Background
Chagas disease is caused by the hemoflagellate parasite Trypanosoma cruzi (T. cruzi), and constitutes a major public health problem in Latin America. It is estimated that 20 million people live in endemic regions and are at risk for the disease and approximately 8 million more are infected. Thirty percent of the infected subjects will develop systemic alterations, mainly cardiac abnormalities.1,2
At present, Chagas disease is a public health concern worldwide, mainly because of the migratory phenomenon of people traveling from endemic areas to non-endemic areas. Infected subjects have been identified in Spain, France, Italy, the United States of America, Australia and Sweden.3 Chagas disease is transmitted by two main pathways: by a triatominae vector or as a result of parasite-infected blood transfusion.4,5
Recent studies have considered other mechanisms of transmission for the parasite, such as organ transplant, transplacentally, laboratory accidents, and oral ingestion.6T. cruzi expresses the enzyme neuraminidase, which acts upon the sialic acid groups of glycosphingolipids located in the membranes of the autonomous system conduction fibers, endocardium endothelial cells and blood vessels.7 The chronic myocarditis produced by this parasite leads to a multifocal and disseminated process of fibrosis and necrosis, which are related to the complex and multifactorial effects of the parasite in the organism. These processes probably interact with each other to generate the disease many years after the initial infection.8
In the rural environment, the main route of transmission is through the vector, which is favored by the living conditions of the inhabitants, such as inadequate housing, close contact with domestic animals (dog, cat, pig and birds), dry stone walls and large grasslands across the country roads.5,9
The main mechanism of infection is when the insect vector feeds from the blood of the people or animals and defecates on the skin. This promotes the entrance of the protozoon located in the feces through the conjunctiva, mucosa or superficial lesions in the skin. Primary or acute stage lasts for approximately 4 to 8 weeks, and only during this period the flagellated parasite can be found in blood. Symptoms may progress unnoticed in most cases; and during the second stage, known as indeterminate, serologic tests reveal anti-T.cruzi antibodies, exposing the infection.10 During the chronic stage, from 10 to 20 years after the infection, the progression of the disease is manifested by the presence of cardiac alterations detected in 30% of the adults by electrocardiogram (EKG) and echocardiogram ECO), often in productive age (38 to 42 years old).11,12
The pathogenic mechanism has not been established, although the alterations observed in this stage are due probably to the damage inflicted by the parasite during the acute phase and to the generation of autoimmune complexes that generate the disease (dilated cardiomyopathy) several years after the acute phase.1,13
The main organs affected are the heart, particularly the myocardium, generating cardiomegaly and changes in the electrical conduction system such as right bundle branch block (RBBB) or left bundle branch block (LBBB) and in the digestive tract dilatation of the esophagus and colon. Visceral alterations are usually present in certain endemic regions, particularly Brazil, Chile and Argentine, and it is postulated that these changes are for the parasite tropism toward the viscera (viscerotropism). The megaesophagus is accompanied by hypersalivation, dysphagia, pain and regurgitation; on the other hand, the megacolon is accompanied by chronic constipation and intestinal obstruction.13
The electrocardiogram (EKG) and echocardiogram (ECG) evaluations in seropositive patients have made possible the identification of the disease stage, and the adoption of the necessary clinical and therapeutic approaches in order to improve the overall quality of life.14
Material and methods
A cross-sectional design was conducted in an endemic area of Querétaro, Mexico for T. cruzi. A total of 1 033 subjects were included. Written informed consent was obtained from all individuals or parental consent in the case of minors. This study was approved by the Committee of Bioethics and Research of the University of Querétaro, Mexico. The subjects were tested to detect anti-T. cruzi and cardiac evaluation with electrocardiogram and echocardiogram with portable equipment.
Serologic determinations. Serum samples were analyzed by conventional serological techniques as follows: a) ELISA, by using antigenic fractions of T. cruzi epimastigote forms (Biomerieux®, Argentina); b) recombinant ELISA, a test that employs 6 types of antigenic fractions from epimastigotes and trypomastigotes (Wiener Lab.®, Argentina); c) indirect hemaglutination, with sensitized erythrocytes with fractions from trypomastigote extracts (Wiener Lab.®, Argentina); d) indirect immunofluorescence, with epimastigote forms of the Y strain from Brazilian origin; and, e) Western-Blot, employing the iron superoxide dismutase as antigen, a detoxifying enzyme secreted by the parasite and present in the Trypanosomatidae family that acts as a defense mechanism for the superoxide radicals generations.15,16
Cardiologic evaluation. Surface electrocardiogram tracing was obtained.14,17
Echocardiography was performed with Toshiba Sonolayer SSH-60® portable equipment with a 2.5 and 3.75 MHz transducer (Japan, Tokyo) with the subject in the left lateral decubitus position. The transthoracic echocardiography study was performed according with conventional echocardiographic views in order to detect any morphological alteration, particularly apical aneurism at the apex of the left ventricle (LV).17
Echocardiography was performed in M and 2D modes to measure the ejection fraction of the LV that was calculated from the LV end-diastolic and end-systolic volumes.18 It was measured the diastolic diameter of the right ventricle, the diastolic diameter of the posterior wall and the systolic diameter of the interventricular septum.
Statistical analysis: Descriptive statistical analysis was performed.
Results
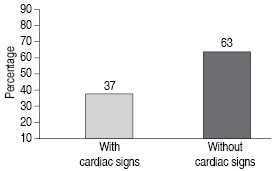
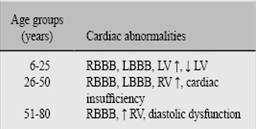
Of 1 033 subjects, 84 between 6 and 88 were positive tested for T. cruzi. The 56% (47) had abormalities relating to conduction system, RBBB or LBBB. All of them had RBBB or LBBB, diastolic dysfunction grades II and III and increased left ventricular volumen. In the case of T. cruzi seronegative subjects, 37% had diastolic disfunction. The 56% of seropositive subjects had cardiac sintomatology (See Figure 1 and 2). Figure 3 shows the frequency of cardiac abnormalities in seropositive and seronegative subjects for T. cruzi, where diastolic dysfunction predominates. In the age group of 26-50 years, most of the cardiac abnormalities ocurred in T. cruzi seropositive subjects (Table I).
According to age, in the group up to 25 years conduction system abnormalities were predominant. In young adult to elderly, heart failure and ventricular diastolic dysfunction grades II and III were found.
Discussion
Dilated cardiomyopathy is the most frequent complication of Chagas disease, and can be prevented with an early diagnosis and monitoring with EKG and ECG evaluations; in addition to an adequate treatment according to the severity of the disease.19,20
In this regard, the conventional serologic techniques to detect anti-T. cruzi antibodies are highly useful tools.
According to the international normativity (WHO/PAHO), the treatment is indicated at pediatric age in the presence of anti-T. cruzi circulating antibodies. In addition close clinical and therapeutic monitoring are recommended in order to avoid the progress of systemic alterations.21
At non-pediatric ages (older than 15 years), the seropositive subjects presented a high variety of alterations, ranging from RBBB and LBBB, cardiac insufficiency and an increase or decrease on the LV.
Cases between 26 to 50 years old, we detected the most characteristic signs of the chronic stage of Chagas disease: RBBB, LBBB, diastolic dysfunction and right cardiac insufficiency. These individuals are in productive age, and represent the group with a higher risk at this stage of the disease.
These findings are in accordance with the described by Pinto-Díaz,11Puigbo12 and Manzullo,22 that reported that the age group between 38 and 42 years has a higher risk of complications. This age group is at risk for sudden death occurs in 2 of each 1 000 persons per year.23 In summary, cardiac alterations are late complications of T. cruzi infection, specific of the chronic phase, and mainly manifested in subjects in reproductive age.11 In the age group of 51 to 88 years, RBBB, diastolic dysfuction, cardiac insufficiency and an increase in the LV were the more relevant findings.
The sociodemographic and epidemiological characteristics of the disease in the subjects of this study, allowed us to identify elements that commonly associated with cardiac alterations reported in other studies in different hospitals or even in other countries like Argentina and Brazil.20,23
In this study, we found a higher proportion of cardiac alterations in seropositive subjects born and raised in a rural poor and endemic region and localities with extreme weather, tropical or subtropical, where almost all of them cohabit with domestic animals like dogs, cats, chicken and pigs; animals identified as T. cruzi reservoirs.23
Migration from rural to urban regions is remarkable, and migrants usually are employed in primary activities. This could be relevant on the development of cardiomyopathy, by the increased demand in physical activity required in these activities. In addition to other environmental factors that can contribute to the development of the cardiomyopathy, like industrial pollution, hazardous waste, etcetera.23
On this regard, it is imperative to avoid health risks mainly in transfusion medicine and to comply with the Mexican Official Norm (NOM-003-SSA2-1993) and the WHO criteria related to donation of blood elements requiring universal serological evaluation of voluntary donors from endemic areas.23
If anti-T. cruzi antibodies are detected the infection will be confirmed and these subjects should be followed up with EKG, echocardiography and chest radiograph every year. Damage to heart muscle due course in children should be identified and prevent complications and to avoid or delay cardiac complications either by mechanic (cardiac pacemakers, artificial heart valves, etc.) or pharmacological treatment.23,24
The social and economic implications of the subject with Chagas disease and heart damage are diverse. One is that it is economically active population with limited resources and without social and political representation. Another aspect is that most are men with family. This situation can lead the family to move to urban areas for medical attention, thus impacting on the budget considered for social welfare.21 The public institution will provide for the assistance and medications, but it will not cover the surgical interventions required by the patients with cardiomiopathy. In addition, it must consider the high cost of medical care and its impact on the health sector over the years of life lost. In this case is a preventable and treatable infection when diagnosed early and avoid the chronic stage of the disease. According to Vallejo et al. (2002) in which there are three posible scenarios of average expenditure in patients with Chagas´s disease, would mean the verage cost of 4 843.44 dollars with a mínimum of 4 463.24 and a máximum of 9 601.10 dollars21 (See Table II).
Table II: Annual approximated costs of medical expenses for patients with Chagas cardiopathy in three different scenarios. (Vallejo M et al, 2002).
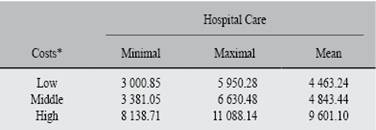
* In US dollars ($).
We found cardiac alterations in seropositive subjects from rural populations in the state of Querétaro. In this regard, a periodic control in patients from T. cruzi endemic regions should be performed to evaluate systemic damage and the level of circulating antibodies. Therefore, we consider this parasitic disease as a health priority for our state, representing an 8% of infection in this geographic region.16
These results suggest a major importance on the directed search for the mechanisms related in the evolution of the disease, considering that some seropositive individuals may display severe manifestations as severe dysrhythmias and even sudden death. In this regard, it is necessary to establish health programs for prevention, diagnosis, and clinical and therapeutical follow up in patients infected with the parasite.23
The present results represent the detection and findings of cardiac abnormalities own Chagas infection in a rural community.
Knowledge of the prevalence in the state demostrate the importance of prevention, early diagnosis to prevent progression of the disease and improve the prognosis of infected subjects.
Conclusion
Our results demonstrated by serology and cardiological findings Chagas infection in an endemic rural population. The elevated prevalence of these cardiac signs in seropositive individuals in working and reproductive age, stresses the importance of promoting programs for prevention, early diagnosis and an adequate treatment in this group of risk. An annual longitudinal evaluation with serologic, clinical and imaging studies is recommended to evaluate the progression and prognosis of the disease in all seropositive patients.











 nueva página del texto (beta)
nueva página del texto (beta)