABTS in residual colored wastewater treatment or denim bleach. Even though ABTS is an expensive mediator, the fact that it is able to accomplish several cycles between laccase an dye, makes it possible to consider its inclusion in the process.
The ABTS–laccase system can be applied for denim industrial discoloration as well as an option for indigo polluted wastewater treatment. In denim discoloration the combination ABTS–laccase offers several advantages over the actual commercial process:
a) Denim fibers are not attacked during discoloration.
b) Avoiding the use of stones will produce savings because machine life will be extended, plus cleaning expenses due to stone recovery are eliminated.
c) Environmental impact will be reduced because the ABTS–laccase system will not produce halogenated byproducts.
d) Indigo oxidation byproducts are easily biode–graded in either aerobic or anaerobic systems; therefore, the residual wastewater treatment is conducted in a simple way, reducing threats to the environment.
ACKNOWLEDGEMENTS
The authors thank Dra. Margarita Teutli, Ing. Jorge Humberto Barrera Macías and Ing. Edgar Gómez Camarillo for their technical help. Myrna Solis had a scholarship from CONACyT.
REFERENCES
Balan D. and Monteiro R. (2001). Decolorization of textile indigo dye by ligninolytic fungi. J. Biotechnol. 89, 141–145. [ Links ]
Bourbonnais R. and Paice M. (1990). Oxidation of nonphenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 267, 99–102. [ Links ]
Bourbonnais R., Leech D. and Paice M. (1998). Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. Biochem. Biophy s. Acta. 1379, 381–390. [ Links ]
Campos R., Cavaco A., Robra K., Schneider M. and Gubitz G. (2001). Indigo degradation with laccases from Polyporus sp. and Sclerotium rolfsii. Textil Res. J. 71, 420–424,. [ Links ]
Cavaco–Paulo A. (1998). Mechanism of cellulase action in textile processes. Carbohydrate Polym. 37, 273–277. [ Links ]
Cheng Ch., Jane–Yii W., Dar–Jen L., Sz–Chwun J. and Kuo–Hwang (2003). Decolorization of the textile dyes by newly isolated bacterial strains. J. Biotechnol. 101, 57–68. [ Links ]
Collins P., Dobson A. and Field J. (1998). Reduction of the 2,2 –Azino–bis (3–Ethylbenzthiazoline–6–Sulfonate) cation radical by physiological organic acids in the absence and presence of manganese. Appl. Environ. Microbiol. 64, 2026–2031. [ Links ]
Greca M., Costa–Ferreira M. and Pessoa M. (2001). Decolorization of an anthraquinone–type dye using a laccase formulation. Biores. Technol. 79, 171–177. [ Links ]
Ho G. and Liao Ch. (1983). Activation of a siliceous carrier for enzyme immobilization. United States Patent 4, 384, 045. [ Links ]
Instituto Nacional de Seguridad Higiene en el Trabajo (2003). Fichas internacionales de seguridad química, ICSC 1295. Ministerio del trabajo y asuntos sociales España. [ Links ]
Lehninger A.L. Bioquímica. (1978). Primera reimpresión, Omega, España. [ Links ]
Novo Nordisk (1999). Deni Lite II S Product Sheet, Enzyme Business. Denmark. [ Links ]
Nyanhongo G., Gomes J., Gubitz G., Zvauya R., Read J. and Steine W. (2002). Decolorization of textil dyes by laccase from a newly isolated strain of Trametes modesta. Water Res. 36, 1449–1456. [ Links ]
Olson A., Gladfelter E. and Burch W. (1993). Decolorizing dyed fabric or garments, United States Patent 5, 268, 002. [ Links ]
Podgornik H., Poljansek I. and Perdih A. (2001). Transformation of indigo carmine by Phanerochaete chrysosporium ligninolytic enzyme. Enzyme Microb. Technol. 29, 166–172. [ Links ]
Potthast A., Rosenau T., Chen.L. and Gratz J. (1995).Selective enzymatic oxidation aromatic methyl groups to aldehydes. J. Org. Chem. 60, 4320–4321. [ Links ]
Solis–Oba M., Ugalde–Saldivar V., González I. and Viniegra–González G. (2005). An electrochemical–spectrophotometrical study of the oxidized forms of the mediator 2,2'–azino–bis–(3–ethylbenzothiazoline–6–sulfonic acid) produced by immobilized laccase, J. Electroanal. Chem. 579, 59–66. [ Links ]
Tan N., Prenafeta–Boldu EX., Opsteeg J.L., Lettinga G. and Field J.A. (1999). Biodegradation of azo dyes in cocultures of anaerobic granular sludge with aerobic aromatic amine degrading enrichment cultures, Appl Micobiol Biotech. 51, 865–871. [ Links ]
Tzanov T., Andreaus J., Guebitz G. and Cavaco–Paulo A. (2003). Protein interactions in enzymatic processes in textiles, Electronic J. Biotechnol. 6, 146–154. [ Links ]
Wong Y. and Jian Y. (1999). Laccase–catalyzed decolorization of synthetic dyes. Water Research, 33, 3512–3520. [ Links ]














