Introduction
Atrial fibrillation (AF) is the most prevalent arrhythmia in clinical practice, and it is associated with significant morbidity and mortality due to cerebrovascular disease (CVD), heart failure (HF), cognitive disease and dementia. In 2010, an estimated 33.5 million persons suffered AF worldwide,1 not only having a significant impact on patients’ health but also representing a considerable economic burden for healthcare systems.2,3 Due to its high economic cost, atrial fibrillation (along with diabetes mellitus and heart failure) is considered one of the three epidemics of the 21st Century.3
According to the world health organization (WHO), the prevalence of obesity has been growing, doubling its prevalence over the last 36 years. In 2014, more than 1.9 billion adults were overweight, with over 600 million people suffering from obesity.4
A possible association between AF and obesity has spurred research, with multiple clinical trials demonstrating obesity as a risk factor for the development of arrhythmias.5-9 A recent study involving 5,282 patients without AF followed for 13.7 years, found that the risk of AF increased 4% for each point increment in the body mass index (BMI) for both male and female patients; the risk persisted even after adjusting for traditional cardiovascular risk factors such as hypertension (HTN) and diabetes mellitus (DM).10 Moreover, studies have shown obesity increases by 49% the risk of developing AF in the general population, and that this risk also increases proportionally with increasing BMI.11 This increased risk is not completely explained by comorbid conditions such as HTN, HF and DM; as such obesity does not appear to be a simple epiphenomenon.2
The Framingham study evaluated the volume of epicardial, intrathoracic and visceral fat in 3,217 patients (54 of them with AF) by computed tomography scan (CT scan). The volume of pericardial adipose tissue, but not the intrathoracic or the visceral, was associated with a higher prevalence of AF in multivariate adjusted models.12 Epicardial fat seems to have a strong association with atrial fibrillation, which appears to be independent of being overweight or obese. Multiple studies have demonstrated a solid relationship of epicardial fat deposits with the presence, chronicity, severity and symptoms of AF12-15 including recurrence after catheter ablation.16-19 To analyze the growing evidence of the relationship between total epicardial fat or epicardial left atrial fat and the prevalence, severity and recurrence of atrial fibrillation, a systematic review was performed.
Methods and materials
A search for articles published in the last 10 years in PubMed, Cochrane (OVID) and EBSCO, was undertaken using the terms atrial fibrillation AND (epicardial fat OR epicardial adipose tissue OR pericardial adipose tissue). A similar search was done in SciELO and LILACS using the terms fibrilación auricular AND (grasa epicárdica OR grasa pericárdica OR tejido graso epicárdico OR tejido graso pericárdico). Observational studies published in the last 10 years evaluating the association between the volume of epicardial adipose tissue (determined by CT scan or magnetic resonance imaging, with results given in cubic centimeters) and atrial fibrillation were included, no language restriction was applied. All articles were evaluated independently by two investigators (JCD, LD, EV or LB) and differences were solved by consensus. The research methodology is shown in Table I.
Table I: Methodology of research.
| Data base | Descriptors | Results |
|---|---|---|
| PubMed | Atrial fibrillation AND (epicardial fat OR pericardial fat OR epicardial adipose tissue OR pericardial adipose tissue) | 103 |
| Atrial fibrillation AND epicardial fat | 16 | |
| Atrial fibrillation AND pericardial fat | 9 | |
| Atrial fibrillation AND epicardial adipose tissue | 24 | |
| Atrial fibrillation AND pericardial adipose tissue | 2 | |
| EBSCO | Atrial fibrillation AND (epicardial fat OR pericardial fat OR epicardial adipose tissue OR pericardial adipose tissue) | 39 |
| Atrial fibrillation AND epicardial fat | 18 | |
| Atrial fibrillation AND pericardial fat | 9 | |
| Atrial fibrillation AND epicardial adipose tissue | 25 | |
| Atrial fibrillation AND pericardial adipose tissue | 2 | |
| Cochrane (OVID) | Atrial fibrillation AND (epicardial fat OR pericardial fat OR epicardial adipose tissue OR pericardial adipose tissue) | 120 |
| Atrial fibrillation AND epicardial fat | 24 | |
| Atrial fibrillation AND pericardial fat | 25 | |
| Atrial fibrillation AND epicardial adipose tissue | 53 | |
| Atrial fibrillation AND pericardial adipose tissue | 0 | |
| SciELO | Fibrilación atrial AND (grasa epicárdica OR grasa pericárdica OR tejido graso epicárdico OR tejido graso pericárdico) | 0 |
| Fibrilación atrial AND grasa epicárdica | 0 | |
| Fibrilación atrial AND grasa pericárdica | 0 | |
| Fibrilación atrial AND tejido graso epicárdico | 0 | |
| Fibrilación atrial AND tejido graso pericárdico | 0 | |
| LILACS | Fibrilación atrial AND (grasa epicárdica OR grasa pericárdica OR tejido graso epicárdico OR tejido graso pericárdico) | 0 |
| Fibrilación atrial AND grasa epicárdica | 0 | |
| Fibrilación atrial AND grasa pericárdica | 0 | |
| Fibrilación atrial AND tejido graso epicárdico | 0 | |
| Fibrilación atrial AND tejido graso pericárdico | 0 | |
| Total | 469 |
Quality of the studies
All articles were submitted for a quality analysis, using the Strobe guideline.20 Articles with a low methodological quality were excluded from the present analysis.
Results
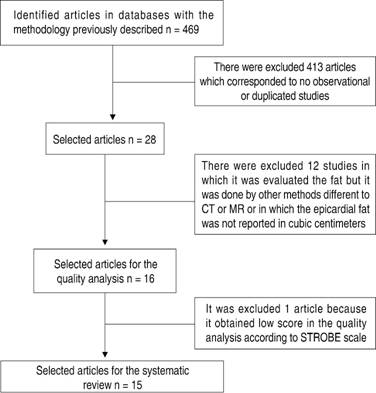
A total of 469 articles were found during the initial search. After eliminating duplicates and articles not fulfilling inclusion criteria based on title and abstract review, 28 articles were considered for full text revision (Table I). After full text revision, 12 studies in which the evaluation of epicardial and periatrial total fat was done by different methods to CT or magnetic resonance imaging (MRI) or those in which the total epicardial or periatrial fat was not measured in cubic centimeters (cm3) were excluded. The 16 remaining articles were submitted to a quality analysis using Strobe guideline, and one article with a low-quality methodology was excluded. Finally, 15 articles were included in the systematic review (Figure 1). Data was extracted and analyzed for three outcome groups: incidence or prevalence, severity (defined as paroxysmal vs non-paroxysmal AF), and recurrence of atrial fibrillation. In each subgroup, a p value for the relationship and the potential confounding variables was estimated. Tables II to IV illustrate the results.
Table II: Characteristics and results of studies in incidence and prevalence of atrial fibrillation.
| Author | Year | Type of study | Sample (n) | Confusion factors | Results | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EAT-T | EAT-LA | ||||||||||
| FA | No FA | FA | No FA | p value | FA | No FA | p value | ||||
| Wong et al(18) | 2011 | Case and controls | 102 | 20 | Age, sex, BMI, valvulopathy, HTN, DM, ischemic heart disease, left ventricular systolic dysfunction (LVSD), Obstructive sleep apnea-hypopnea syndrome (OSAHS) | 299.9 ± 53.85 | 168.8 ± 19.2 | < 0.001 | 118.5 ± 23.85 | 69.7 ± 11 | < 0.001 |
| Shin et al(28) | 2011 | Case and controls | 80 | 80 | Age, sex, BMI, HTN, DM, total cholesterol, HDL, LDL, triglycerides | 83.8 ± 26.8 | 67.2 ± 23.1 | < 0.001 | ND | ND | ND |
| Nagashima et al(19) | 2011 | Case and controls | 40 | 37 | Age, sex, BMI, HbA1c, HDL, LDL, triglycerides | 185.6 ± 76.1 | 138.3 ± 45.2 | < 0.01 | 51.5 ± 27.8 | 32.9 ± 14.5 | < 0.01 |
| Stojanovska et al(17) | 2015 | Case and controls | 169 | 62 | Age, sex, BMI, HTN, CAD, DM, dyslipidemia, left auricle diameter | 79 ± 12.5 | 50 ± 6 | < 0.0001 | ND | ND | ND |
| Mahabadi et al(27) | 2014 | Case and controls | 46 | 3809 | Age, sex, BMI, systolic blood pressure (SBP), antihypertensive treatment | 147.1 ± 64.4 | 92.7 ± 46.1 | < 0.0001 | ND | ND | ND |
| Nakanishi et al(30) | 2012 | Cohort | 17 | 262 | Age, sex, HTN, DM, hypercholesterolemia, BMI, C reactive protein (CRP), cigarette, antihypertensive y LVEF | 80 ± 22 | 53 ± 20 | < 0.001 | 34 ± 8 | 18 ± 7 | < 0.001 |
| Sevinc et al(31) | 2016 | Case and controls | 58 | 74 | Age, BMI, hyperlipidemia, LV volume | ND | ND | ND | 54.33 ± 23.43 | 42.99 ± 20.76 | 0.004 |
| Al Chekakie(14) | 2010 | Case and controls | 197 | 76 | Age, sex, structural cardiac illness, HTN, DM, BMI, LV diameter, LVEF | 101.6 ± 44.1 | 76.1 ± 36.3 | < 0.001 | ND | ND | ND |
| Tsao et al(23) | 2011 | Case and controls | 68 | 34 | Age, sex, triglycerides, cholesterol HTN, DM, CAD, LVV | ND | ND | ND | 29.9 ± 12.1 | 20.2 ± 6.5 | < 0.001 |
| Kanazawa et al(24) | 2014 | Case and controls | 120 | 120 | Age, sex, BMI, dyslipidemia, HTN, DM, smoking, abdominal circumference abdominal | 148.8 ± 46.1 | 113.9 ± 32.5 | < 0.001 | ND | ND | ND |
| Kusayama et al(25) | 2016 | Case and controls | 32 | 32 | Age, sex, BIM, HTN, dyslipidemia, DM, triglycerides, HDL, LDL, HbA1C, LV diameter | ND | ND | ND | 111.6 ± 5.5 | 108.1 ± 6.7 | 0.02 |
| Drossos et al(32) | 2014 | Cohort | 28 | 55 | Age, sex, BMI, HTN, DM, COPD, CVA, recent AMI, peripheral vascular disease | 195 ± 80 | 126 ± 47 | 0.0001 | ND | ND | ND |
AF = atrial fibrillation; EAT-T = total epicardial adipose tissue; EAT-LA = left atrial epicardial adipose tissue; BMI = body mass index; HTN = high blood pressure; DM = diabetes mellitus; OSAHS = obstructive sleep apnea-hypopnea syndrome; HDL = high density lipoproteins; LDL = low density lipoproteins; CAD = coronary artery disease, SBP = systolic blood pressure, LVEF = left ventricle ejection fraction; LV = left ventricle; LVV = left ventricle volume, ND = no data.
Table III: Characteristics and results of studies in severity of atrial fibrillation.
| Author | Year | Type of study |
Following time | Sample (n) | Confusion factors | Results | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EAT-T | EAT-LA | |||||||||||
| Without Recurrence | With Recurrence |
Without recurrence | With recurrence |
p Value | Without recurrence | With recurrence |
p value | |||||
| Nagashima et al(19) | 2011 | Cohort | 10.2 months | 25 | 15 | Age, sex, BMI, HbA1C, HDL, LDL, triglycerides | 153.5 ± 42.7 | 239 ± 90.2 | 0.0002 | 40.7 ± 13.9 | 69.6 ± 35.5 | 0.0008 |
| Masuda et al(22) | 2015 | Cohort | 16 ± 4 months | 24 | 29 | Age, sex, BMI, persistent AF, months with AF, HTN, DM, heart failure, CVA/TIA, CHADS2 and CHADSVASC score, medicine prescription previous procedure, atrial volume, LVEF, procedures characteristics | 94.5 ± 35.2 | 98.5 ± 45.7 | 0.72 | 25 ± 9.5 | 35.1 ± 13.1 | 0.0002 |
| Tsao et al(23) | 2011 | Cohort | 3 months | 44 | 24 | Age, sex, triglycerides, total cholesterol, HTN, DM, coronary disease, LV volume | ND | ND | ND | 26.8 ± 11.1 | 35.2 ± 12.5 | 0.007 |
AF = atrial fibrillation; EAT-T = total epicardial adipose tissue; EAT-LA = left atrial epicardial adipose tissue; BMI = body mass index; HTN = hypertension; DM = diabetes mellitus; CVA = cerebrovascular accident; COPD = chronic obstructive pulmonary disease; OSAHS = obstructive sleep apnea-hypopnea syndrome; HDL = high density lipoproteins; LDL = low density lipoproteins; CAD = coronary artery disease; SBP = systolic blood pressure; LVEF = left ventricle ejection fraction; LV = left ventricle; LVV = left ventricle volume; ND = no data.
Table IV: Characteristics and results of studies in recurrence of atrial fibrillation.
| Author | Year | Type of study |
Following time | Sample (n) | Confusion factors | Results | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EAT-T | EAT-LA | |||||||||||
| Without recurrence | With recurrence |
Without recurrence | With recurrence |
p value | Without recurrence | With recurrence |
p value | |||||
| Nagashima et al(19) | 2011 | Cohort | 10.2 months | 25 | 15 | Age, sex, BMI, HbA1C, HDL, LDL, triglycerides | 153.5 ± 42.7 | 239 ± 90.2 | 0.0002 | 40.7 ± 13.9 | 69.6 ± 35.5 | 0.0008 |
| Masuda et al(22) | 2015 | Cohort | 16 ± 4 months | 24 | 29 | Age, sex, BMI, persistent AF, months with AF, HTN, DM, heart failure, CVA/TIA, CHADS2 and CHADSVASC score, medicine prescription previous procedure, atrial volume, LVEF, procedures characteristics | 94.5 ± 35.2 | 98.5 ± 45.7 | 0.72 | 25 ± 9.5 | 35.1 ± 13.1 | 0.0002 |
| Tsao et al(23) | 2011 | Cohort | 3 months | 44 | 24 | Age, sex, triglycerides, total cholesterol, HTN, DM, coronary disease, LV volume | ND | ND | ND | 26.8 ± 11.1 | 35.2 ± 12.5 | 0.007 |
BMI = body mass index; HDL = high density lipoproteins; LDL = low density lipoproteins; AF = atrial fibrillation; HTN = hypertension; DM = diabetes mellitus; CVA = cardiovascular; TIA = transient ischemic attack; LVEF = left ventricle ejection fraction; LV = left ventricle; ND = no data.
Of the 15 studies included in the analysis, eight included patients who were undergoing AF ablation procedures,17,19,21-26 with the remaining seven studies including patients with and without AF from specialized clinical centers. CT was used to determine fat volume in all the studies, except in one study, which used MRI.21
Impact of EAT-T or EAT-LA on AF incidence/prevalence
Baseline characteristics of patients included in the AF incidence-prevalence subgroup were non-significantly younger and had a lower frequency of HTN, hyperlipidemia, DM, HF and coronary illness. Only two studies found a significantly higher age, male sex, BMI and obesity in the AF group than in the control group.17,27 All studies demonstrated a statistically significant association of EAT-T and EAT- LA with AF (Table II).
Impact of EAT-T or EAT-LA on AF severity
In the severity subgroup, there were no statistically significant differences in baseline characteristics between patients with paroxysmal AF vs no paroxysmal AF. Six of the seven studies1417,19,24,26,28 proved that EAT-T was noticeably greater in patients with no paroxysmal AF vs paroxysmal AF, only one study failed to demonstrate such association.29 A significantly higher EAT-LA volume in patients with non-paroxysmal AF was also found in tree of the four included studies,19,26,29 with only one study finding a non-significant difference.
Impact of EAT-T or EAT-LA on AF recurrence
In the AF recurrence subgroup in only one study BMI was significantly higher for patients with recurrence versus patients without recurrence,19 no other significant differences were found in the baseline characteristics of patients with vs patients withouth AF recurrence in the included studies. Two studies19,22 evaluated the association between total epicardial fat and AF recurrence, with one finding a statistically significant association.19 Periatrial epicardial fat volume was also significantly associated with AF recurrence,19,22,23 an association that remains significant after multivariate analysis.
Discussion
The association between AF and obesity has been a subject widely discussed. However, the results of the Framingham study12 which demonstrated that pericardial fat (but not the intrathoracic or abdominal visceral fat) was associated with the AF prevalence, generated research interest in the importance of pericardial adipose tissue in the genesis and maintenance of AF.
Due to the increasing number of new studies on the subject, the importance of understanding the different mechanisms of AF and its potential clinical applications, we performed this systematic review including 15 studies. Both EAT-T and EAT-LA appear to have a statistically significant association with the prevalence of atrial fibrillation. Regarding AF severity (defined as paroxysmal versus non-paroxysmal AF) almost all studies included found that patients with non-paroxysmal AF (a term that includes persistent, long standing persistent and permanent AF) have considerably more epicardial total and periatrial fat than patients with paroxysmal AF. The number of recurrences after catheter ablation was limited, but several studies have demonstrated a statistically significant higher risk of recurrence after AF ablation associated with increasing volume of epicardial fat.
A recently published metaanalysis found a statistically significant difference in the epicardial total fat between patients with AF versus patients without AF (32 mL, IC 95% 21.5-42.5) and in patients with persistent AF versus paroxysmal AF (29.6 mL, IC 95%: 12.7-46.5).33 Zu et al reported similar results in another metaanalysis, finding statistically significant differences for EAT-T and EAT-LA between patients with AF versus patients without FA and patients with persistent AF versus paroxysmal AF.34
It is important to highlight that the results from the fifteen selected studies were heterogeneous, with epicardial fat values notably dissimilar, in part due to the technique used to determine fat volume (for example, the study by Wong et al used MRI, currently considered the gold standard) and partly because of the difference in the epicardial fat definition according the Hounsfield units (HU). Additionally, differences in studied populations could also explain study heterogeneity.
Cardiac adipose tissue is composed by epicardial fat (EAT), which lies between the visceral pericardium and the epicardium, and the paracardial fat that is located outside the visceral pericardium. In this way, the EAT is the one which is in intimate contact with myocardium, different to pericardial adipose tissue which is separate by the pericardium itself. This is a key concept for having a well physiopathologically understanding of the influence of EAT over the atrial myocardium. Literature frequently uses terms such as epicardial adipose tissue and pericardial adipose tissue in an interchangeably way, fact that represents a problem that go beyond a linguistic discussion. Both tissues are anatomically situated in different heart locations and have different embryologic origins and irrigation.13,35-37 This is the reason why this systematic review only included articles referring to epicardial adipose tissue.
The epicardial adipose tissue is implicated in certain physiologic functions such as providing energy, protection and thermoregulation.13,35 Nevertheless, the excessive accumulation of it results in a cascade of physiopathological events, taking into account that EAT represents a metabolically active tissue, source of cytokines and other inflammatory mediators who have paracrine influence over myocardium.
For atrial fibrillation to develop it is necessary the presence of a substrate, a trigger and the activation of the autonomic nervous system (ANS) and all of them can be influenced by the epicardial fat.3,13,35 Keeping this in mind, EAT contributes to the development of a substrate, directly through the adipose infiltration of atrial myocardium and indirectly by releasing pro inflammatory mediators and pro fibrotic factors.35 Some studies, have demonstrated that the EAT volume is associated with adipose infiltration of the atrial myocardium, even founding in a sheep model that sustained obesity leads to an increase of the epicardial fat with important adipose infiltration specially in the posterior wall or the left atrium. Understanding this, it can be explained physiopathologically why this could contribute to the disorganization and alteration of the harmonious electric conduction of the wave of propagation, ending up in the development of atrial fibrillation.35,37-39 In the other hand, the epicardial fat releases multiple inflammatory mediators such as IL6, IL8, TNF alfa, IL1B and MCP1, attracting monocytes which releases cytokines, expanding the phenomena.3,13,35,36,38,40-43
EAT is associated with a significant inflammatory response in the adjacent atrial myocardium, which in turn promotes and sustains AF. An increased immune cellular response has been demonstrated, with studies revealing increased 18-fluorodeoxyglucose uptake in atrial tissue adjacent to EAT (which is caused by the high metabolic activity of inflammatory cells) and infiltration of atrial tissue by inflammatory cells, as demonstrated by atrial biopsy.44-46 Pro fibrotic factors such as activin A (which induces the expression of TGF B1 and TGF B2, leading to fibrosis in the atrial myocardium) and metalloproteinases (especially 2 and 7) released by the epicardial adipose tissue, also contribute to the formation of a suitable substrate for atrial fibrillation.35,41,46 Thus, inflammatory mediators and pro fibrotic factors released by EAT have an important role in atrial remodeling and arhythmogenesis of AF, promoting substrate formation through multiple mechanisms.3,18,35,41,42
Additionally, EAT promotes AF by enhancing atrial trigger activity and modulation of the autonomic nervous system.3,13 Increased adipose tissue has been associated with a shortened atrial refractory period and a rise in the resting voltage of myocites within the pulmonary veins, resulting in activity heightened automaticity. The anatomical proximity of the adipose EAT with the pulmonary vein ostia allows inflammatory mediators secreted by EAT to contact with myocardial cells within the pulmonary veins, increasing the firing rate of ectopic foci.3,35,36,40 By removing periesophageal fat during posterior pericardiectomies postoperatory AF rates are reduced, a fact that further supports this hypothesis.47
Additionaly, ganglionic plexuses within the epicardial fat modify the electrophysiologic properties of the atrial myocites, both by modification of the sympathetic (increasing the inward calcium current) and parasympathetic (reducing the atrial refractory period, slowing the electrical conduction and promoting reentries) nervous system.3,13,35,36,38,40,41 Adipokines released by EAT, that seem to have a significant effect on nerve growth regulation, have been implicated in AF arrhythmogenesis associated with epicardial fat.3,48 Pokushalov et al49 reported a reduction in the incidence of postoperative AF after injection of botulinum toxin (a neurotoxin that blocks acetylcholine release) in the epicardial fat pads of patients undergoing coronary bypass. This appeared to reduce the autonomic activity and potentially suppress the ganglion plexus, evidencing the important role of the autonomic nervous system in maintaining atrial fibrillation.35
Although different methods have been used to measure epicardial fat, the most precise method is volumetric quantification. Area and fat pad thickness could be more available methods and less expensive, but these meassurements do not well-correlate with ex-vivo mass and present significant inter and intra observer variability.35,50 Magnetic resonance imaging is currently the gold standard for EAT measurement, allowing volumetric quantification with exceptional spatial resolution and radiation-free. Nevertheless, its cost and in many cases limited availability, along with prolonged imaging time limit its widespread use.51 Computerized tomography (CT) allows volumetric quantification of EAT in an easy and reproducible way. Although spatial resolution is excellent, its major drawback is the use of radiation.52 Although echography is a widely available method, volumetric quantification of epicardial fat using this method has not been validated and also, it has a low reproducibility and image quality is limited.53,54 Taking these considerations into account, we limited our systematic review to studies in which EAT was quantified in volume measurement with CT or MRI, thus allowing for a more accurate EAT volume quantification. Nonetheless, although the included studies used the same imaging technique, differences in quantification directly contributed to study heterogeneity.
Our findings are similar to previously published meta-analysis.33,34 The available evidence supports the influence of epicardial total and periatrial fat on the incidence and severity of atrial fibrillation, but also in recurrence posterior to ablation by catheter. Furthermore, they set important basis for the physiopathological AF theory which has been widely studied in last decades and demands new clinical challenges, considering its potential implications on risk stratification of patients with atrial fibrillation,55 weight loss strategies56-61 and targeted therapies to adipokines.
Limitations
Significant interstudy heterogeneity constituted an important limitation, as it limitated the quantitative synthesis of data and the capacity to dimension the size and effect that seems to be important. Moreover, the results are limited to stablish that it exists a statistically significant difference of measurements between groups subjected to study, fact that interferes with the determination of a cut value of epicardial total and periatrial fat volume from which incidence, prevalence, severity and recurrences are higher. Nonetheless, this is the first systematic review in strictly analyzing the tree mentioned outcomes based on high quality studies, situation that favors feeding the evidence already available until this moment and promoting future investigations which could impact over wellness of patients.
Conclusions
The present systematic review analyzed fifteen high quality studies according to evaluating the impact of epicardial adipose tissue (EAT-T) and periatrial epicardial adipose tissue (EAT-LA) on AF. Most studies report a statistically significant relationship between EAT-LA and EAT-T with increased incidence and prevalence of atrial fibrillation, increased arrhythmia severity (defined as a greater probability of suffering non-paroxysmal AF) and AF recurrence after catheter ablation. These findings are in line with previously published evidence and support previous hypothesis regarding the genesis and maintenance of atrial fibrillation. More studies are needed to define a «normal» cutoff value for EAT-T and EAT-LA, thus helping to identify at-risk populations. Moreover, with currently available evidence supports ongoing trials on modulation of these fat deposits to reduce the incidence and recurrence of AF.











 nueva página del texto (beta)
nueva página del texto (beta)