Introduction
Although introduced over 20 years ago by Campeau,1 the radial artery has only recently received major attention as an alternative to the transfemoral access for coronary angiography and transcatheter angioplasty coronary interventional procedures. The current availability of miniaturized catheters (down to 4 Fr) with improved navigability has positioned radial access as the preferred option of interventional cardiologists.2 As compared to transfemoral access, a transradial approach provides the advantages of smaller puncture holes, decreased bleeding and thrombotic rates, shorter time to homeostasis with early patient ambulation which translate to a shortened hospital stay.2 These advantages are augmented by minimal adverse events such as arterial wall thickening, stenosis.3 Radial artery thrombosis is the major complication of vascular closure, occurring in ~2%3 to 9%4 of cases. However, total radial artery occlusion is mitigated by the physiology of the hand, which receives dual blood supply by the ulnar and radial arteries.2
In the last decade multiple vascular compression devices for transradial access have been developed and evaluated.2 The primary objective of this observational study was to compare the time required to achieve hemostasis in three different radial vascular compression devices. A secondary endpoint was to identify and compare among these devices adverse clinical events such as superficial hematoma, bleeding at the access site, pain, paresthesia, and acute and sub-acute (24 hrs post procedure) presence of radial pulse.
Methods
Patients enrolled in this study consisted of those undergoing cardiac catheterization presenting with ST elevation and non-ST elevation myocardial infarction, unstable and stable angina as well as diagnostic coronary angiograms between June 2010 to November 2010. All patients received interventional procedures via transradial vascular access; surgeries were performed at the Christus Muguerza Hospital and OCA Hospital in Monterrey, Mexico. All patients read, understood and agreed with the procedure by providing a signed informed consent. Patients were divided among three groups, each receiving a different vascular compression device following transradial catheterization procedure. Group I received TR Band(tm) (Terumo, Tokyo, Japan), Group II received Neptuno(tm) (Biotronik, Berlin, Deutschland) and Group III received Finale(tm) (Merit Medical, South Jordan, UT). A Barbeau's and Allen's test was performed at baseline in all patients, documenting an appropriate dual arterial circulation and patency of palmar arch. Criteria for patient exclusion from study included those patients that rejected enrollment, tested abnormally for either the Barbeau's or Allen's tests, and/or tested positively for renal function impairment (Table I).
Transradial catheterization procedure
Radial vascular access was performed following standard procedures. In brief, following sterile preparation and injection of 2% lidocaine at the puncture site, a 20-gauge needle was inserted following Seldinger technique in the right hyperextended wrist. An introductory sheath of 5 or 6 Fr (French gauge) was advanced in the radial artery between 6 to 8 cm above the crease of the wrist. A guidewire was inserted through the sheath. Once the guidewire was in position the initial access system was removed and replaced by a radial artery glide sheath. All catheterization procedures were performed through 5 to 6 Fr catheters. All patients received calcium channel blocker or nitroglycerin to relieve any potential arterial spasm as well as heparin at a dose between 2,000 to 5,000 UI at the discretion of the operator. These medications were supplied intra-arterially. Following the procedure, the sheath was removed and one of the radial compression devices were placed according to the group assigned. Clinical parameters including time to achieve hemostasis, superficial hematoma, bleeding at access site, pain, paresthesia and presence of radial pulse were noted and evaluated immediately after vascular compression device implantation with a 24 hour post-procedure follow up.
Device description
Three devices were utilized in this observational study. Group I received the TR Band(tm) (Terumo Interventional Systems, Tokyo, Japan), a commercially available radial hemostasis device that applies precise pressure to the radial artery (via dual compression balloons) without damaging adjacent structures. The inflatable chambers, through a unidirectional valve, provide selective compression to the radial artery, leaving the ulnar artery patent while hemostasis is achieved. The device is transparent, allowing constant monitoring of the access site. Group II received the Neptune(tm) Hemostasis Pad (TZ Medical, Portland, OR) which is a product derived from seaweed. Calcium alginate provides the calcium ions that aid the hemostasis process. The calcium ions are activated when in contact with blood to the hemostatic pad and accelerate clot formation at the puncture site. Group III receive the Finale(tm) (Merit Medical, South Jordan, UT) radial compression device which consists of a wrist band, secured with Velcro, that provides non-occlusive compression while maintaining adequate blood flow to the hand. The degree of compression is regulated using an onboard dial and, like the TR Band(tm), the device is transparent which allows easy monitoring. All three devices aim to achieve homestasis while avoiding total arterial closure. In all three devices, pressure was gradually released in a staggered fashion by either decreasing the atmospheres (TR Band(tm)), turning the selector dial of the device (Finale(tm)) or simply by gradual removal of the device (Neptune(tm)). In cases where hemostasis was not achieved by visual observation the device was returned to the previous level of pressure and maintained in place for a longer period of time (Figure 1).

Figure 1: Representative image of the devices utilized in this study. In the upper panel (image A, D and G) the device is presented ready to use. The middle panel (image B, E and H) demonstrate how the devices are applied to the patient and the bottom panel (image C, F and I) show representative examples of how hemostasis was achieved post device removal. Group I (image A, B, C) received the TR Band(tm) (Terumo Interventional Systems, Tokyo, Japan) provides selective radial hemostasis by transparent dual inflatable chambers which permit ulnar artery flow while radial artery hemostasis is achieved. Group II (image D, E and F) received the Neptune(tm) Hemostasis Pad (TZ Medical, Portland, OR) seaweed derived radial compression device which aids with the hemostasis process when the calcium alginate is in contact with blood. Group III (image G, H and I) received the Finale(tm) (Merit Medical, South Jordan, UT) transparent radial compression device which consists of a velcro-secured wrist band that applies selective non-occlusive compression. In our study, hemostasis was achieved at approximately 180.7 ± 62.4 mins in all groups with no or minimal immediate clinical adverse events (images C, F and I) or 24 hrs following the procedure.
Results
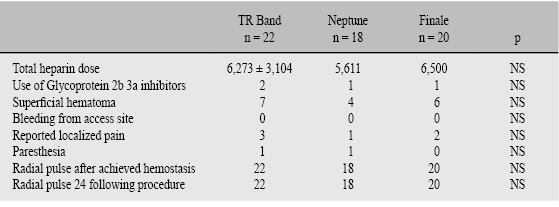
A total of 60 patients (n = 60) were enrolled in this observational study. Patients presented with diagnosis of stable ischemic cardiomyopathy (68%), myocardial infarction with (7%) and without (18%) ST segment elevation, and others (6%; i.e. patients with silent ischemic cardiomyopathy, status post cardiac transplant). Patients were not on an anticoagulant regimen (i.e. warfarin) and did not present any platelet count abnormality in their routine blood panel. Presence of radial pulse as well as Barbeau's and Allen's tests were performed at baseline, demonstrating proper perfusion of the hand. All patients underwent successful cardiac catheterization without any complications. Patients in all three groups received approximately 6,268 ± 2,490 units of heparin (Group I, 6,273 ±3,104 IU, Group II 5,611 ± 2,033 IU, Group III 6,500 ± 2,212 IU; p = not significant [NS]). All enrolled patients were available for the 24 hour follow-up evaluation.
The distribution among treatment groups is described as follows: 22 patients received TR Band(tm) (Group I), 18 received Neptune Pad(tm) (Group II) and 20 received Finale(tm) (Group III). All patients were subject to comparable duration of total compression time. All groups received approximately 180.7 ± 62.4 mins (Group I, 200 ± 74 mins, Group II 175 ± 67 mins, Group III 172 ± 37.8 mins; p = 0.05) (Figure 2) . All patients demonstrated evidence of radial pulse immediately after hemostasis was achieved as well as 24 hours following the procedure (Group I, 100% of patients, Group II 100% of patients, Group III 100% of patients, p = NS). However, 28% of the enrolled patients developed a superficial hematoma. Both Group I (31%, n = 7) and Group II (30%, n = 6) had similar incidences while Group III had a slight, non-statistical decreased incidence (22%, n = 4). Pain at the access site was uncommon among patients in both immediate and follow-up evaluations (10% of patients Group I: 13% of patients, Group II: 5% of patients, Group III: 10% of patients, p = NS), however, 3% (1 patient in Group I and 1 patient in Group II) presented paresthesia immediately following the procedure. The noted paresthesia resolved by the 24-hour evaluation (Table II).

Figure 2: Time to achieve hemostasis. There was no statistical difference between the groups when comparing the amount of time to achieved hemostasis following the interventional procedure. All groups showed no signs of bleeding or oozing ~4 minutes after VCD application.
Discussion
Arterial radial access has gained increasing popularity for interventional procedures since its inception 20 years ago. The advantages of access via the radial artery over the transfemoral artery have been demonstrated in multiple studies over the last decade, which has led to its dominance as preferred arterial access in multiple cardiovascular centers.5 Furthermore, the learning curve of performing the radial access technique is not significantly steeper for either high-volume experienced operators6 or interventional cardiologists in training.7 While the reduction in access site complications, increased comfort for the physician and patient during and after the procedure, and diminished hospital cost makes radial access the ideal arterial access site, some adverse vascular complications (i.e. arterial spasm, forearm hematoma, access failure, pseudoaneurysm formation, radial artery avulsion) and permanent total arterial occlusion remain as pitfalls for this procedure. While there is no significant threat to the perfusion of the hand due to its dual arterial supply (radial and ulnar arteries), the total occlusion of the artery will limit the possibility of future reintervention. It has been speculated that some demographics, such as female and elderly patients,(8),(9) may show more predisposition to radial access occlusion. However, available data from observational studies have demonstrated that a prolonged compression of the radial artery, completely impeding the arterial flow, was the only independent factor for radial artery occlusion following radial access.10 Following this rationale, partial and radial artery selective compression devices (while leaving the ulnar artery flow patent) were developed that would assist in ensuring hemostasis without creating an aggressive, prolonged arterial occlusion. In our study, all three evaluated radial compression devices successfully achieved hemostasis regardless of the slight alterations of mechanism, yet similarity in aim of non-occlusive compression. Group I (TR band) had a slight increase in compression time recorded but all groups required an approximate three hours to display no evidence of bleeding. Our data is concordant with literature; none of the patients in the study presented major vascular complications. Some patients reported paresthesia and localized pain which resolved by 24 hours. Superficial hematoma with likelihood of resolution and no consequence to the patient was the only relatively common finding and had no clinical relevance. We consider that further investigation of radial compression devices as compared to manual compression are necessary to evaluate their advantages and may further simplify the procedure.
Study limitations
This observational study was performed to evaluate three different radial vascular compression devices in the clinical setting. However, due to its observational nature the study was not designed to demonstrate statistical differences between the groups. For this reason, while the results obtained demonstrate that all the devices evaluated seem to be efficient in achieving hemostasis, a more controlled study (i.e. with larger sample size per group) would be required to further support any statistical differences. Since the sample size and study design was solely performed as an observational study the statistical evaluation was performed only with the intentions to see if statistical trends were present. Another limitation is that manual compression alone was not included as a control group in this observational study. This is a particularly important limitation as Group I and III only provide vascular compression while Group II (Neptune Pad) have an active component (calcium alginate), which would require further investigation to demonstrate its efficacy and advantages versus manual compression alone.











 nueva página del texto (beta)
nueva página del texto (beta)