Introduction
The complete AV block that produces a dissociation of atrial and ventricular contractions,1 is a heart pathology associated with the trans-placental transmission of maternal antinuclear antibodies (ANA’s) being a severe manifestation of neonatal lupus.2 Besides, the cardiac lesions most frequently associated to this pathology are the isomerism of the left atrial appendages (LAI) and discordant atrioventricular connections, affecting about 60% of the diagnosed cases. During pregnancy women detected with ANA’s particularly anti Ro/SSA, La/SSB and anti-Smith antibodies have been linked to fetal heart damage.3-6 Seropositive Ro/SSA or La/SSB woman have a 2-3% of probability of getting a child that develops congenital AV block or abnormalities of the myocardium7 and a 17-20% of risk in case of having a previous child with the condition, this situation have its relevance in the prognosis for each case. Complete AV block involves an immune mediated inflammatory process which results in a damage of the conduction tissue and myocardium during the heart developing of the product.8 For the diagnosis of this pathology the use of ultrasound technology during prenatal care allows an early finding between 18 and 24 weeks of gestation, propitiating a better prognosis and for defining an adequate therapy.8,9 The principal treatment for a congenital heart block is aimed to a prophylactic sense that includes maternal administration of steroids, plasmapheresis, sympathomimetic, and in utero cardiac pacing during an adequate gestational age,10 added to this, a prenatal progression of the cardiac anomaly is important in case that a perinatal management could be necessary, since a pacemaker is generally needed in most children with congenital heart block.11 Due to this situation, the medical treatment for this pathology is focused on identifying the optimal timing for the placement of a pacemaker in order to ensure a positive outcome.12
Case report
The case refers to a 22 years old patient (G3P2C0A0) who was admitted to the department of gynecology and obstetrics of this hospital with 31.3 weeks period of gestation (POG) and a probable lupus nephropathy, without previous history of smoking or alcoholism, prenatal control started at the second trimester, without complications during pregnancy, whit a fetal heart rate between 51 and 105 bpm.
Results of the hematological parameters of the patient resulted in a reduction in erythrocyte mass, hemoglobin and hematocrit levels (2.89 10^6/mL, 8.10 g/dL and 26.5% respectively). The liver function panel resulted in 5 U/L of TGP, high levels of GGT 102 U/L and LDH 1,288 U/L; a renal biopsy was performed showing morphologic changes compatible with diffuse lupus nephritis class IV-S (A). In the analysis of ANA’s the results where positive for anti-Sm and anti-Ro/SSA (166.3 U/mL, 160.64 U/mL respectively). Obstetric echocardiogram showed a dissociation in the heart rhythm with apparent atrioventricular block, with an atrial frequency in the range of 130-140 bpm and at ventricular level with a bradycardia of 58 bpm, without a septal defect or cardiomegaly. After 33 weeks of POG the patient, gave birth by cesarean to a female with weight of 1,380 g, height of 40 cm and a heart rate of 48 bpm. The Apgar score was 6 at first minute and 7 at minute five, while the Silverman score was 3 at the first minute and 2 at minute five.
After birth, she was transferred to the Neonatal Intensive Care Unit (NICU). With ventilatory support phase II, nasal Continuous Positive Airway Pressure (CPAP). The patient was active and reactive to external stimuli. On the first day after birth, she still have CPAP support and a heart rate of 50 bpm without support of vasoactive amines (Figure 1), a radiography was taken as a control of the cardiopulmonary state (Figure 2). On the second day after birth, the patient remains bradycardic (60-65 bpm) with support of dobutamine, nonspecific human immunoglobulin and three doses of dexamethasone were added to the therapy.
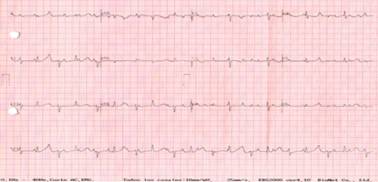
Figure 1: Electrocardiographic trace of the newborn. A complete dissociation between auricular and ventricular rhythm is observed with an auricular beat rate of 140 and a ventricular beat rate of 50 bpm.
Figure 2: Control radiography taken at the first day after birth showing the normal findings in a newborn child.
On the third day after birth was placed a temporary external pacemaker, the electrodes where attached to the right ventricle (Figure 3), fixed to the skin and connected to an external generator, the procedure was performed by a pediatric cardiologist; omeprazole, cefotaxin and paracetamol were added to the management. Subsequently, the patient went to the (NICU) for surveillance, expecting a weight increase for the placement of a definitive pacemaker. On the fourth day, a 1-minute apnea episode was reported, a cephalic helmet was placed with oxygen at 35%, 3 L per minute and enteral stimulation was initiated. On the sixth day, the oxygen saturation was 93% with ambient air and weighted 1,100 g. A radiography with gastromegalia and distention of intestinal loops were reported. On the tenth day the cephalic helmet was retired. On the eleventh day, onfaloclisis were removed and was the last day of cefotaxime. At the next day the pacemaker is set to 125 bpm to improve her cardiac output.

Figure 3: Radiography taken after the placement of epicardial electrodes connected to the temporal pacemaker.
Two months after surgery, the external pacemaker begins to fail, drops to 60 bpm and the pacemaker generator was replaced, the weight of the baby was of 2,730 g. A week later, she undergoes surgery. The external definitive pacemaker was placed and the heart rate is set at 100 bpm (Figure 4), it was supported with phase III ventilator with 42 cycles, FiO2 at 50%. Endopleural catheter was placed and removed the next day. One day after surgery, the baby was extubated and a cephalic helmet was placed, the same was removed after three days, maintaining good oximetry.

Figure 4: Radiography taken after the implantation of the definitive pacemaker in retroperitoneal position.
The baby was discharged at 78 days due to clinical improvement with a weight of 3,150 g.
Discussion
The prevalence of congenital AV block it’s found between 2 and 5% of fetuses and infants whose mothers are seropositive to ANA’s,1 who are usually diagnosed after the detection of AV block.13 A prospective study which included 186 individuals, conformed by 146 serially screened fetuses with normal pregnancy outcomes, and 40 fetuses/neonates with a diagnosis of heart block or endocardial fibroelastosis, resulting that mothers of children with cardiac involvement had moderate to high anti-Ro/SSA antibody levels (≥ 50 U/mL) as compared with only 44% of mothers of healthy infants (p < 0.0001), and for fetuses with exposure to anti-Ro levels an odds ratio (OR) of 7.8 (range 0.4 to 159) was described.14 Other studies have been reported with similar results, in which the recurrence rate of complete heart block (CHB) is at least two- to three-fold higher than the rate for a mother with anti-Ro/SSA and anti La/SSB antibodies.1,15-17 In terms of prognostic a retrospective study in which they included sixty fetuses with heart block of which thirty-two had complex cardiac malformations and 31 of them were associated with left isomerism. The outcomes of these fetuses were 22 terminations of pregnancy (TOP), three intrauterine fetal deaths (IUFD), three neonatal deaths (NND), two childhood deaths (CD) and only two survivors. They propose the fetal hydrops and the association with complex cardiac malformations as predictors of adverse outcome in cases of heart block where cases without cardiac malformations have a significantly better prognosis.18 The diagnostic of congenital AV block is associated with high fetal and neonatal mortality, and independently whether the diagnosis is made before or after birth, both, undergo for the implantation of a pacemaker with earlier intervention and a significantly greater need for intervention among those diagnosed in utero.14 With a mortality estimated around 8-16% in infants and half of this proportion in children and adults.13,14
For the fetal diagnosis of congenital AV block, the echocardiography has been determined as the gold standard, however, all M-mode and Doppler echocardiographic techniques that are based on the relationship between the atrial and ventricular mechanical events, can be well applied,19 besides clinical application of fetal electrocardiography and fetal magnetocardiography are promising noninvasive and highly precise tools. Referent to the treatment, dexamethasone is currently used, a study in which was included maternal treatment with dexamethasone was associated with normalized AV conduction in fetuses with first-degree AV Block,20 however its administration is also associated with potential side effects for both mother and fetus, especially potential negative effects in osteogenesis in the embryo developing. Coupled with this prenatal treatments may also include intravenous immunoglobulins and plasmapheresis, used alone or together in combination with steroids for the treatment of AV block21,22 it could be considered in asymptomatic high degree AV blocks with specific risk conditions. Literature reports that two thirds of the affected pediatric population diagnosed with congenital heart block requires a pacemaker23 in order to prevent sudden cardiac death and to restore heart rate to avoid longstanding bradycardia.
We report a clear case of neonatal lupus syndrome according to the parameters of clinical, radiological, electrocardiographic diagnosis, the need of the implantation of a pacemaker was supplied, all the characteristics presented by the patient with a persistent bradycardia, made her fit for surgery. This procedure is presented as an excellent option for patients with structurally normal hearts instead of treatments with steroids of which there is great controversy around its use, however there is still discussion about whether the pacing should be permanent or temporary. Supporting the implementation of this procedure, there are reports of permanent pacemaker implantation in term and preterm newborns with very acceptable success and survival rates.(24)-(26) There is limited information about the success of implantation of pacemakers in neonates with AV block, therefore it is important to report the procedures performed and important points to achieve success of the surgery, although this success may be due to the pathophysiological aspects of each individual. The detection of more cases of this rare pathology, the refining of the diagnosis with the use of documented tools and the generation of devices adapted to the needs of the patient are integral factors for good care.











 nueva página del texto (beta)
nueva página del texto (beta)


