Introduction
Drug-eluting stents are currently considered as the selected endovascular devices to treat patients with ischemic heart disease and acute coronary syndrome, due to significant reduction of intra-stent restenosis (ISR) against non-medicated bare-metal stents, and such action has been clinically translated as a lower rate of target lesion reoperation. However, first-generation drug-eluting stents were related to an increased incidence of very late stent thrombosis.1,2 Clinical outcomes3 have then improved with second-generation drug-eluting stents, although there is a need to be treated with dual antiplatelet therapy (DAPT) for an extended period of time (from six months to one year) reducing systematic use of such stents; so the development of new technologies has been enhanced with advantages and satisfactory clinical outcomes from a drug-eluting stent including more biocompatible polymers and bioabsorbable polymers, bioabsorbable stents and titanium-nitride-oxide-coated bioactive stents.4
Titanium-nitride-oxide coated bioactive stents (Titan BAS) safety has been already included in many reports about different kinds of groups of patients in different real world clinical scenarios.5,6
Titanium has better biocompatibility against stainless steel, gold or other materials that have been used as the stent surface’s coating since titanium provides minimum toxic ion release, so tissue reaction and inflammatory process would be both reduced. Titanium oxide blood compatibility, regarding platelet adhesion and fibrinogen adsorption, can be enhanced by adding nitrogen.
Previous prospective studies comparing both the bioactive stents and the paclitaxel-eluting stents showed better clinical outcomes in bioactive stent group for patients with complex coronary lesions and patients with acute myocardial infarction.5
Titan bioactive stent (Hexacath Company, France) has a stainless-steel platform and it is titanium-nitride-oxide-coated to reduce inflammatory reaction. Titanium is biologically inert because of its low electrochemical surface and it also has an excellent biocompatibility. Neither does it allow for the fibroblast growth nor does it stimulate platelet adhesion.6
Regarding second-generation drug-eluting stents, both the design and the used drug type are being considered as a new development to avoid high rates of documented restenosis with bare-metal stents and first-generation drug-eluting stents. Therefore, the Endeavor zotarolimus-eluting stent has significantly reduced the safety-efficacy combined target against them.7,8 Endeavor stent is made of cobalt alloy with phosphorylcholine polymer coating allowing for zotarolimus release to reduce neointimal hyperplasia. In addition, it does not produce thrombosis because of its phosphorylcholine polymer composition.9,10
As recognized in the international literature, clinical studies results have been already reported by comparing bioactive stent (Titan) versus a second-generation drug-eluting stent in patients with ST-segment elevation myocardial infarction (STEMI) subjected to a percutaneous transluminal coronary angioplasty, having similar outcomes up to five-year follow-up and demonstrating the non-inferiority in such stents.11 In our group of patients, however, there is not enough information about this kind of bioactive stents in such specific clinical scenario or their comparison with current selected stents.
Objective
The main purpose of this study is to evaluate and compare immediate, in-hospital and one-year use clinical outcomes of the titanium-nitride-oxide-coated stent (Titan) versus Endeavor stent (zotarolimus eluting stent) in patients with ST-segment elevation myocardial infarction (STEMI) that were treated at the Hemodynamics and Interventional Cardiology Department of the High Specialty Medicine Unit No.34-the Mexican Social Security Institute in Monterrey City, the State of Nuevo Leon.
Material and methods
From January 2011 to December 2014, a descriptive, comparative, longitudinal, retrospective, observational study was performed by analyzing database from the Hemodynamics and Interventional Cardiology Department of the High Specialty Medicine Unit No.34-the Mexican Social Security Institute in Monterrey City, the State of Nuevo Leon, related to patients with ST-segment elevation myocardial infarction that were subjected to primary, pharmacoinvasive and rescue angioplasties, using Titan stent against Endeavor stent.
18 year-older female and male patients were included to be subjected to a percutaneous coronary intervention in any of those clinical scenarios.
Patients who were treated with a zotarolimus-eluting stent (Endeavor) or titanium-nitride-oxide-alloy stent (Titan) were all considered. 256 patients were included and divided in two groups: 135 patients in Titan stent group and 135 patients in Endeavor stent group.
Inclusion clinical criteria: ST-segment elevation myocardial infarction type acute coronary syndrome (ACS) diagnosed 18 year-older patients who were treated with a percutaneous transluminal coronary angioplasty (PTCA) at the hemodynamics laboratory. The proper informed consent letter was obtained for all the patients.
ST-segment elevation acute coronary syndrome diagnosis was defined according to the guidelines related to the presence of ST-segment persistent elevation (2 mm within two adjacent precordial leads at least, or 1 mm within two limb leads at least), new or presumably new left bundle branch block or new pathological Q waves within two adjacent leads on the electrocardiogram (ECG) at least, and it was related to an increase in biochemical markers of myocardial necrosis-creatine phosphokinase-MB (CPK-MB) enzymes or troponin I, twice the upper limit of normal at least.12
Definite stent thrombosis was defined according to the Academic Research Consortium criteria.12
Most information was obtained from the Hemodynamics and Interventional Cardiology Department database. Its monitoring was updated based on medical and electronic records, in-hospital stay and outpatient clinic’s medical notes, as well as six-month or one-year telephone follow-up’s medical notes. Patients were divided in two groups to be compared: the ones who were subjected to coronary angioplasty using Titan stent(s) and those who were subjected to coronary angioplasty using Endeavor stent(s) (zotarolimus eluting stent).
Patients having different stents from the ones established in the study cohorts were excluded. In addition, patients having two different types of stent in the same vessel, or using two different types of stent, having incomplete records or being unable to have a higher six-month follow-up were all excluded.
Following primary points were determined in this study: Major adverse cardiac events (MACEs), death, myocardial infarction, need for target lesion revascularization (TLR), target vessel revascularization (TVR), cerebrovascular event (CVE) and stent thrombosis. Secondary points: Dual antiplatelet therapy (DAPT) usage time.
Stent implantation was performed by a certified interventional cardiologist having a previous informed consent. Determination on which endovascular device would be employed (type of stent) as well as the use and type of adjuvant pharmacological treatment were the sole responsibility of the operator.
Following demographic variables were analyzed in both groups: present or non- present systemic arterial hypertension (SAH), diabetes mellitus (DM), smoking, dyslipidemia (DLP), previous ischemic heart disease (IHD), left ventricular ejection fraction (LVEF), number of diseased coronary vessels, percutaneous transluminal coronary angioplasty and/or previous coronary revascularization surgery (CRS) and procedure indication. Immediate, in-hospital and one-year follow-up primary points were analyzed as follows: Major adverse cardiac events (MACEs), death, myocardial infarction, need for target lesion revascularization (TLR), target vessel revascularization (TVR), cerebrovascular event (CVE) and stent thrombosis (ST). Secondary points: Dual antiplatelet therapy (DAPT) usage time.
Presence of events was directly reported to the Hemodynamics Department service and such events were included in the department’s database. Stent thrombosis, reinfarction, target lesion revascularization and target vessel revascularization events were all evaluated either by control coronary angiography or autopsy study. The need for target vessel revascularization (TVR) was defined as the secondary repeat revascularization (percutaneous or surgical intervention) to intra-stent restenosis.
Inclusion angiographic criteria: All lesions were included according to the AHA/ACC (American Heart Association/American College of Cardiology) classification (A, B1 B2 and C types), including vessels ≥ 2.25 mm and ≤ 4.0 mm estimated by quantitative angiography. Exclusion angiographic criteria: Patients showing contraindications or being sensitive to aspirin, heparin, clopidogrel, or being hypersensitive to contrast media and platelet count lower than 60,000 and higher than 700,000 cells/mm3.
Employed stent system:
Endeavor stent - zotarolimus eluting stent - (Medtronic; Indianapolis, Indiana, USA) is a phosphorylcholine-coated cobalt-chromium alloy stent which transfers zotarolimus drug to 10μg per 1mm stent length. The phosphorylcholine polymer is considered as a synthetic copy of predominant phospholipids on the red blood cells membrane. Therefore, it shows a high biovascular compatibility.9
Titan-2 stent - bioactive stent - (Hexacath; Paris, France) has a TITANOX (titanium-nitride-oxide coating) coated Helistent platform. It also has significant effects when reducing inflammation, inhibiting platelet aggregation and minimizing both thrombogenicity and endothelial cell growth.8,10
Stent implantation: Stent implantation was performed according to the standard coronary interventional procedure. Before such procedure is performed, patients were treated with 300 mg aspirin (acetylsalicylic acid) by oral intake and a 600 mg clopidogrel loading dose by oral intake. Unfractionated heparin was used in a dose of 70-100 IU/kg throughout this procedure. The operator considered and decided to use glycoprotein IIb/IIIa inhibitors. Likewise, the operator considered and decided to implant stent(s) either by direct stenting technique or pre-dilatation technique.
Available stent length measurements were as follows: 9, 12, 13, 16, 18, 19, 22, 24, 28, 30 and 38 mm, including 2.25, 2.5, 3.0, 3.5, 4.0 and 4.5 mm diameters.
An electrocardiogram and serial cardiac enzymes measurement were carried out after the procedure was performed.
Patients took aspirin (75 mg by oral intake, indefinitely) while 75 mg clopidogrel by oral intake were prescribed for 12 months in relation to drug-eluting stents and 6 months in relation to bioactive stents. Angiographic clinical success was defined as having a residual angiographic stenosis < 20% which involves a TIMI grade 3 flow without showing any major adverse cardiac event (MACE) at the end of the procedure (fatal acute myocardial infarction, emergency surgery and cardiovascular death).
Definitions about primary and secondary endpoints: The primary endpoint was the presence of immediate, in-hospital and 12-month follow-up major adverse cardiac events, which were defined as reinfarction compound (acute myocardial infarction), target lesion revascularization (TLR), target vessel revascularization (TVR), cerebrovascular event and death.12
Target lesion revascularization: It is defined as the ischemia repeat revascularization due to stenosis (> 50%) of stent luminal diameter, inside the stent itself or 5 mm away from stent proximal or distal segment estimated either by quantitative coronary angiography, intravascular ultrasound or taken to target vessel coronary bypass surgery due to intra-stent restenosis.
Target vessel revascularization: It is defined as the ischemia repeat revascularization due to stenosis lesion (> 70%) in a different segment from the previously target lesion segment.
Acute myocardial infarction: It is defined as the ST-segment persistent elevation (2 mm within two adjacent precordial leads at least, or 1 mm within two limb leads at least), new or presumably new left bundle branch block or new pathological Q waves within two adjacent leads on the electrocardiogram (ECG) at least, and it was related to an increase in biochemical markers of myocardial necrosis-creatine phosphokinase-MB (CPK-MB) enzymes or troponin I, twice the upper limit of normal at least.
Cardiac death: It was defined as the cardiovascular cause death or unknown cause death.
Stent thrombosis (ST): It was defined as an acute coronary syndrome having vascular occlusion angiographic record and including a thrombus inside or close to the previous stent segment. In the absence of angiography, stent thrombosis could be defined by the presence of acute myocardial infarction (AMI) on target vessel section, or by cardiac cause death for 30 days after procedure was performed.
Stent thrombosis was classified as acute grade (< 24 hours after stent implantation), subacute grade (1-30 days after stent implantation) or late grade (> 30 days after stent implantation) according to the Academic Research Consortium (ARC).
Statistical analysis: Descriptive variables are expressed according to measures of central tendency and dispersion (mean ± standard deviation, median and percentiles) as appropriate. As for the differences in proportions of categorical variables, they were considered according to the Pearson’s chi-squared test (χ2) or Fisher’s exact test as per number of patients. Frequency of major cardiovascular events, target lesion revascularization (TLR) or target vessel revascularization (TVR) and binary restenosis will be all expressed as percentages. Numerical variables were evaluated by Student’s t- test. A p < 0.05 value with 95% confidence interval (95% CI) was considered statistically significant. Statistical analysis was performed by IBM® SPSS Statistics program, Mac OS X version 24.
Results
Demographic and angiographic features
From January 2011 to December 2014, 256 patients with ST-segment elevation myocardial infarction (STEMI) were analyzed. These patients were treated with Titan bioactive stent (135 patients) or Endeavor stent (121 patients) at the Hemodynamics and Interventional Cardiology Department of the High Specialty Medicine Unit Num. 34-the Mexican Social Security Institute in Monterrey City, the State of Nuevo Leon. Baseline demographic features in both study cohorts are showed in Table I.
Table I: Demographic variables.
| Variables | Titan stent group n = 135 patients (%) |
Endeavor stent group n = 121 patients (%) |
p Value |
|---|---|---|---|
| Age (years) | 62.36 ± 12.95 | 57.50 ± 10.42 | 0.001 |
| Male patients | 109 (80.7) | 105 (86.8) | 0.193 |
| Female patients | 26 (19.3) | 16 (13.2) | |
| Diabetes mellitus | 33 (24.4) | 35 (28.9) | 0.418 |
| DM diagnosis time (years) | 1.15 ± 0.48 | 1.13 ± 0.40 | 0.775 |
| Hypertension | 98 (72.6) | 56 (46.3) | < 0.0001 |
| Dyslipidemia | 68 (50.4) | 59 (48.8) | 0.797 |
| Smoking | 71 (52.6) | 56 (46.3) | 0.313 |
| Pre-acute myocardial infarction | 84 (62.2) | 71 (58.7) | 0.562 |
| Pre-PTCA | 13 (9.6) | 10 (8.3) | 0.703 |
| Pre-coronary revascularization surgery | 0 (0) | 0 (0) | |
| Chronic stable angina | 36 (28.3) | 44 (37.0) | 0.149 |
| Angina or post-chronic heart failure | 47 (34.8) | 41 (33.9) | 0.876 |
| Acute myocardial infarction starting time | 16.60 ± 22.27 | 19.08 ± 22.81 | 0.380 |
| Killip-Kimball 1-2 classification | 78 (57.8) | 47 (38.8) | 0.010 |
| Killip-Kimball 3-4 classification | 57 (42.2) | 74 (61.2) | 0.010 |
| Cardiogenic shock (CS) | 17 (12.6) | 12 (9.9) | 0.500 |
| Cardiogenic shock diagnosis time | 1.44 ± 6.05 | 2.88 ± 12.83 | 0.245 |
| Serum creatinine (mg/dL) | 1.11 ± 0.56 | 1.06 ± 0.60 | 0.485 |
| Total cholesterol (mg/dL) | 203.96 ± 28.76 | 200.36 ± 28.76 | 0.294 |
| Low-density lipoprotein cholesterol (mg/dL) | 157.64 ± 24.71 | 153.23 ± 28.28 | 0.188 |
| High-density lipoprotein cholesterol (mg/dL) | 35.55 ± 6.71 | 33.58 ± 5.38 | 0.010 |
| Left ventricular ejection fraction | 43.20 ± 9.29 | 44.79 ± 7.83 | 0.142 |
| Number of diseased vessels | 1.62 ± 0.71 | 1.56 ± 0.68 | 0.491 |
| Number of target vessels | 1.31 ± 0.52 | 1.24 ± 0.42 | 0.237 |
| Pre-PTCA reference diameter | 3.24 ± 0.48 | 3.21 ± 0.40 | 0.550 |
| Pre-PTCA stenosis diameter | 0.32 ± 0.32 | 0.34 ± 0.36 | 0.607 |
| Stenosis percentage | 89.22 ± 11.22 | 89.71 ± 11.27 | 0.729 |
| Lesion length | 22.39 ± 13.31 | 20.66 v 10.32 | 0.244 |
| Post-PTCA reference diameter | 3.26 ± 0.52 | 3.19 ± 0.40 | 0.222 |
| Post-PTCA stenosis diameter | 3.27 ± 0.53 | 3.14 ± 0.57 | 0.053 |
| Final stenosis percentage | -0.20 ± 1.29 | 0.11 ± 1.28 | 0.058 |
| Follow-up period (months) | 11.60 ± 5.22 | 10.30 ± 4.49 | 0.033 |
PTCA = Percutaneous translumnal coronary angioplasty.
Male patients were predominant in this study with 109 patients (80.7%) in Titan stent group and 105 patients (86.8%) in Endeavor stent group, p = 0.193.
The average age in Titan stent group was 62.3 ± 12.9 years old, and in Endeavor stent group was 57.5 ± 10.4 years old, having a statistically significant difference, p = 0.001.
Smoking incidence, diabetes mellitus, diabetes mellitus diagnosis time (years of diagnosis) and dyslipidemia were all found in similar proportions with no significant differences between both groups.
A higher incidence of systemic arterial hypertension (SAH) was demonstrated in Titan stent group, and such incidence was shown in 98 patients (72.6%) against 56 patients (46.3%) in Endeavor stent group, having a significant difference of p < 0.0001 value.
History of previous acute coronary syndrome, previous myocardial infarction (MI) and chronic stable angina was similar in each group with no statistical significance. History of previous percutaneous coronary intervention was also similar by showing 9.6% in Titan bioactive stent group (13 patients) versus 8.3% in Endeavor drug-eluting stent group (10 patients), p = 0.70.
Regarding the development time of ST-segment elevation myocardial infarction (acute coronary event), there was no significant difference, so it showed an average time of 16.6 ± 22.2 development hours in the Titan stent group, and 19 ± 22.8 development hours in the Endeavor stent group; p = 0.38. However, there was a significant difference concerning the clinical presentation severity (Killip-Kimball classification-KK). This way, 57.8% and 42.2% patients in Titan stent group vs 38.8% and 61.2% patients in Endeavor stent group were classified as KK 1-2 and KK 3-4, p = 0.01.
Incidence of cardiogenic shock as clinical presentation during procedure was produced up to 11.3% total patients, and it was present in 17 patients from Titan stent group (12.6%) and 12 patients from Endeavor stent group (9.9%), p = 0.50. Cardiogenic shock showed similar development times with no statistical significance.
Some laboratory parameters such as serum creatinine, total cholesterol, high-density lipoprotein cholesterol and low-density lipoprotein cholesterol showed no difference between both groups.
Similarly, left ventricular ejection fraction (LVEF) was similar in both groups: 43 ± 9% in Titan stent group versus 44 ± 7% in Endeavor stent group, p = 0.142.
Certain angiographic features such as the number of diseased vessels, number of target vessels, pre-and post-PTCA reference diameters, pre- and post-PTCA stenosis percentage, and target lesion length were all similar in both groups.
Follow-up period was 11.60 ± 5.22 months in Titan stent group versus 10.30 ± 4.49 months in Endeavor stent group, p = 0.033.
Regarding both the clinical scenario and the acute myocardial infarction development, Table II shows the type of employed strategy in coronary intervention performed in each study cohort as a myocardial reperfusion method. Incidence of different PTCA invasive strategies were similar in each group: primary PTCA 62.2% versus 60.3% (p = 0.459); pharmacoinvasive PTCA 15.6% versus 10.8% (p = 0.257); and rescue PTCA 22.2% versus 28.9% (p = 0.219) in Titan stent group vs Endeavor stent group, respectively.
Table II: Type of invasive strategy performed for ST segment elevation acute coronary syndrome.
| Employed strategy | Titan stent group n = 135 patients (%) |
Endeavor stent group n = 121 patients (%) |
p Value |
|---|---|---|---|
| Primary PTCA | 84 (62.2) | 73 (60.3) | 0.459 |
| Pharmacoinvasive PTCA | 21 (15.6) | 13 (10.8) | 0.257 |
| Rescue PTCA | 30 (22.2) | 35 (28.9) | 0.219 |
During interventional procedure, a percutaneous coronary intervention was performed on the anterior descending artery in 65.9% patients in Titan stent group against 84.3% patients in Endeavor stent group, p = 0.001. Remaining target vessels showed no statistically significant differences between both study cohorts (Table III). According to the AHA/ACC classification, coronary lesions causing ST-segment elevation myocardial infarction were considered as type A in 3% versus 3.3% (p = 0.875); type B in 34.6% versus 56.2% (p = 0.001) and type C (complex lesions) in 62.4% versus 40.5% (p = 0.001) patients in Titan stent group versus Endeavor stent group, respectively.
Table III: Angiographic-anatomic variables and outcomes.
| Variables | Titan stent group n = 135 patients (%) |
Endeavor stent group n = 121 patients (%) |
p Value |
|---|---|---|---|
| Target vessel | |||
| Coronary artery | 7 (5.2) | 3 (2.5) | 0.265 |
| Anterior descending artery | 89 (65.9) | 102 (84.3) | 0.001 |
| Circumflex artery | 48 (35.6) | 33 (27.3) | 0.155 |
| Right coronary artery | 70 (51.9) | 48 (39.7) | 0.051 |
| Ramus intermedius artery | 3 (2.3) | 0 (0) | 0.097 |
| Diagonal artery | 14 (10.5) | 7 (5.8) | 0.171 |
| Posterior descending artery | 11 (8.1) | 12 (9.9) | 0.621 |
| Posterolateral artery | 7 (5.3) | 1 (0.8) | 0.043 |
| Venous hemo-duct | 0 (0) | 3 (2.5) | 0.068 |
| Dominant artery | 0.553 | ||
| Right coronary artery | 113 (83.7) | 107 (88.4) | |
| Circumflex artery | 16 (11.9) | 10 (8.3) | |
| Codominance | 6 (4.4) | 4 (3.3) | |
| Types of lesion | 0.002 | ||
| A | 4 (3) | 4 (3.3) | 0.875 |
| B | 47 (34.6) | 68 (56.2) | 0.001 |
| C | 84 (62.4) | 49 (40.5) | 0.001 |
| Pre-PTCA TIMI 0-1 | 107 (79.3) | 110 (90.9) | 0.100 |
| Pre-PTCA TIMI 2-3 | 28 (20.7) | 11 (9.1) | 0.100 |
| Thrombus | 46 (34.6) | 32 (26.4) | 0.160 |
| Chronic occlusion | 2 (1.5) | 5 (4.1) | 0.201 |
| Stent restenosis | 2 (1.5) | 5 (4.1) | 0.201 |
| Stent thrombosis | 2 (1.5) | 1 (0.8) | 0.358 |
| Late thrombosis | 0 (0) | 2 (1.7) | 0.134 |
| Treated segment | 0.548 | ||
| Proximal segment | 56 (41.5) | 58 (47.9) | |
| Middle segment | 70 (51.9) | 57 (47.1) | |
| Distal segment | 9 (6.7) | 6 (5.0) | |
| Small vessel disease* | 39 (28.9) | 22 (18.2) | 0.045 |
| Single vessel disease | 109 (80.7) | 97 (80.2) | 0.908 |
| Multi-vessel disease | 38 (28.1) | 29 (24) | 0.447 |
Additional angiographic features of the vessel causing myocardial infarction such as the initial TIMI flow, thrombus load, presence of chronic occlusions, stent restenosis, and previous stent thrombosis were similar in both groups. Likewise, there were no differences regarding target coronary artery segment; although there was a statistically significant difference in the small vessel disease treated in both groups, so this feature became more frequent when using Titan bioactive stent group (28.9%) against 18.2% patients in Endeavor stent group, p = 0.045.
Significantly, a greater number of stents was used in Titan stent group with an average of 1.40 ± 0.61 against Endeavor stent group, where an average of 1.23 ± 0.66 stents was reported, p = 0.036. Implanted stents diameters were similar in each group: 3.19 ± 0.54 mm versus 3.15 ± 0.43 mm (p = 0.596). Implanted stents length was also similar: 21.27 ± 4.28 mm versus 22.23 ± 4.98 mm (p = 0.100) in Titan stent group versus Endeavor stent group, respectively.
Endovascular device implantation was performed by direct stenting technique in 18.5% patients in Titan stent group (25 patients), while such implantation was performed with Endeavor stent in 14% patients (17 patients) with no statistical difference (p = 0.335). Employed atmospheres for stent release and over-impaction were similar in each group: 13.28 ± 1.87atm in Titan stent group versus 13.53 ± 1.57atm in Endeavor stent group, p = 0.256. Final TIMI grade 1 flow in 1.7% patients in Endeavor stent group and 0% patients in Titan stent group, final TIMI grade 2 flow in 9.1% versus 9.6%, and final TIMI grade 3 flow in 89.3% versus 90.4%, respectively, were all observed with no significant difference (p = 0.323) (Table IV).
Table IV: Stents and subsequent release outcome.
| Variables | Titan stent group n = 135 patients (%) |
Endeavor stents group n = 121 patients (%) |
p Value |
|---|---|---|---|
| Employed stents | 1.40 ± 0.61 | 1.23 ± 0.66 | 0.036 |
| Number of stents | 0.003 | ||
| 1 | 90 (66.7) | 100 (82.6) | |
| 2 | 36 (26.7) | 19 (15.7) | |
| 3 | 9 (6.7) | 0 (0%) | |
| 4 | 0 (0) | 0 (0%) | |
| 5 | 0 (0) | 1 (0.8) | |
| 6 | 0 (0) | 1 (0.8) | |
| Stent diameter | 3.19 ± 0.54 | 3.15 ± 0.43 | 0.596 |
| Stent length | 21.27 ± 4.28 | 22.23 ± 4.98 | 0.100 |
| Direct stenting | 25 (18.5) | 17 (14.0) | 0.335 |
| Employed atmospheres |
13.28 ± 1.87 | 13.53 ± 1.57 | 0.256 |
| Final TIMI flow | 0.323 | ||
| 1 | 0 (0) | 2 (1.7) | |
| 2 | 13 (9.6) | 11 (9.1) | |
| 3 | 122 (90.4) | 108 (89.3) | |
| PTCA successful outcome | 135 (100) | 119 (98.3) | 0.134 |
A PTCA successful outcome was reported in 100% of the Titan stent group cases (135 patients) and in 98.3% of the Endeavor stent group cases (119 patients), p = 0.134.
Regarding the adjuvant pharmacological treatment (Table V), patients were treated with 600 mg clopidogrel loading dose by oral intake in 56.3% in Titan stent group vs 63.6% in Endeavor stent group (p = 0.232), and with aspirin loading dose in 98.5% versus 98.3%, respectively (p = 0.912).
Table V: Pre-procedural employed drugs and intra-aortic balloon pump.
| Variables | Titan stent group n = 135 patients (%) |
Endeavor stent group n = 121 patients (%) |
p Value |
|---|---|---|---|
| Clopidogrel | 76 (56.3) | 77 (63.6) | 0.232 |
| Nitrates | 93 (68.9) | 86 (71.1) | 0.703 |
| Statins | 113 (83.7) | 104 (86) | 0.617 |
| Aspirin | 133 (98.5) | 119 (98.3) | 0.912 |
| ACE inhibitors | 71 (52.6) | 79 (65.3) | 0.039 |
| ARA | 3 (2.3) | 8 (6.7) | 0.085 |
| Beta blockers | 59 (44.4) | 60 (49.6) | 0.405 |
| Calcium antagonists | 2 (1.5) | 1 (0.8) | 0.628 |
| Thrombolysis | 4 (3.0) | 3 (2.5) | 0.813 |
| IIb-IIIa inhibitors | 24 (18) | 12 (9.9) | 0.064 |
| IABP | 18 (13.3) | 17 (14.0) | 0.868 |
| Duration of dual antiplatelet therapy | 6.46 ± 4.11 | 10.98 ± 2.51 | < 0.0001 |
ACE-inhibitors = Angiotensin-converting enzyme inhibitors, ARA = Angiotensin receptor antagonists, IABP = Intra-aortic balloon pump.
Use of other drugs such as nitrates, statins, angiotensin receptor antagonists, beta blockers and calcium antagonists was similar between both groups.
A difference in the use of angiotensin-converting enzyme inhibitors (ACE-inhibitors) was observed, so it was used in 52.6% patients in bioactive stent group against 65.3% patients in zotarolimus-eluting stent group, p = 0.039.
Glycoprotein IIb/IIIa inhibitors were administered in 24 patients in Titan stent group (18%) and 12 patients in Endeavor stent group (9.9%) with no statistical significance (p = 0.64).
Use of intra-aortic balloon pump during interventional procedure was similar in both groups; 13.3% vs 14%, Titan stent group vs Endeavor stent group, respectively (p = 0.868).
Duration of dual antiplatelet therapy (DAPT) was significantly lower in Titan bioactive stent group with a mean of 6.46 ± 4.11 months against Endeavor stent group, which mean was 10.98 ± 2.51 months with a p < 0.0001 value.
Peri-procedural immediate clinical outcomes: 2 deaths were registered in the hemodynamics room (1.5%) in Titan stent group, whereas Endeavor stent group showed no deaths with no statistical significance (p = 0.179) (Table VI).
Table VI: Immediate, in-hospital and 12-month follow-up clinical outcomes.
| Variables | Titan stent group n = 135 patients (%) |
Endeavor stent group n = 121 patients (%) |
p Value |
|---|---|---|---|
| Acute myocardial infarction in hemodynamics room | 0 (0) | 0 (0) | |
| Deaths in hemodynamics room | 2 (1.5) | 0 (0) | 0.179 |
| In-hospital acute myocardial infarction | 1 (0.7) | 0 (0) | 0.343 |
| In-hospital death | 3 (2.2) | 2 (1.7) | 0.742 |
| In-hospital cerebrovascular events | 0 (0) | 0 (0) | |
| Hematoma | 1 (0.7) | 0 (0) | 0.343 |
| Target lesion revascularization | 0 (0) | 3 (2.5) | 0.066 |
| Target vessel revascularization | 0 (0) | 1 (0.8) | 0.908 |
| PTCA on another vessel | 3 (2.2) | 3 (2.5) | 0.892 |
| Stent thrombosis | 2 (1.5) | 1 (0.8) | 0.627 |
| Major adverse cardiac event | 17 (12.6) | 12 (9.9) | 0.500 |
| Major adverse cardiac event-free (months) | 13.49 ± 5.93 | 12.13 ± 5.18 | 0.049 |
| Total death | 10 (7.4) | 7 (5.8) | 0.603 |
Regarding in-hospital major adverse cardiac events, in-hospital reinfarction appeared in one single patient in Titan stent group (0.7%), p = 0.343. During hospitalization, 3 deaths were registered in Titan stent group (2.2%) and 2 patients died in Endeavor stent group (1.7%), p = 0.742. No in-hospital cerebrovascular events were registered in any of the examined patients.
Both TVR and TLR rates were not statistically significant; 0% versus 2.5% and 0% versus 0.8% in Titan stent group against Endeavor stent group, respectively, during 12-month follow-up. A percutaneous transluminal coronary angioplasty was required to be performed on another vessel in 2.2% patients in Titan stent group vs 2.5% patients in Endeavor stent group, p = 0.892.
Stent thrombosis was registered in 2 patients using Titan stent (1.5%) and 1 patient using Endeavor stent (0.8%), p = 0.627.
A major adverse cardiac event was produced in 12.6% patients in Titan bioactive stent group and 9.9% patients in Endeavor stent group with no statistical significance (p = 0.50) throughout follow-up.
Major adverse cardiac event-free average time was 13.49 ± 5.93 months in patients using Titan stent, and 12.13 ± 5.18 months in patients using Endeavor stent, p = 0.049.
There were no significant differences in overall mortality during 12-month follow-up, which was registered in 7.4% patients using Titan stent versus 5.8% patients using Endeavor stent, p = 0.603.
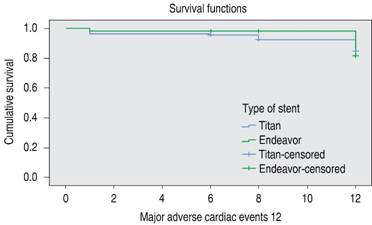
There were no significant differences in major adverse cardiac event-free survival analysis when performing the Log-rank Mantel-Cox test, p = 0.090 (Figure 1).
Discussion
Nowadays, use of drug-eluting stents is preferred in most of the ischemic heart disease clinical scenarios, and they are also predominant in acute coronary syndromes since they show better outcomes in terms of stent restenosis when reducing TLR and TVR mainly.2,3,9 However, it has been demonstrated that use of drug-eluting stents has not significantly reduced hard endpoints such as death and myocardial infarction consistently.10
Based on previous information, bioactive stents (BAS) have been selected to be manufactured with new technologies on titanium-nitride-oxide coating design to improve biocompatibility.8,11
Nowadays, safety in use of bioactive stents has been confirmed when treating coronary lesions, and their efficacy has also been confirmed when reducing major adverse cardiac event rates, having a lower rate of stent thrombosis even when using short dual antiplatelet therapy (DAPT) schemes previously reported within controlled prospective studies in non-selected study cohorts and within some records. TiNOX13 trial employed a dual antiplatelet therapy for one month at least; whereas TITAX AMI14 trial compared the dual antiplatelet therapy outcome in 7.6 months using bioactive stents versus 10.1 months using paclitaxel-eluting stents (PES) (p < 0.001), having a thrombosis rate in 0.5% in bioactive stents versus 6.6% in paclitaxel-eluting stents used, p < 0.001.
As recognized in the international literature, use of titanium-nitride-oxide-coated bioactive stents has been already compared in the BASE-ACS trial since 2012 initially, whose original publication reported a head-to-head comparison in a randomized trial against a second-generation drug-eluting stent - everolimus - (everolimus-eluting stents) in the context of acute coronary syndrome - acute myocardial infarction.15 In such trial, 827 patients with acute myocardial infarction (1:1) were randomized in order to be treated with bioactive stent (BAS) or everolimus-eluting stent, and as for patients subjected to early percutaneous coronary intervention to treat acute myocardial infarction, a bioactive stent implantation was not lower than everolimus-eluting stent implantation regarding the primary endpoint’s occurrence estimation on a major adverse cardiac event compound (cardiac death, non-fatal acute myocardial infarction or ischemia-driven target lesion revascularization) in 12-month follow-up. Analysis was performed under treatment purposes. The primary endpoint compound occurred in 9.6% patients in bioactive stent group and 9% patients in everolimus-eluting stent group (HR [hazard ratio] 1.04, 95% CI [confidence interval] 0.81-1.32, p = 0.81, p value for non-inferiority = 0.001). Major adverse cardiac event relative risk ratio in bioactive stent was 1.07 (0.6% absolute risk difference) against everolimus-eluting stent, which is a difference that met the main purpose of this non-inferiority trial regarding bioactive stents when reducing MACEs in this patient category. Authors, however, affirm that such trial had no adequate or required power to address individual elements in terms of safety and efficacy. Authors emphasize that non-fatal myocardial infarction was significantly less frequent in bioactive stent group (2.2% versus 5.9%, p = 0.007), and that stent thrombosis (ST) tended to be lower in bioactive stent group against everolimus-eluting stent group.15
Concerning our analysis, which compared use of Titan stent against Endeavor stent (zotarolimus-eluting stent) in patients with ST-segment elevation myocardial infarction (STEMI), there were no significant differences regarding major adverse cardiac events, death, myocardial infarction, stent thrombosis or cerebrovascular events, either in-hospital or one-year follow-up.
A major adverse cardiac event was produced in 12.6% patients in bioactive stent group and 9.9% patients in Endeavor stent group with no statistical significance (p = 0.50) throughout the follow-up, having major adverse cardiac event rates closer to the ones reported by Karjalainen et al.15
Both TVR and TLR rates were not statistically significant; 0% versus 2.5% and 0% versus 0.8% in Titan stent group against Endeavor stent group, respectively. A percutaneous transluminal coronary angioplasty was required to be performed on another vessel in 2.2% patients in Titan stent group versus 2.5% patients in Endeavor stent group, p = 0.892.
We must emphasize that a significant difference was registered in dual antiplatelet therapy usage time (6.46 ± 4.11 months in Titan stent group versus 10.98 ± 2.51 months in Endeavor stent group, p= < 0.0001).
It is also important to emphasize that Titan stent was significantly and more frequently employed in elderly patients (62.36 ± 12.95 years old versus 57.59 ± 10.42 years old in Endeavor stent group, p = 0.001); to treat AHA/ACC-based more complex type C lesions (62.4% in Titan stent group versus 40.5% in Endeavor stent group, p = 0.010) and small vessels (28.9% in Titan stent group versus 18.2% in Endeavor stent group, p = 0.045).
Karjalainen PP et al16 performed a post hoc analysis about BASE-ACS trial, which was published in 2013, focusing more on the stent vs patient-oriented outcome in 24-month follow-up. They defined stent-oriented outcome as a cardiac death element, non-fatal myocardial infarction related to the target vessel, or ischemia-driven target lesion revascularization. On the other hand, patient-oriented outcome was defined as an all-cause death element, any non-fatal myocardial infarction or revascularization. 24-month clinical follow-up was completed in 406 patients in bioactive stent (BAS) group (97.4%) and 398 patients in everolimus-eluting stent group (97.1%). 24-month follow-up stent-oriented outcomes were produced in similar frequencies in both stent groups (10.1% in bioactive stent group versus 11.2% in everolimus-eluting stent group, p = 0.53). Similarly, 24-month follow-up patient-oriented outcome was similar in both groups (16.3% versus 19.8%, respectively, p = 0.2).
In addition, 4-year clinical follow-up was completed in 753 patients (91.1%). For 4 years, bioactive stent continued to be not lower than everolimus-eluting stent concerning the incidence of major adverse cardiac events (14.7% versus 17.8%, p = 0.001 for non-inferiority). Non-fatal myocardial infarction continued to be less frequent in bioactive stent group (5.0% versus 9.2%, respectively, p = 0.025). Both cardiac death and ischemia-driven target lesion revascularization were similar (2.9% versus 3.5% and 8.6% versus 9.2%, p = 0.62 y p = 0.80, respectively). It was reported in this study that independent predictors of major adverse cardiac event were the presence of calcified lesions (HR [Hazard Ratio] 1.54, p = 0.021), number of target vessels (HR 1.53, p = 0.025) and reference vessel diameter (HR 0.54, p = 0.006).
More recently, at the end of 2016, final 5-year follow-up of this BASE-ACS study was published, substantially confirming that bioactive stent was not lower than everolimus-eluting stent to primary endpoint of major adverse cardiac events (14.4% versus 17.8%, respectively, and hazard ratio (HR) for bioactive stent versus everolimus-eluting stent was 0.82 with 95% confidence interval, 0.58-1.16, p = 0.26 for superiority, p = 0.001 for non-inferiority). Non-fatal myocardial infarction rate remained consistently lower in bioactive stent group (5.9% versus 9.7%, respectively, p = 0.028). Both cardiac death and ischemia-driven target lesion revascularization rates remained as well without showing any significant differences between both groups (2.8% versus 3.8% and 8.3% versus 9.9%, p = 0.76 and p = 0.58, respectively).11
Main limitations in this study are as follows: Retrospective nature, analysis performance in one single high specialty unit, which outcomes might not be considered as reproducible in other healthcare facilities or to overall patients, as well as the number of examined patients and limited one-year follow-up. Despite such limitations, this study provides a general overview of clinical outcomes in our patients with ST-segment elevation myocardial infarction (STEMI) subjected to percutaneous transluminal coronary angioplasty and treated with Titan bioactive stent against second-generation drug-eluting stent (Endeavor stent).
Conclusion
It was demonstrated in this study that no superiority was registered in the use of a second-generation drug-eluting stent such as the Endeavor stent (zotarolimus-eluting stent) versus Titan bioactive stent (titanium-nitride-oxide-coated stent) in patients with ST-segment elevation myocardial infarction (STEMI) subjected to coronary angioplasty regarding immediate, in-hospital and one-year follow-up clinical outcomes.
The Titan stent seems to be a good choice for this kind of ST-segment elevation acute coronary syndrome in both efficacy and safety against new drug-eluting stents, and it could be used in elderly patients and/or patients with high bleeding risk requiring less time of dual antiplatelet therapy (DAPT).











 nueva página del texto (beta)
nueva página del texto (beta)