Introduction
The aorta is the largest blood vessel in the human body, is affected by multiple pathologies. Aortic aneurysms are one entity with an increasing incidence in our population;1-3 It is estimated that the 18th most common cause of death in all individuals and the 15th most common in individuals older than age 65 years.4-6 The ascending aorta is affected in 50.0 to 60.0% of cases.7,8 The greatest number of cases occur in the seventh decade of life with a clear predominance in the male gender.6,7 The etiology of aneurysms is divided into degenerative, related to cystic medial degeneration, inflammatory, congenital anomalies, micotic, traumatic and post-stenotic (Table I).6-8 Aortic dissection, intramural aortic hematoma, penetrating aortic ulcer, ruptured aortic aneurysm and intraaortic atheromatous masses are the main complications.9-12
The clinical suspicion of an aneurysm is difficult due to the variety of symptoms that present, the diagnosis is usually incidental to perform basic imaging studies such as chest X-ray.10-13 Transthoracic echocardiography plays a transcendental role in the initial approach to aortic aneurysms;4-6 Although new technologies such as magnetic resonance nuclear or computed tomography have somewhat displaced its role; We consider it important to retake it because many of the regions of our country do not have these resources. The information provided by the transthoracic echocardiogram is relevant since it is not limited to the confirmation of the diagnostic suspicion, it has an acceptable correlation with the etiological process, it allows to evaluate the evolution, the prognosis and even to determine the moment for the surgical approach.14-16
A transthoracic echocardiographic examination carried out with an adequate technique and based on the knowledge and recognition of the complexity of the aortic geometry in normal and pathological conditions allows to apply known parameters that will orient us in the diagnosis improving our detection but especially recognizing the probability that some complication that costs the life to the patient.16,17
For this reason, we consider it important to present this clinical case, which is not only representative but rather didactic; Allows to make known to the doctors and to the specialists in formation how the calculations and the necessary equations are realized in the evaluation of a structure as complex as the aorta. It is also a reminder for specialists, but especially it is an attempt to reclaim the role of transthoracic echocardiography in an era of great technological advances.
Case report
The patient is a 33-year-old man who presented to echocardiography department sent from your general area hospital, with diagnosis of precordial pain under study. Two months prior to presentation, he began noting episodes of chest pain and dyspnea. He initially attributed these to his stressful lifestyle, and did not seek medical attention. He had no history of hypertension, diabetes mellitus, or hyperlipidemia. The patient was taken to general hospital, where a chest radiograph demonstrated clear lung fields, but with great cardiomegaly. The electrocardiogram showed left ventricular hypertrophy with diastolic overload data. Your doctor requests echocardiographic evaluation for suspected diagnosis of dilated cardiomyopathy. An echocardiogram was subsequently performed to assess his ventricular function; the patient’s weight was 108 kilograms with a height of 1.93 meters.
Echocardiographic measurements
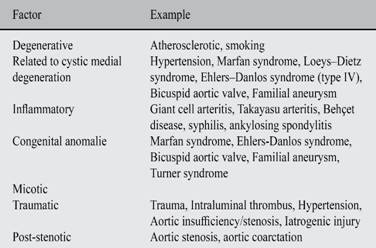
According to the recommendations to American Society of Echocardiography (ASE) and European Association of Echocardiography (EAE);18-20 the echocardiographic exam was being performed with equipment General Electric Model Vivid T8. The patient lying the left lateral decubitus position, you get the windows and basic views as they are: parasternal window (long-axis view, short-axis view, right ventricular inflow and outflow view); apical window (four-chamber view, five-chamber view, two-chamber view, and long-axis view); subcostal window and suprasternal notch. Using the mode technique two-dimensional (B-Mode), M-mode, spectral pulsed wave (PW) and spectral continuous wave (CW). It immediately becomes evident in B-mode the parasternal long axis view (Figure 1) a large dilatation of the aortic root and the ascending aorta. The diameters obtained at the level of the annulus is 3.2 cm; in sinuses of Valsalva 7.3 cm; the sinotubular junction is 8.1 cm; and ascending aorta 8.4 cm. In short-axis parasternal view the diameters of the ascending aorta were 8.7 for 9.5 cm (Figure 2). In a modified long-axis suprasternal view it is observed that the dilation extends to the ascending aorta (Figure 3) spanning the proximal half of the aortic arch (Figure 4).
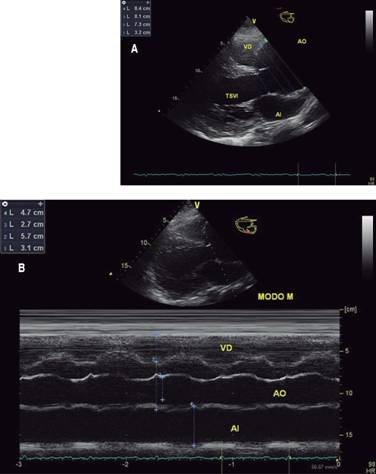
Figure 1: Echocardiographic images obtained in a long axis using two-dimensional and M-mode. On the left, the image A in two-dimensional mode shows the diameter acquired at the level of the aortic ring, the sinuses of Valsalva, the sinotubular junction and the proximal third of the ascending aorta at end-diastole; Photograph B on the right using the M-mode corroborates dilation of the aortic root and the left atrium.
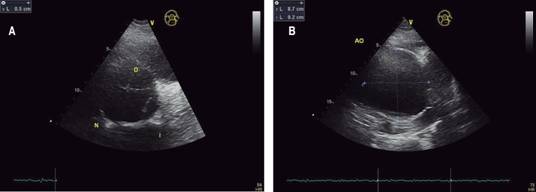
Figure 2: Echocardiographic images obtained in short axis parasternal at the level of the great vessels, focusing on the aortic valve and the sinotubular junction. The image A in two-dimensional mode shows large dilation of the aorta at the level of the sinus of Valsalva and at the same time appearances a trivalva aorta. On the right in the image B, the diameter of the aorta is observed at the level of the sinotubular junction, but in a cross section, which also allows us to see the complexity of the aortic geometry. Note the difference in diameters.

Figure 3: Transthoracic echocardiogram in two-dimensional mode from a suprasternal window in a modified long axis where the dilatation of ascending aorta is reduced in its final third; The initial portion of the aortic arch is not affected with a diameter of 4.1 cm.

Figure 4: Transthoracic echocardiogram in two-dimensional mode from a suprasternal window in a long axis where it is seen that the aortic arch and the proximal portion of the thoracic descending aorta are not dilated.
M-mode examination suggests dilation of the left ventricular cavity and possible systolic dysfunction (Figure 5); which is corroborated in the apical window with a view of four chambers, resulting in mild systolic dysfunction with a left ventricular ejection fraction estimated at 56.0% by the modified Simpson method (Figure 6). Using the seventeen-segment model it was analyzed regional wall motion finding a mild generalized hypokinesia, intensity was mild. According to the simplest definition we are before a thoracic aortic aneurysm (diameter > 4.0 cm), which affects the annulus, the aortic root, the ascending aorta and the proximal half of the aortic arch (Table II). This is specifically an annuloaortic ectasia; it is a true aneurysm since it affects the three laminas of the wall.18-22

Figure 5: Transthoracic echocardiogram in M-mode obtained a long parasternal view at the level of the mitral valve where an increase in the E-septum distance is observed suggesting systolic dysfunction and dilation of the left ventricle.
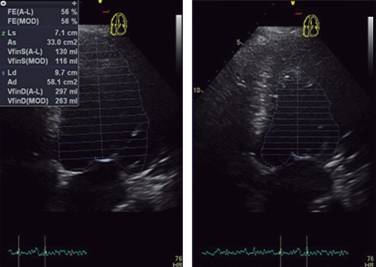
Figure 6: Transthoracic bidimensional echocardiogram in four chamber, showing left ventricular function evaluated by area-length method and modified Simpson method.
Table II: Thoracic aortic diameters.

Adapted from: Roman et al,20 and Davies RR et al,22 and Vasan RS et al.23
However, in order to adequately establish the diagnosis of aortic aneurysm using qualitative methods, we must first know some anthropometric parameters such body surface area or BSA, according to Du Bois and Du Bois in square meter (m2) is equal to 0.007184 × (Height)0.725 × (Weight)0.425; replacing for the presented clinical case we have to BSA = 0.007184 × (193)0.725 × (108)0.425; therefore, BSA is (0.007184 × 45.397 × 7.315) = 2.386 m2. The patient’s body mass index according to the formula Adolphe Quetelet, is 29.0 kg/m2 of BSA. According to the results of the studies carried out by Roman MJ20 and Devereux RB,21 using the equation one we can calculate mean predicted aortic root diameter or PARD, was calculated on 4.987 cm. To know the Z-score of level of the sinuses of Valsalva by the method of Roman et al.,20 which was equivalent to 9.63, in the sinotubular junction is 12.97; and the ascending aorta 14.22. The new Z-score with the technique developed by Devereux et al,21 is obtained in sinuses of Valsalva 14.33, in the sinotubular junction is 17.39; and the ascending aorta 18.54. Optionally you can apply the new Z-score normalized for height has been calculated PARD, where the results are: in sinuses of Valsalva 17.52, in the sinotubular junction is 21.24; and the ascending aorta 22.64.22-24 The diameters obtained from the echocardiographic examination of the patient confirm together with the Z-score and the new Z-score the presence of a true aortic aneurysm (PARD 4.987 cm, that is to say enlargement to more than 1.5 times; the aortic root diameter ratio 3.52 cm/m2 BSA; and area height ratio 13.2).25-28 It is unknown because of the absence of previous studies, the expansion rate of dilation of the aorta.
The analysis to determine the risk of complications or rupture of the aneurysm was calculated using formula of Juvonen T et al;29,30 with risk of rupture at one year of 4.94%. This is consistent when compared to other risk scales as: a) thoracic aneurysms rupture at an annual rate, where risk rupture is great 7.0% (diameter ≥ 6.0 cm); b) aortic root diameter ratio: 1.62 (greater probability of rupture with an index ≥ 1.30); c) area height ratio of 13.5 (high percentage of complication with a result above 10.0); d) Z-score and New Z-score calculated up to 18.0 (a higher value of ≥ 2.0). The evolution is unknown since it does not have previous echocardiographic analyzes that allow to know the rate of growth of the aneurysm.
Based on a detailed observation that includes the morphology, location, extent and aggregate lesions found in the echocardiographic examination, we can highlight the following findings: the dilation extends from the annulus to the aortic arch but concerns the descending aorta; exist dilated sinuses with enlargement or effacement of the sinotubular junction; the aortic insufficiency is the result of a deficient coaptation secondary to the affection of the geometry of the aortic root; no lesion of the valve is observed and shows three valves. There is no thickening or calcification of the valvular apparatus or aortic walls. The alterations previously mentioned as well as the height and age of the patient orient to an etiology by congenital anomaly with cystic medial degeneration; in other words, the patient meets criteria for diagnosis of Marfan syndrome. Which must be corroborated according to the criteria of Ghent nosology (Tables III, IV and V).25,28
Table III: Ghent criteria used to establish the diagnosis of Marfan syndrome.

Adapted from: Roland RJ et al,26 Cabrera BF et al,28 and Elefteriades JA et al.29
CT = Computer tomographic, MRI = Magnetic resonance imaging, FB1 = Fibrillin-1 gene.
Table IV: Revised Ghent criteria for diagnosis of Marfan syndrome.

Adapted from: Roland RJ et al,26 Cabrera BF et al,28 and Elefteriades JA et al.29
Ao = Aortic diameter at the sinuses of Valsalva above indicated Z-score or aortic root dissection. EL = ectopia lentis. ELS = Ectopia lentis syndrome. FBN1 = Fibrillin-1 mutation. MASS, myopia, mitral valve prolapse, borderline (Z < 2) aortic root dilatation, striae, skeletal findings phenotype, MFS = Marfan syndrome, MVPS = Mitral valve prolapse syndrome, Syst = Systemic score and Z = Z-score.
Table V: Scoring of systemic features.
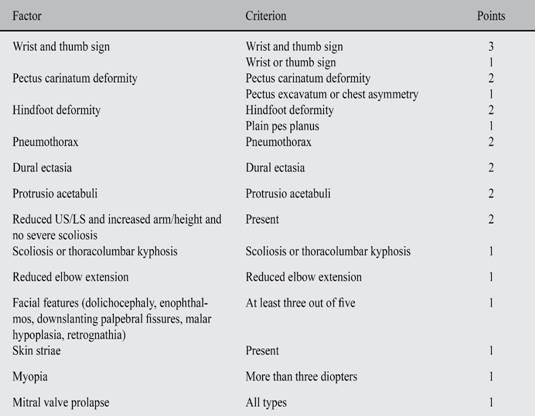
Maximum total: 20 points; greater than or equal to 7 indicates systemic involvement.
US = Upper segment, LS = Lower segment, US/LS = Upper segment/lower segment ratio.
Adapted from: Cabrera BF et al.28
Discussion
The aorta is the largest blood vessel in the human body and absorbs the impact of 2.3-3 billion heart beats a year while delivering roughly 200 million liters of blood to the various parts of the body.1,2 The aorta is classically divided into two major anatomic segments, the thoracic and abdominal aorta. Several distinct anatomic regions form the thoracic aorta including the aortic root, ascending, arch, and the descending aorta.3,4 The aortic root includes the aortic valve annulus, the aortic valve cusps, and the sinuses of Valsalva; the ascending aorta which contains the tubular portion of the ascending aorta beginning at the sinotubular junction and extending to the brachiocephalic artery origin; the aortic arch which initiates at the origin of the brachiocephalic artery, and is the origin of the head and neck arteries, coursing in front of the trachea and to the left of the esophagus and the trachea; and the descending aorta which commences at the isthmus between the origin of the left subclavian artery and the ligamentum arteriosum and courses anterior to the vertebral column, and then through the diaphragm into the abdomen.5
Aortic wall is composed of three layers, listed from the blood flow surface outward: a) Intima (endothelial layer on a basement membrane with minimal ground substance and connective tissue); b) Media (bounded by an internal elastic lamina, a fenestrated sheet of elastic fibers; layers of elastic fibers arranged concentrically with interposed smooth muscle cells; bounded by an external elastic lamina, another fenestrated sheet of elastic fibers); and c) Adventitia (a resilient layer of collagen containing the vasa vasorum and nerves. Some of the vasa vasorum can penetrate into the outer third of the media).6 The thoracic aorta has a greater concentration of elastin and is therefore more compliant than the abdominal aorta. The adventitia is the outermost layer and consists of collagen fibers and fibroblasts that provide support and structure to the aortic wall.7 The vasa vasorum supply oxygen to the outer one-third to one-half of the thoracic aorta while the inner half is supplied from the lumen. The abdominal aorta has an absence of medial vasa vasorum and is thinner than the thoracic aorta, supporting less than 28 layers of vasa vasorum, while the thoracic aorta has approximately 35 layers of elastic lamellae.9
Age-related changes demonstrate a progressive decline in the elastic properties of the aortic wall with fragmentation of elastin followed by an increase in collagenous remodeling, resulting in an increase in collagen to elastic ratio that leads to loss of aortic compliance.9,10 The aortic changes in elastic tissue incurred by old age are reflected in further gross and microscopic abnormalities with tortuous due to an increase in length, and its intimal surface doubles between the second and the sixth decade.9
The ascending aorta is not truly or totally vertical; the aortic arch poses an additional challenge because an axial image through the aortic arch will produce an oblong rather than a circular contour. Also, even in a given cross section, the aorta will not be a true circle (the only truly annular, or circular, anatomic structure found within the aortic root is the sinutubular junction); the human aorta is geometrically intricate.3 The asymmetry of the aortic root is more evident at the level of the sinuses of Valsalva, where the variation of the diameter obtained in the parasternal long axis and in the short axis can be observed.10 Recent research highlights the importance of the interleaflet triangles in determining valvar morphology and function;10,11 as well as of the variation in the morphology of the aortic ring during the cardiac cycle (has its largest size, and most circular shape, in midsystole, becoming smallest and most elliptical at end-diastole).3,11
A true aneurysm represents a dilatation o all three layers of the aorta, creating a large bulge o the vessel walls. True aneurysms are characterized as either fusiform or saccular, depending on the extent of the vessel’s circumference within the aneurysm.13 A fusiform aneurysm, the more common type, is characterized by symmetrical dilation o the entire circumference of a segment of the aorta. A saccular aneurysm is a localized outpouching involving only a portion of the circumference. A pseudo-aneurysm, on the other hand, represents a contained rupture, and the wall does not contain the normal histologic components of the aorta.3,13 That size is not the only important imaging criterion loss of normal «Waist» at sinotubular junction is a sign of intrinsic aortic disease.10
The media layer of the aortic wall is composed of smooth muscle cells within a matrix of elastin, collagen, and other structural proteins including fibrillin, laminin, glycosaminoglycans, proteoglycans, and fibronectin.2 The total mechanisms responsible for the aortic dilatation are not clear, but may at least be partially related to smooth muscle cell apoptosis, a form of programmed cell death.6 The aortic aneurysms are associated with degenerative changes with fragmentation and loss of elastic fibers.3 An increased local production of enzymes capable of degrading these fibrillar extracellular matrix proteins.7 Much of this evidence is derived from studies on matrix metalloproteinases (MMPs); four MMPs, including 72-kD gelatinase (MMP-2), 92-kD gelatinase (MMP-9), matrilysin (MMP-7), and macrophage elastase (MMP-12), are capable of degrading elastic fibers, whereas at least three other MMPs are specific for interstitial collagens.3,6,7 There is also significant reduction of smooth muscle cells with accumulation of basophilic lakes of mucopolysaccharide, hence the term cystic medial necrosis.9 These changes may be associated with inherited mutations in individual components of this extracellular network, notably the Marfan syndrome.13 The combination of inherited, degenerative, mechanical, and hemodynamic factors adversely affects the medial layer of the aortic wall, leading to dilatation and setting the stage for the catastrophes of aortic dissection or rupture.11
The incidence of aortic aneurysms has a minimal variation depending on the geographic area, in The Olmsted county study the frequency estimated to be 5.9 per 100,000 person-years compared with 350 cases for abdominal aortic aneurysms.13 The most recent data available from the Centers for Disease Control and Prevention indicate that aneurysm disease is the 18th most common cause of death in all individuals and the 15th most common in individuals older than age 65 years, accounting for 13,843 and 11,147 deaths in these 2 groups, respectively.14 The ascending aorta is affected in 50.0 to 60.0% of cases, the aortic arch in less 10.0%, and the descending thoracic aorta in 30.0 to 40.0%; the mean age of diagnosis is 59-69 years with a male predominance of 2:1 to 4:1.15 The incidence of aortic disease is certain to increase as our population ages.3,14
The principal conditions associate to with true aneurysm aortic are mentioned in Table one. The etiology of aortic aneurysm formation varies depending on the location of the lesion:
a) Aortic root
The most common type is supracoronary aneurysm, in which the aortic annulus is normal in size, as is the short segment of aorta between the annulus and the coronary ostia.10 Annuloaortic ectasia, this type of aneurysm is typical of Marfan syndrome and other related disorders characterized by cystic medial necrosis of the aortic wall. The third category, the tubular type of ascending aortic aneurysm, has features midway between the other two configurations.1,3,9
b) Ascending aortic
The majority of ascending aortic aneurysms are associated with degenerative changes in the media layer of the aortic wall.16,17 There is also significant reduction of smooth muscle cells with accumulation of basophilic lakes of mucopolysaccharide, hence the term cystic medial necrosis.17
Aneurysms of the ascending aorta are also commonly associated with stenotic congenitally bicuspid aortic valves or hypertension. However, the frequent occurrence of dissection and ascending aortic dilatation in the setting of a bicuspid aortic valve supports the theory that disease of the aortic valve and the aorta in these situations actually reflect a common development abnormality.16,17
c) Aortic arch
Sixty percent of arch aneurysms are located in the proximal arch and involve the distal ascending aorta in the majority of these cases. Non-atherosclerotic medial degeneration is common in this region as the result of repetitive aortic injury and aging, but ascending aneurysms associated with Marfan’s syndrome rarely extend to the arch, unless an acute dissection has occurred.17,18 Ascending aortic dilatation is common in patients with bicuspid aortic valves, but extension into the arch is rare. Atherosclerotic aneurysms are common in the proximal arch as the distal-most extension of an ascending aortic aneurysm, and, not infrequently, fusiform atherosclerotic or degenerative aneurysms will span the entire arch. Distal arch aneurysms are most commonly atherosclerotic, and can either be limited to the arch or act as the proximal extent of an aneurysm that extends into the descending thoracic aorta.11 Tertiary syphilis and other forms of idiopathic aortitis are rare, but when present, these aneurysms routinely involve multiple segments of the aorta.11-13
d) Descending thoracic aortic
The majority of descending thoracic aortic aneurysms are degenerative in etiology and associated with atherosclerosis (most aneurysms are fusiform; less frequently, saccular aneurysms). Other causes of descending thoracic aortic aneurysms include traumatic aneurysms, mycotic or infectious aneurysms and post-operative pseudoaneurysms.11,13
e) Miscellaneous
Many cases that were once considered idiopathic or attributed to atherosclerosis can now be traced to genetic abnormalities affecting the aortic. In many cases, there is a family history of aneurysmal disease, with multiple genetic mutations including the thoracic aortic aneurysm and dissection 1 (TAAD1) mutation, which accounts for 20% to 30% of familial cases; the TGF-β receptor 2 (TGF-βR2) mutation, which accounts for approximately 5% of cases; the familial aortic aneurysm 1 (FAA1) mutation; and the myosin heavy chain 11 (MYH11) mutation.7 Inherited connective tissue disorder associated with aneurysm formation in Ehlers-Danlos syndrome and Loeys-Dietz syndrome, caused by mutations in the transforming growth factor β (TGF-β) receptor; individuals with this mutation develop aneurysms that enlarge rapidly and are prone to rupture.7,11,13 Infrequent causes o aortic aneurysms include weakness of the media from infections of the vessel wall by Salmonella species, staphylococci, streptococci, tuberculosis, syphilis, or fungi. Inflammatory diseases such as Takayasu arteritis or giant cell arteritis may similarly weaken the vessel and result in aneurysm formation.3,11,12
The aneurysms thoracic aorta expansion grows very slowly at approximately 0.10 cm per year; the descending aorta grows a bit faster at approximately 0.30 cm per year. In conjunction with an average of 0.12 cm per year.14 Studies have shown that the risk of rupture is related to the size of the aneurysm, as predicted by the Laplace relationship (wall tension is proportional to the product of pressure and radius). Thoracic aneurysms rupture at an annual rate of 2.0% or aneurysms less than 5.0 cm in diameter, 3.0% or aneurysms 5.0 to 5.9 cm, and 7.0% or aneurysms greater than 6.0 cm.3,14 Although diameter is the main marker of complications, have been identified dissections do occasionally occur at small aortic sizes (diameter < 5.50 cm); the presence of symptoms and diameter ≥ 5.0 cm is very indication of treatment.
The mechanical properties of the dilated aorta can be determined via measurement of 6 independent variables: aortic pressure in systole and diastole, aortic diameter in systole and diastole, and aortic wall thickness in systole and diastole; the aorta enlarges, distensibility of the aortic wall decreases, so that by approximately 6 cm in size, the aorta becomes a rigid tube; this phenomenon is that the enlarged aorta demonstrates a very high wall tension.3,16
• Echocardiographic examination
The echocardiographic examination is achieved with supine patient is instructed to slightly hyperextend the neck and, if necessary, turn the head to the right or to the left, facilitating placement of the ultrasound probe in the suprasternal notch. The long axis of the aorta is displayed using a 2-MHz to 5-MHz multifrequency phased-array transducer with system settings to maximize resolution. High-resolution harmonic imaging is beneficial, as is sacrificing frame rate in favor of improved image detail, because the temporal resolution normally required for dynamic cardiac motion is not an issue with these structures. Usually, the arch vessels are readily apparent on grayscale imaging and can be visualized in their longitudinal orientation either simultaneously with the arch or with minimal manipulation.23,24
Qualitative color flow Doppler imaging is routinely used to differentiate and locate the arch vessels and discern laminar versus disturbed flow patterns. The color Doppler scale should be set to 40 to 60 cm/sec and optimized by adjusting the color box size, image depth, sector size, and gain. More extensive quantitative Doppler sampling is reserved for absent or disturbed flow signals.26 The proximal aorta can be seen in its long and short axis from the parasternal view. The parasternal long axis view with superior angulation emphasizes visualization of the ascending aorta.20,24 Transesophageal echocardiography provides a substantially broader window to aortic anatomy. The aorta can be visualized from the annulus through the ascending aorta, arch, and descending thoracic aorta to the level of the gastroesophageal junction25 (Table VI).
Table VI: Recommendations for examination of the aorta.
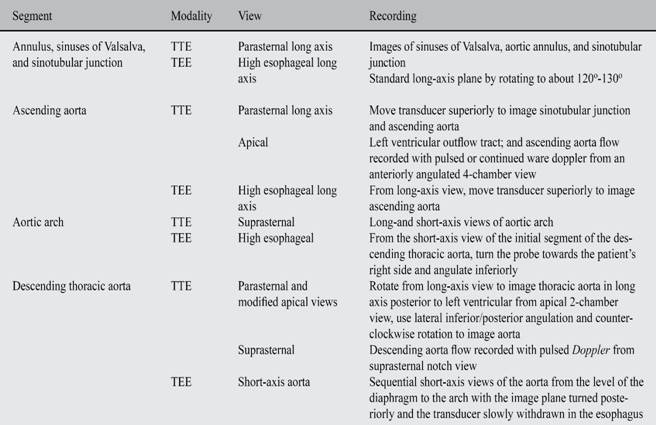
Adapted from: Vasan RS et al,23 Triulzi M et al,24 and Willens HJ et al.25
TEE = Transesophageal echocardiography; TTE = Transthoracic echocardiography.
The normal aorta diameters at the level of the annulus is 23.0-29.0 mm (13.0 ± 1.0 mm/m2 BSA); in sinuses of Valsalva 29.0 to 38.0 mm (19.0 ± 1.0 mm/m2 BSA); the sinotubular junction is 22.0-36.0 mm (15.0 ± 1.0 mm/m2 BSA); ascending aorta 22.0-36.0 mm (15.0 ± 1.0 mm/m2 BSA); aortic arch 26.0-34.0 mm (15.0 ± 1.0 mm/m2 BSA); descending aorta is 24.0-30.0 mm or 13.0 ± 1.0 mm/m2 BSA.18-24 For sex differences see Table one. An aneurysm is defined as enlargement to more than 1.5 times the normal dimension for that aspect of the aortic anatomy or level ≥ 2.3 cm/m2 BSA.26 Dilation of the aorta can either be isolated or associated with other cardiovascular diseases such as hypertension or aortic valve disease. Other useful parameters to define the presence of an aneurysm are: a) aortic root diameter ratio: observed diameter/maximum predicted diameter ≥ 1.30; b) area height ratio: aortic area observed/height ≥ 10.0; c) expansion rate of dilation of the aorta ≥ 2.0 mm/year; and d) Z-score ≥ 2.0.18-26
Aneurysm (true aneurysm): a permanent localized dilatation of an artery, having at least a 50% increase in diameter compared to the expected normal diameter of the artery in question. Although all 3 layers (intima, media, and adventitia) may be present, the intima and media in large aneurysms may be so attenuated that in some sections of the wall they are undetectable.11Pseudoaneurysm (false aneurysm): contains blood resulting from disruption of the arterial wall with extravasation of blood contained by periarterial connective tissue and not by the arterial wall layers. Such an extravascular hematoma that freely communicates with the intravascular space is also known as a pulsating hematoma.9Ectasia: arterial dilatation less than 150% of normal arterial diameter.10Arteriomegaly: diffuse arterial dilatation involving several arterial segments with an increase in diameter greater than 50% by comparison to the expected normal arterial diameter.11 The combination of dilation of the whole ascending aorta and aortic annulus is termed annuloaortic ectasia.13
The diagnose aortic root dilatation, it is essential to have clearly defined normal values of aortic diameters. These normal values were first established by Roman and colleagues in 1989; the proposed equation for adults relates to body surface area (BSA) and consists of two formulas for calculating normal aortic sinus diameters. A dilated aortic root is defined as a Z-score ≥ 2.0, which corresponds with a diameter ≥ 2.0 standard deviation above normal. The equation by Roman et al.,20 discriminates between subjects younger than 40 years and those older than 40 years, which may have important consequences when evaluating aortic root diameters in subjects around their 40th birthday.
Echocardiographic evaluation included measurements of the diameters at the levels of aortic valve annulus, the sinuses of Valsalva, the sinotubular junction, and ascending aorta from leading edge to leading edge in the parasternal long axis view. Measurements were performed in end-diastolic, and the average of three measurements was used for Z-score calculation using equation previously described.20,21
Using aortic size index, patients were stratified into three risk groups: less than 2.75 cm/m2 BSA are at low risk (approximately 4.0% per year); 2.75 to 4.24 cm/m2 BSA are at moderate risk (approximately 8.0% per year); and those above 4.25 cm/m2 BSA are at high risk (approximately 20.0% per year).14,22 The Mount Sinai group recently developed an excellent mathematical model to predict the risk of rupture for patients with descending thoracic aneurysms based on aneurysm size, age, and the presence of chronic obstructive pulmonary disease (COPD) or atypical chest or back pain not attributable to the aneurysm itself. The rate of rupture (rR) was estimated by the formula previously described.30
The aortic aneurysm secondary to hypertensive heart disease show changes as dilation of the ascending aorta with normal sinuses and sinotubular junction.3 In the atherosclerosis focal irregular thickening of the aortic wall with areas of calcification; associated thrombus is seen as a mobile echo-density.27 Enlargement of the aortic sinuses and/or the ascending aorta, usually with a preserved sinotubular junction bicuspid aortic valve disease is typical.9 Marfan and Loeys-Dietz syndrome are associated to dilated sinuses with enlargement or effacement of the sinotubular junction; which are sometimes accompanied with long anterior mitral leaflet or prolapsed.28 Systemic inflammatory disease, dilated thick walled aorta with characteristic thickening extending onto base of the anterior mitral valve leaflet.4 Takayasu or giant cell arteritis, exist dilation of thoracic and abdominal aorta.13 Sinus of Valsalva aneurysm, may be a smooth dilation of one sinus or may have a «windsock» appearance; rupture may occur into the right ventricular outflow tract from the left coronary cusp, into the right atrial from the right coronary cusp or into the left atrial from the noncoronary cusp.2,3 Finality, in the syphilitic aortitis dilated aorta with calcification may involve proximal coronary arteries.2,9
Conclusions
The incidence of aortic disease is truly increasing, can be considered as a «virulent disease»; despite which thoracic aortic aneurysms are an indolent process.3 It is important to intervene before the aorta reaches these hinge-point dimensions. Specifically, an individual with thoracic aortic aneurysm incurs a 34% lifetime risk of rupture or dissection by the time that his or her ascending aorta reaches a diameter of 6.0 cm.22,23
The majority of ascending aortic aneurysms are detected as incidental findings on chest X-rays or by other imaging studies performed for other diseases. The most common symptom is excruciating chest or interscapular pain, with subsequent migration. Echocardiography is most useful for evaluating the size of the aneurysm, degree of root dilatation and aortic regurgitation, presence of intimal flap, and left ventricular function; although it is sometimes believed that magnetic resonance imaging or computed tomographic are better techniques, echocardiography plays a very important role in the process of diagnosis, treatment, evolution and stratification of the risk of thoracic aneurysms. The main problem faced by echocardiography is the proper measurement of aortic diameters given the complexity of aortic root geometry, and its limited vision spanning only the proximal third of the descending thoracic aorta.
The Z-score and de the New Z-score are important tools in the evaluation of aortic aneurysms, but underused. When the Z-score is combined with other evaluation parameters such as: the diameter ratio (≥ 1.40); area height ratio (≥ 10.0); expansion rate (> 2.0 mm/year); and the Mount Sinai group risk of rupture; we significantly improve detection capacity. These parameters are necessary to determine the need for surgical management, but also to define the surgical technique used for the treatment of the aortic aneurysm that varies depending on the underlying pathology and the quality of the aortic wall, the ability of the surgeon, the state of the aortic valve and the age, the expected survival and the general condition of the patient.
The initial approach of an aneurysm is by echocardiography, which despite its technical limitations is a fundamental tool. The transthoracic echocardiogram establishes the diagnosis, the probable etiology, the progression, the risk of rupture, the need for intervention and the response to treatment.
The predicted aortic root diameter or PARD, the diameter ratio, area height ratio, expansion rate and Z-score; improve the detection and limit the error rates so we must apply them routinely in the echocardiographic examination of patients with suspected or diagnosed aortic aneurysm; and not limit ourselves to the simple measurement of the diameter. The role of echocardiography in thoracic aneurysms is far from over or limited.











 nueva página del texto (beta)
nueva página del texto (beta)