Introduction
Aortic stenosis is the most common valvular heart disease worldwide. About 7% of the population over 65 years-old suffers degenerative aortic stenosis. The prognosis of patients with symptomatic severe aortic stenosis is dismal without valve replacement. Approximately one third of patients over 75 years are not referred for surgery.1 Severe symptomatic aortic stenosis (AS) is fatal, the mortality rate is about 50% after two years.2 Aortic valve replacement (AVR) is the only treatment that has proven to be helpful strengthening the survival prospects.3 In 2002, Cribier and colleagues performed the first transcatheter aortic valve implantation (TAVI).3 More recently, technology has developed rapidly, by now more than 200,000 transcatheter valves have been implanted worldwide.4 The results of several prospective and randomized multicenter registries and trials5-10 have provided definitive data confirming this treatment as an alternative to standard surgical aortic valve replacement (SAVR) for inoperable and high-risk patients. Moreover, other recent trials highlighted the non-inferiority of TAVI compared with open surgery even in patients with intermediate surgical risk,11,12 leading an expansion of TAVI to the treatment of an even broader spectrum of patients. Herein we present a clinical case of aortic stenosis and intermediate surgical risk, being the first experience with this technology in this type of patients reported in our country.
Case report
Male, 73-year-old, history of dyslipidemia and hypertension. Chronic coronary artery disease diagnosed in 2013 and requiring percutaneous coronary intervention and implantation of a zotarolimus-eluting stent in the left anterior descendant artery.
Insidiously started one year after the last coronary angiogram with progressive impairment of functional class. A transthoracic echocardiogram was performed and reported severe aortic stenosis. Initially treated with hydric depletion with loop diuretics and aldosterone receptor antagonist with no improvement despite treatment, being the reason of the patient reference to our institution to assess aortic valve replacement.
Physical examination: blood pressure 120/65 mmHg, heart rate 65 beats/min, respiratory rate 18 breaths/min, temperature 36.5 oC, weight: 78 kg, height 1.65 meters. Jugular plethora grade II, chest auscultation with subscapular and bilateral rales, anterior chest with apex lifting on fifth intercostal space at left midclavicular line, rhythmic cardiac sounds with aortic meso-telesystolic murmur, positive Gallavardin sign, limbs with bilateral ankle oedema, immediate capillary filling and parvus-et-tardus pulse.
Results of laboratory studies: leukocytes 6,500/mm3, Hb 12.3 g/dL, hematocrit 38.2%, platelets 116,000/mm3, neutrophils 4,600/mm3, ureic nitrogen 36 mg/dL, glucose 83 mg/dL, creatinin 0.97 mg/dL, sodium 144 mEq/L, potassium 4.1 mEq/L, chloride 110 mEq/L, trombine time 13.5 secs, tromboplastine time 37.9 secs, alanine amine-transferase levels 32 U/L, aspartate amine-transferase 29 U/L, total bilirrubin 0.57 mg/dL.
Results of paraclinic studies: 12-lead electrocardiogram (ECG): in sinus rhythm, with posterior right-fascicle hemiblock and non-specific repolarization disorders. Chest X-ray in standard projection with apparent cardiomegaly, increased bilateral vascularity and prominent pulmonary cone (Figure 1A).

Figure 1: Chest X-ray in anteroposterior projection with cardiomegaly, increased bilateral vascularity and prominent pulmonary cone (A), radiographic outcome after valve implantation (B).
Transthoracic and transesophageal echocardiography: showed left ventricular end-diastolic volume of 55 milliliters, systolic volume of 36 milliliters, ejection fraction 68%, with no changes in global or segmental mobility at rest (Figure 2), without thrombi, concentric remodeling data; left atrium without thrombi and calculated left atrial volume index (LAVI) of 36 mL/m2; aortic valve trivalve, heavily calcified, with severe stenosis, valve area 0.7 cm2, indexed 0.4 cm2/m2, mean gradient of 40 mmHg, with no regurgitation (Figure 3). Aorta: aortic annulus 20 mm, aortic root 28 mm. Mitral valve: structurally normal, mild regurgitation. Right ventricle: preserved systolic function, tricuspid annular plane systolic excursion (TAPSE) 22 mm/m2, S wave 14 cm/s. Right atrium: without thrombi. Tricuspid valve: structurally normal, no regurgitation, systolic pulmonary artery pressure (SPAP) of 25 mmHg.
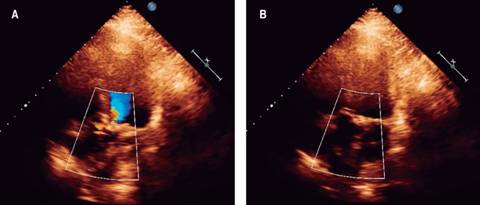
Figure 2: Transthoracic 2D-echocardiogram, four-chamber view and echo-Doppler: showing increased acceleration jet in the left ventricle outflow tract in systole (A) and no regurgitation in diastole (B). See text for further details.

Figure 3: Transthoracic 2D-echocardiogram, long parasternal axis view: showing aortic valve thickened, heavily calcified, with severe stenosis, limited opening in systole (A) and diastole (B). See text for further details.
Coronary angiogram: no significant stenoses in epicardial vessels (Figure 4).
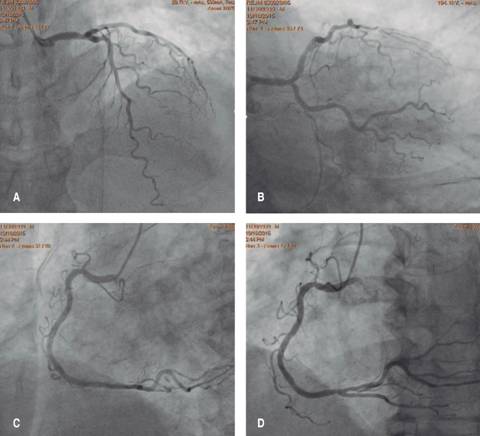
Figure 4: Diagnostic coronary angiogram: without significant angiographic significant stenosis and normal distal flow in both the left coronary system (A and B) and right coronary system (C and D).
Femoral angiogram: right common femoral artery with minimum diameter of 7.05 mm, left femoral with minimum diameter of 6.6 mm, with no angiographic stenoses or tortuosity (Figure 5).

Figure 5: Angiography of aorta in left anterior oblique projection showing radiographic aortic annulus and root diameters (A). Femoral angiogram: showing iliac and femoral vessels without stenosis or tortuosity (B). See text for further details.
Cardiac computed tomography (cardiac CT): performed in an ECG-gated multi-slice 64-detector equipment. The multiplanar reconstruction in axial plane showed a maximum aortic annulus diameter of 26.6 mm, minimum diameter of 21.6 mm, mean diameter 24.1 mm, annulus perimeter 73.9 mm and annulus area of 42.3 mm2 (Figures 6A and 6B). At the level of the coronary cusps it was measured a diameter of 29 mm for the right and non-coronary cusps, 27 mm for the left coronary cusp and aortic root area of 64.6 mm2 (Figures 6C and 6D). Right coronary ostia distance of 13.3 mm from the aortic annulus plane, and a 15.3 mm distance from the left main coronary artery ostia (Figures 7A and 7B). In coronal axis it was measured a 41 degree aortic root angulation (Figure 7C). The 3D-reconstruction showed several calcification in the aortic arch (Figure 7D). Peripheral vessels: Right iliac artery diameter of 11 x 13 mm, left iliac artery 11.8 x 14.8 mm, right common femoral artery 10 x 11 mm and left common femoral artery 9 x 11 mm.

Figure 6: Cardiac computed tomography (cardiac CT) with multiplanar reconstruction in axial plane showing the aortic annulus diameters (A and B) and aortic cusps diameters (C and D). See text for further details.
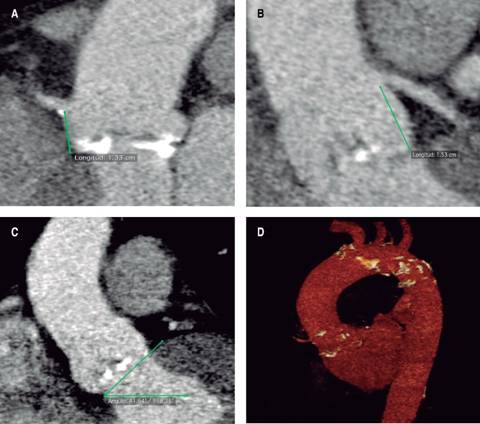
Figure 7: Cardiac CT with multiplanar reconstruction in coronal plane showing right coronary ostia with a distance of 13.3 mm from the aortic annulus plane (A), and a 15.3 mm distance from the left main coronary artery ostia (B), measuring a 41o aortic root angulation (C). The 3D-reconstruction with several calcification in the aortic arch (D). See text for further details.
Surgical risks: EuroSCORE II risk of mortality 3.87%, score of the Society of Thoracic Surgeons predictive risk of mortality (STS-PROM) 4.8%. In Heart Team session was proposed to perform TAVI via a transfemoral approach as the best treatment in this patient.
Procedure
Held on in our facilities, with prior informed consent. With modified Seldinger technique are punctured right and left common femoral artery and right femoral vein, placing a 6 and 7 French (fr) introducers and a temporary pacemaker. The aortic valve was intersected with a 0.035 mm interchange teflonated guidewire, a multipurpose catheter 6 fr was advanced and through in an «Amplatz extra-stiff» guidewire is interchanged and swapped to the left ventricle. In the right femoral artery a 18 fr introducer was placed, advancing the NovaFlex Delivery System (Edwards Lifesciences, Irvine, California) and an Edwards Sapien XT #26 (Edwards Lifesciences, Irvine, California) valve within, after corroborating the proper position (Figure 1B) with fluoroscopy in left anterior oblique projection and pacing at 180 beats per minute the valve is released successfully with mild regurgitation as a result (Figure 8). Transthoracic echocardiography after the procedure shows adequate valve deployment, identifying no paravalvular leak, appropriate opening/closure and mild regurgitation, mean gradient of 6 mmHg and peak velocity 1.7 m/seg. Introducers and delivery system are removed, considering the procedure as successful.

Figure 8: Angiography of aorta in left anterior oblique projection during the TAVI procedure: A) The aortic valve is intersected with an «Amplatz extra-stiff» guidewire and is swapped to the left ventricle. B) The NovaFlex Delivery System (Edwards Lifesciences, Irvine, California) is advanced with an Edwards Sapien XT #26 (Edwards Lifesciences, Irvine, California) valve, corroborating the proper position. C) Balloon valve expansion. D) The valve is released successfully. E) Resultant mild regurgitation observed in aortography. F) Fluoroscopic control after procedure.
The patient was transferred to an intensive coronary care unit, without immediate complications, with no need of amines or mechanical ventilation. Within 72 hours pacemaker was removed remaining in sinus rhythm and adequate heart rate. The hospital discharge is decided five days after the procedure to continue outpatient follow-up being in NYHA functional class I.
After two-year follow-up the patient remains in NYHA functional class I. Transesophageal echocardiogram shows a non-dilated left ventricle with ejection fraction of 80%; normo-functional percutaneous aortic prosthesis with peak velocity of 2.2 m/seg, maximum gradient 20 mmHg, mean gradient 10 mmHg, valve area 1.2 cm2 and mild posterior paravalvular aortic leak; mitral valve with mild regurgitation.
Discussion
Nowadays TAVI is an accepted alternative to surgery in patients with severe aortic stenosis in high surgical risk. In 2016 and 2017, the first randomized studies were published to address the outcomes in intermediate surgical risk patients and showed that TAVI was not inferior to SAVR.11-13
In PARTNER 2A, 2,032 intermediate-risk patients with severe aortic stenosis were randomly assigned to undergo either TAVI with the Edwards SAPIEN XT valve (as this case) or surgical replacement. The primary endpoint was death from any cause or disabling stroke at two years.11 STS risk score was similar in both treatments. After two years the all-cause mortality or disabling stroke was similar in the TAVI group and the SAVR group (19.3% vs. 21.1%, p = 0.33 and p = 0.001 for non-inferiority). In the transfemoral access cohort, TAVI demonstrated a lower mortality and disabling stroke (hazard ratio = 0.79; 95% CI = 0.62-1.00; p = 0.05). TAVI resulted in larger aortic valve areas than did SAVR and also resulted in lower rates of acute kidney injury, severe bleeding, and new-onset atrial fibrillation; SAVR resulted in fewer major vascular complications and less paravalvular aortic leak.
Another study performed a propensity-score analysis including 963 patients treated with SAPIEN-3 TAVI and 747 with SAVR.13 For the primary composite endpoint of mortality, stroke, and moderate or severe aortic regurgitation, TAVI was both non-inferior and superior to SAVR. In summary, the propensity score analysis indicated a significant superiority for the composite outcome with TAVI compared with surgery, suggesting that TAVI might be the preferred alternative treatment in intermediate-risk patients.
The SURTAVI trial evaluated the clinical outcomes of 1660 intermediate-risk patients in a randomized trial comparing TAVI using CoreValve (the Evolut-R valve was used in less than 20% of patients) with SAVR.12 The combined primary endpoint (all-cause mortality or disabling stroke) at 24 months was 12.6% in the TAVI group and 14.0% in the surgery group. SAVR was associated with higher rates of atrial fibrillation, acute kidney injury, and transfusion requirements, whereas residual aortic regurgitation and need for pacemaker implantation were more frequent among TAVI patients. The TAVI resulted in lower mean gradients and larger aortic valve areas than surgery did. Structural valve deterioration at 24 months did not occurred in either group. SURTAVI revealed that CoreValve TAVI was not inferior to surgery in patients with intermediate surgical risk.
In the last two years, the PARTNER II and SURTAVI trials demonstrated non-inferiority of TAVI compared with SAVR in intermediate-risk patients, whereas the NOTION trial and some national observational registries laid the foundation for treatment of low-risk patients. Actually, due to the results of these trials, the 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease14 grants a I-B indication for TAVI in patients at intermediate surgical risk and no other risk factors included in the EuroSCORE II or STS scores (frailty, porcelain aorta, sequelae or chest radiation) if the decision is made by a Heart Team, unlikely the 2017 AHA/ACC Update15 grants a IIa-BR indication to these patients.
Confirming this, Auffret et al analyzed temporal trends of TAVI in France from 2010 to 2015 and underlined the tendency to treat lower-risk patients, with a reduction in median logistic EuroSCORE from 20.3% (interquartile range, 12.1-30.8%) to 13.6% (interquartile range, 9-21%) over the study period.16
However, whilst these results are ostensibly encouraging, important caveats remain. In both trials, the mean age of patients was > 80 years and a significant proportion were considered to be frail. As such, external generalisability may be limited when encompassing younger cohorts. In view of this, and the fact that valve durability beyond five years remains largely untested, guidelines continue to favour AVR in patients aged < 75 years.17
In the moment when this procedure was performed the only existent evidence was the PARTNER II trial and the decision was made by a Heart Team consensus because of the intermediate surgical risk (STS-PROM 4.8%) without any other risk factor and without Cardiac CT contraindication. An Edwards Sapien XT #26 (Edwards Lifesciences, Irvine California) valve was implanted with optimal clinical and echocardiographic outcomes after two-year follow-up.
As in our case, consistent postoperative management strategy is essential. Early discharge (within 24-72 hours) after TAVI is not associated with an increased risk of rehospitalization or sudden cardiac death18,19 suggesting that 24-hour continuous electrocardiographic monitoring (instead of 72 hours as recommended in European Society of Cardiology guidelines) may be sufficient in patients with no conduction abnormalities immediately following the procedure.20
Finally, early mobilization after the procedure promotes rapid return to baseline status, minimizes nosocomial complications, and decreases length of stay in the frail elderly. Early discharge after TAVI, therefore, seems to be a reasonable option in patients who do not experience procedure-related complications.
Conclusions
Transcatheter aortic valve implantation has established as an effective interventional strategy for severe AS in those patients with high-surgical risk. A combination of increased procedural familiarity and progression in device technology has enabled improvements in complication rates, morbidity and mortality. This has led to a commensurate expansion in the use of TAVI, including in patients at intermediate surgical risk or even low risk. A lack of conclusive evidence relating to this issue can present challenges in the decision-making process. Ongoing and upcoming large-scale clinical trials are anticipated to provide more definitive evidence, and may herald the emergence of a new era in the field of TAVI in Mexico.











 nueva página del texto (beta)
nueva página del texto (beta)


