Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de cardiología
versão impressa ISSN 0188-2198
Rev. Mex. Cardiol vol.27 no.1 México Jan./Mar. 2016
Original Research
Effect of metformin on obesity associated metabolic phenotypes
Efecto de la metformina sobre la obesidad asociada a fenotipos metabólicos
Ulises Navarrete-Tapia,* Carlos Narváez,** Francisco Izaguirre-Gutiérrez,*** Andrés Domínguez-Borgua,*** Gabriela Gutiérrez-Salmeán,**** Guillermo Ceballos,* Eduardo Meaney*
* Laboratorio de Investigación Integral Cardiometabólica, Sección de Estudios de Postgrado e Investigación de la Escuela Superior de Medicina del Instituto Politécnico Nacional.
** Especialidad de Cardiología. Hospital General Tacuba, ISSSTE.
*** Servicio de Medicina Interna. Hospital Regional Tlalnepantla, ISSEMyM.
**** Facultad de Ciencias de la Salud. Universidad Anáhuac México Norte.
Correspondence to:
Eduardo Meaney, MD, PhD
Laboratorio de Investigación Integral Cardiometabólica,
Sección de Estudios de Postgrado e Investigación,
Escuela Superior de Medicina del Instituto Politécnico Nacional.
Plan de San Luis y Díaz Mirón s/n,
Col. Casco de Santo Tomás,
Del. Miguel Hidalgo, 11340, México, D.F.
Phone: (52) (55) 57296300, ext. 62820
E-mail: lalitomini@prodigy.net.mx
Recibido: 08/09/2015
Aceptado: 11/01/2016
ABSTRACT
Background: It is known that there are different metabolic phenotypes associated with obesity, each of them imposing distinct cardiovascular risk. Metformin is a drug with wide and diverse applications in cardiometabolic disorders. Aims: To determine the effect of metformin on lipid profile, glucose metabolism and anthropometric variables of different phenotypes of obesity. Material and methods: We conducted a "before and after" clinical trial in order to evaluate the response to metformin treatment (850 mg/day for 24 weeks). Variables like body weight, body mass index (BMI), waist-hip index (WHI), blood pressure, glycemia, total cholesterol (TC) and its fractions, HDL, LDL as well as triglycerides and TC/HDL-c and TG/HDL-c lipoprotein indexes were analyzed at baseline and at the end of the trial. Results: HDL-c and TC/HDL-c ratios showed a considerable improvement after treatment. A reduction in LDL fraction was observed. Triglyceride levels had no significant changes. An average weight reduction of 0.48 Kg was obtained alongside with an improvement of the BMI. Both systolic and diastolic blood pressures did not show meaningful changes. Conclusions: Sustained administration of metformin had a beneficial effect on lipid profile, weight reduction and cardiovascular risk predictors.
Key words: Metformin, metabolic phenotypes, obesity.
RESUMEN
Antecedentes: Se sabe que los diferentes fenotipos metabólicos asociados a la obesidad imponen un riesgo cardiovascular distinto. La metformina es un fármaco con amplias y diversas aplicaciones en trastornos cardiometabólicos. Objetivos: Determinar el efecto del tratamiento con metformina sobre el perfil lipídico, las alteraciones del metabolismo de carbohidratos, y sobre las variables antropométricas observadas en los fenotipos metabólicos de la obesidad. Material y métodos: Se realizó un ensayo clínico "antes y después" para evaluar la respuesta al tratamiento con metformina (850 mg/día durante 24 semanas). Variables como peso, índice de masa corporal (IMC), índice cintura-cadera (ICC), presión arterial, glucemia, colesterol total y sus fracciones HDL, LDL, triglicéridos e índices lipoproteicos CT/c-HDL y TG/c-HDL fueron analizados al inicio y al final del estudio. Resultados: El c-HDL y el índice CT/c-HDL tuvieron una mejoría significativa posterior al tratamiento. Se observó un descenso en c-LDL. Se obtuvo un descenso de peso promedio de 0.48 kg y paralelo a ello una mejora del IMC. La presión arterial tanto sistólica como diastólica no mostró cambios significativos. Conclusiones: La administración de metformina tuvo un efecto benéfico sobre el perfil lipídico, la reducción del peso corporal y algunos predictores de riesgo cardiovascular.
Palabras clave: Metformina, fenotipos metabólicos, obesidad.
BACKGROUND
Obesity has become a worldwide health problem. According to the World Health Organization, only in 2014, more than 1900 million adults were overweight and more than 600 million were obese.1 It is also known that pathologies like metabolic syndrome (MS), Type 2 diabetes mellitus (T2DM) and cardiovascular diseases (CVD) are directly related to obesity.2 While inflammatory and metabolic alterations strongly associated to central adiposity have been thoroughly described,3 it is important to observe that not all individuals with metabolic dysfunction are obese, nor do all obese patients express metabolic anomalies.4 Thus, the individuals metabolically obese but with normal weight (MONW), initially identified by Ruderman5 are characterized by hyperinsulinemia, hyperglycemia, resistance to insulin, dyslipidemia (hypercholesterolemia and hypertriglyceridemia) but with normal adipose mass and body mass index (BMI). Meanwhile, individuals who are metabolically healthy obese (MHO) have in fact visceral obesity but not the characteristic comorbidities of the so-called metabolic syndrome.6 Although initially it was believed that this phenotype was not associated to an augmented cardiovascular risk,7 recent studies have suggested an incremented cardiovascular and global risk in these subjects.8,9 In general, the prevalence of MHO in the general obese adult population is about 25-30%.10 However, several reports suggest differences in this percentage.11-13 The first report regarding the prevalence of different metabolic phenotypes associated with obesity in Mexican population had been shown in the Opus Prime Study.14 This trial found the MHO phenotype in just 5.4% of its cohort and MONW individuals in 5.8%.14 An important highlight of this study was the identification of intermediate phenotypes, composed by subjects with only one or two of the determinant traits of the MS, who represented the more ample segment of the cohort.
Metformin and lifestyle modifications are the first line treatment of T2DM.15 The effect of metformin is exerted through mechanisms dependent on AMP-activated protein kinase (AMPK) and from a transitory and mild inhibition of complex I of the mitochondrial oxidative chain.16 These effects are mostly expressed on carbohydrate metabolism by decreasing hepatic production of glucose and improving insulin sensitivity, enhancing the recruitment and activity of type 4 glucose transporter (GLUT-4), modulating the incretin system (with increased concentrations of the glucagon like peptide, GLP-1),17 and exerting an antioroxigenic effect, as well as a reduction of intestinal absorption of carbohydrates,18 among others. On lipid metabolism, it increases the esterification of free fatty acids and inhibits lipolysis on adipose tissue, providing protection for the β cell against glucotoxicity and lipotoxicity.19 Also, a cardioprotective effect secondary to body weight loss and improvement of lipid profile has been reported with the prolonged administration of metformin.20 Other clinical studies21,22 have also shown an improvement of anthropometric and metabolic variables with the use of metformin in non-diabetic population. However, some reports have pointed out the failure of metformin in attaining weight control in overweight and obesity.
In this work we analyze the effects of metformin treatment (850 mg/day for 24 weeks) in obesity-associated phenotypes, to explore the possibility of using it as a preventive tool in subjects with an altered metabolism.
MATERIAL AND METHODS
A non-controlled (i.e., "before and after") clinical trial was performed with the objective of determining the effect of metformin on doses of 850 mg/day for 24 weeks on metabolic and anthropometric abnormalities seen in metabolic phenotypes associated to obesity. The study was approved by both; the institutional Research and Ethic Committees, and was conducted according with the Helsinki Declaration, and the Mexican Federal Regulations.
Individuals of any gender, ≥ 20 years old, thin, overweighed or obese (according to their BMI) who had one or more of the MS traits (defined with the last harmonized criteria23) were recruited on convenience. In addition, only those subjects with glucose metabolism abnormalities (impaired fasting glucose or impaired glucose intolerance) were selected, excluding subjects with diagnosed T2DM or under pharmacological treatment. Non-inclusion criteria include hypertriglyceridemia > 500 mg/dL, total plasma cholesterol > 400 mg/dL, serum creatinine > 1.4 mg/dL (women) and > 1.5 mg/dL (men), serum transaminases > 3 times above the normal superior limit, total bilirubin > 2.0 mg/dL or malign diseases. All individuals with clinical contraindications for the use of metformin were excluded from the study.
Fasting glycemia, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and serum triglycerides (TG) obtained during the initial visit were considered as basal determinations. The TC/HDL-c and TG/HDL-c ratios were calculated. Anthropometric features as body weight, height, waist and hip circumferences, BMI and waist-hip relationship (WHI) were obtained at the baseline. Patients were weighed barefoot with a calibrated clinical scale; they were asked to stand in the center of the scale, with separated feet for uniform distribution of weight and the result was recorded to the closest 0.1 kg. Waist circumference was measured with a non-elastic fiber-glass tape placed between the iliac spine and the last rib, at mid expiration, while hip circumference was obtained placing the measuring tape at the trochanter level. Blood pressure was measured by means of a calibrated aneroid sphygmomanometer on sitting position, following the recommendations of the American Heart Association,24 registering the average number obtained from three determinations.
Thin, overweight and obese individuals with one or more of the MS features entered the active phase of the study, receiving 850 mg/day of metformin. Thus, the following categories of patients were considered: 1) Intermediate thin (1-2 metabolic anomalies), 2) Dysmetabolic thin (> 2 metabolic anomalies), 3) Intermediate overweight (1-2 metabolic anomalies), 4) Dysmetabolic overweight (> 2 metabolic anomalies), 5) Intermediate obese (1-2 metabolic anomalies) and 6) Dysmetabolic obese (> 2 metabolic anomalies). At the first visit, diet and lifestyle modifications were recommended. At the end of the study, laboratory and anthropometric measurements were repeated. Each individual served as his or her own control.
Statistical analysis. Sample size was determined through comparison of two paired means in one single group, taken as a reference the value change of HDL-c in the MEFISTO study.25 To compare differences between the continuous numeric variables at the beginning and end of the study, the Student's t test for related samples was used. In order to reveal the differences in basal characteristics of the different sample groups the ANOVA test was used, followed by Tukey's post hoc test. The variable contrast in the different metabolic phenotypes was carried out through Wilcoxon's test. Statistical significance was defined as a value of p < 0.05.
RESULTS
Fifty six patients were included in the study, corresponding to the following categories: 2 patients of thin dysmetabolic phenotype; 6 individuals intermediate overweight; 19 dysmetabolic overweight; 12 intermediate obese and 17 dysmetabolic obese. The age of the sample was 44.13 ± 11.39 years with a range from 20 to 75 years old. According to the gender condition, a predominantly female distribution (~60%) was found.
The mean values and standard deviations of variables (Table I) were: glycemia 88.46 ±12.46 mg/dL, HDL-c 39.73 ± 9.08 mg/dL, LDL-c 147.64 ± 37.26 mg/dL, TG 181.80 ± 74.75 mg/dL; systolic and diastolic blood pressures 117.8 ± 14.15 and 75.81 ± 8.51 mmHg, respectively. An average body weight of 80.99 ± 15.17 Kg was found, minimum 57.30 and maximum 126 kg, the average waist perimeter was of 102.30 ± 9.57 cm.

The most frequent anthropometric abnormality at baseline was the increased waist perimeter in 96% of the patients. Regarding the biochemical defects, the reduction of HDL-c was the more frequent, followed by hypertriglyceridemia and hypercholesterolemia. Thirteen individuals (23%) exhibited impaired fasting glucose, the less observed initial biochemical abnormality.
In an analysis of the forty-eight patients that completed the study, an increase of 1.89 mg/dL of serum glucose was found, with a mean initial value of glucose of 88.46 ± 12.46 mg/dL and a final one of 90.35 ± 13.12 mg/dL, p = 0.34. In the lipid profile, it was observed an increase of the HDL-c of 3.77 mg/dL at the end of the study, (from an initial value of 39.73 ± 9.08 mg/dL to 43.50 ± 6.85 mg/dL, p = 0.005).
The TC/HDL-c ratio showed an average decrease of 0.58 with an initial average of 5.02 ± 1.36 and at the end of the study of 4.44 ± 0.97 (IC: 0.20 to 0.96), p = 0.003 (Figure 1). An average decrease of 8.50 mg/dL of the LDL-c fraction was observed. The average concentration of triglycerides had an increase of 7.10 mg/dL, going from 181.80 ± 74.75 mg/dL to 188.91 ± 98.16 mg/dL (IC: -32.02 to 17.73), p = 0.56. The TG/HDL-c index went from the initial value of 4.90 ± 2.62 to 4.41 ± 2.97 at the end of the study (IC: -0.45 to 1.43) p = 0.302 (Figure 2).

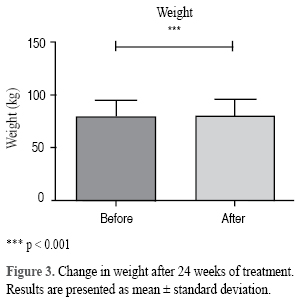
A mean body weight loss of 0.46 kg (from 80.99 ± 15.17 kg to 80.53 ± 14.97 kg, p < 0.001) was observed, with a maximum reduction range of -2.75 kg (Figure 3). Parallel to the loss of body weight, a slight improvement of BMI was also observed, from 30.82 ± 4.62 kg/m2 to 30.62 ± 4.60 kg/m2 at the end of the study p = 0.001, (IC: 0.77 to 0.30). The waist perimeter was reduced, from 102.47 to 102.29 cm (IC: -0.10 to 0.42), p = 0.229.
Both systolic and diastolic pressures increased non-significantly, 4.13 and 2.54 mmHg, respectively. The general results of the study are summarized in table I.
There were dissimilarities of several variables among subjects pertaining to different phenotypes. For example, waist circumference between subjects with normal weight in comparison with the other groups (Table II).
Upon follow up, the next findings were made: the patients of dysmetabolic thin phenotype were lost in the follow-up. In the intermediate overweight group a reduction of 0.61 kg in body weight was observed after 24 weeks of treatment, from 72.58 ± 2.22 kg to 71.97 ± 2.24 kg, p = 0.035. Alongside with the weight reduction, a BMI significant improvement was also observed with an initial value of 27.33 ± 1.59 and final of 27.06 ± 1.61 (IC: 0.172 to 0.368) p = 0.0009.
In overweighed individuals belonging to the dysmetabolic phenotype, no significant changes in biochemical patterns, lipoprotein quotients or anthropometric measurements were observed. On the contrary, obese subjects within the dysmetabolic phenotype experienced the most remarkable changes in concentrations of HDL-c (from 36.65 ± 1.50 mg/dL at the beginning of the study to 40.79 ± 1.17 mg/dL at the end of it) (IC: 20.50 to 84.50, p = 0.47). Also, the TC/HDL ratio diminished from 5.25 ± 0.33 at base data to 4.31 ± 0.25 at the end of the trial (CI: 96.0 to -9.0, p = 0.004) (Figure 4).

DISCUSSION
The aim of this study was to explore the impact of metformin on anthropometric and metabolic abnormalities in different phenotypes associated with obesity. Metformin was selected due to its known beneficial effect on carbohydrate and insulin metabolism26,27 and its documented pleitropic effects capable to reduce cardiovascular risk in both diabetic and non-diabetic subjects.28,29
Within the defining anthropometric and metabolic characteristic of the MS found at the beginning of the study, the increase in the abdominal waist perimeter was the most prominent, 96% patients of the sample were overweighed or obese. The second and third most frequent anomalies found were low concentrations of HDL-c and hypertriglyceridemia, which are in line to several recent surveys are the characteristic lipid abnormalities in contemporary Mexican urban population.30,31 According with those studies half of Mexican subjects with hypertriglyceridemia also have mixed dyslipidemia or low concentrations of HDL-c.31 These findings are consistent with our own study in which 60% of patients at base had hypertriglyceridemia and 93% hypoalphalipoproteinemia (low HDL-c).
After the administration of 850 mg/day of metformin for 24 weeks, an increase of HDL-c was observed (3.77 mg/dL). This increase is similar to the one observed by Quintero-Castillo et al,32 who found in obese patients with polycystic ovaries syndrome, significant changes in TC, LDL-c, HDL-c and triglycerides after the administration of metformin for a period identical of the one used in our study, but with doses up to 2,000 mg/day. The increase in HDL-c reported was of 7.24 mg/dL, an increase close to double of the figures in our study. Giugliano33 found an increase of 28.32% (p < 0.01) of HDL-c after the daily administration of 1,700 mg of metformin in a sample of non-diabetics, hypertensive, obese, female patients, a much larger increment than the one observed in our study (9.5%) that comprised subjects with similar characteristics. Interestingly, other studies in non-obese population with polycystic ovaries syndrome failed tofind significant differences in the HDL-c concentrations after 16 weeks with doses up to 2,500 mg/day of metformin.34 Lastly, the MEFISTO25 study, whose objective was to evaluate nitroxidative stress, vascular stiffness and inflammation (PCR) as well as the carotid intima-media's thickness after the treatment with metformin, did not show any significant change in the values of HDL-c, LDL-c, glucose, weight and BMI in the group treated with metformin. However, a significant reduction of the TC concentrations was observed. Differences can be attributed to the distinct doses used or perhaps to methodological differences. Thus, the improvement of HDL-c after administration of metformin in non-diabetic patients has given inconsistent results. Still, the increase on HDL-c in our study is far from what was reported in other studies, however, unlike our study, most of the clinical essays that have tried to prove the effect of metformin on MS parameters have used medium-high doses of metformin (> 1,500 day). Anyhow, the Framingham35 and other studies, have shown that an increase of 1 mg/dL of HDL leads to a 2-3% of reduction of cardiovascular risk. In our study, an increase of 14.6 mg/dL of the serum TG concentrations was observed. This is in contradiction with Quintero-Castillo report, who found a significant reduction of TG and LDL-c concentrations in obese patients with polycystic ovaries syndrome.32 The obtained results could be related to several factors: 1) a large proportion of patients included in the present study, had a history of mixed dyslipidemia and the suspension of hypolipidemic drugs was requested upon entering the study, 2) bad alimentary habits, 3) lack of adherence to the study drug and 4) biological variability of serum TG.36,37 However, the rise of TG did not reach statistical difference.
On the other hand, the importance of evaluating the TC/HDL-c index lies in that it is an excellent coronary heart disease predictor, better than the isolated fractions LDL-c and TC.38 Thus Gaziano et al39 together with the Physicians Health Study40 evidenced that an increase of just one unit of the TC/HDL-c ratio is associated to an increase of 49% of coronary acute syndromes. In our study a reduction of less than half of a unit of the TC/HDL-c ratio was observed. We believe that this small reduction has a beneficial impact, reducing coronary risk. Changes in the TC/HDL-c ratio were obtained with a low metformin dose. On the other hand, the TG/HDL-c index, aside from being considered as good cardiovascular risk marker,41,42 it is also an acceptable sign of insulin resistance. This index is in inverse relationship with β-cell function suggests that can be used as a relevant predictive marker of T2DM.43 In our study the ratio decreased almost a half of a unit, although the descent was not significant.
The small doses of metformin used in this study had proved useful in other trials25 done by our group, reducing nitroxidative processes, left ventricular hypertrophy and carotid intima thickness, improving diastolic dysfunction and antioxidant effect of HDL. Evidently, in order to better improve BMI, waist circumference and lipid profile greater doses of metformin have to be used. However, with the results reported here, it is difficult to determine the prognostic impact of cardiovascular and cardiometabolic risks and longer and larger studies are necessary to assess this possibility.
CONCLUSIONS
The sustained administration of metformin at a dose of 850 mg/day had a beneficial effect in the lipid profile, and cardiovascular risk predictors. Within the metabolic phenotype categories, the greatest impact was observed in the dysmetabolic obese, where an increase of plasma concentrations of HDL-c and an improvement of the TC/HDL-c ratio were observed.
REFERENCIAS
1. Mendis S, Davis S, Norrving B. Organizational update: the world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke. 2015; 46 (5): e121-e122. [ Links ]
2. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006; 444 (7121): 875-880. [ Links ]
3. Bays H. Central obesity as a clinical marker of adiposopathy; increased visceral adiposity as a surrogate marker for global fat dysfunction. Curr Opin Endocrinol Diabetes Obes. 2014; 21 (5): 345-351. [ Links ]
4. Hashimoto Y, Tanaka M, Okada H, Senmaru T, Hamaguchi M, Asano M et al. Metabolically healthy obesity and risk of incident CKD. Clin J Am Soc Nephrol. 2015; 10 (4): 578-583. [ Links ]
5. Ruderman NB, Schneider SH, Berchtold P. The "metabolically-obese", normal-weight individual. Am J Clin Nutr. 1981; 34 (8): 1617-1621. [ Links ]
6. Samaropoulos XF, Hairston KG, Anderson A, Haffner SM, Lorenzo C, Montez M et al. A metabolically healthy obese phenotype in hispanic participants in the IRAS family study. Obesity (Silver Spring). 2013; 21 (11): 2303-2309. [ Links ]
7. Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud'homme D et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005; 90 (7): 4145-4150. [ Links ]
8. Manu P, Ionescu-Tirgoviste C, Tsang J, Napolitano BA, Lesser ML, Correll CU. Dysmetabolic Signals in "Metabolically Healthy". Obes Res Clin Pract. 2012; 6 (1): e9-e20. [ Links ]
9. Cherqaoui R, Kassim TA, Kwagyan J, Freeman C, Nunlee-Bland G, Ketete M et al. The metabolically healthy but obese phenotype in African Americans. J Clin Hypertens (Greenwich). 2012; 14 (2): 92-96. [ Links ]
10. Denis GV, Obin MS. Metabolically healthy obesity': origins and implications. Mol Aspects Med. 2013; 34 (1): 59-70. [ Links ]
11. Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med. 2008; 168 (15): 1617-1624. [ Links ]
12. Lee K. Metabolically obese but normal weight (MONW) and metabolically healthy but obese (MHO) phenotypes in Koreans: characteristics and health behaviors. Asia Pac J Clin Nutr. 2009; 18 (2): 280-284. [ Links ]
13. Gomez-Huelgas R, Narankiewicz D, Villalobos A, Warnberg J, Mancera-Romero J, Cuesta AL et al. Prevalence of metabolically discordant phenotypes in a mediterranean population-The IMAP study. Endocr Pract. 2013; 19 (5): 758-768. [ Links ]
14. Fanghanel-Salmon G, Gutierrez-Salmean G, Samaniego V, Meaney A, Sanchez-Reyes L, Navarrete U et al. Obesity phenotypes in urban middle-class cohorts; the prit-lindavista merging evidence in Mexico: the opus prime study. Nutr Hosp. 2015; 32 (01): 182-188. [ Links ]
15. American Diabetes A. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes. 2015; 33 (2): 97-111. [ Links ]
16. Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013; 56 (9): 1898-1906. [ Links ]
17. Kappe C, Patrone C, Holst JJ, Zhang Q, Sjoholm A. Metformin protects against lipoapoptosis and enhances GLP-1 secretion from GLP-1-producing cells. J Gastroenterol. 2013; 48 (3): 322-332. [ Links ]
18. Ikeda T, Iwata K, Murakami H. Inhibitory effect of metformin on intestinal glucose absorption in the perfused rat intestine. Biochem Pharmacol. 2000; 59 (7): 887-890. [ Links ]
19. Lupi R, Del Guerra S, Fierabracci V, Marselli L, Novelli M, Patane G et al. Lipotoxicity in human pancreatic islets and the protective effect of metformin. Diabetes. 2002; 51 (Suppl 1): S134-S137. [ Links ]
20. Cicero AF, Tartagni E, Ertek S. Metformin and its clinical use: new insights for an old drug in clinical practice. Arch Med Sci. 2012; 8 (5): 907-917. [ Links ]
21. Yanovski JA, Krakoff J, Salaita CG, McDuffie JR, Kozlosky M, Sebring NG et al. Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial. Diabetes. 2011; 60 (2): 477-485. [ Links ]
22. Diehl LA, Fabris BA, Barbosa DS, De Faria EC, Wiechmann SL, Carrilho AJ. Metformin increases HDL3-cholesterol and decreases subcutaneous truncal fat in nondiabetic patients with HIV-associated lipodystrophy. AIDS Patient Care STDS. 2008; 22 (10): 779-786. [ Links ]
23. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009; 120 (16): 1640-1645. [ Links ]
24. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005; 111 (5): 697-716. [ Links ]
25. Meaney E, Vela A, Samaniego V, Meaney A, Asbun J, Zempoalteca JC et al. Metformin, arterial function, intima-media thickness and nitroxidation in metabolic syndrome: the mefisto study. Clin Exp Pharmacol Physiol. 2008; 35 (8): 895-903. [ Links ]
26. Zhang Y, Hu C, Hong J, Zeng J, Lai S, Lv A, et al. Lipid profiling reveals different therapeutic effects of metformin and glipizide in patients with type 2 diabetes and coronary artery disease. Diabetes Care. 2014; 37 (10): 2804-2812. [ Links ]
27. Hollenbeck CB, Johnston P, Varasteh BB, Chen YD, Reaven GM. Effects of metformin on glucose, insulin and lipid metabolism in patients with mild hypertriglyceridaemia and non-insulin dependent diabetes by glucose tolerance test criteria. Diabetes Metab. 1991; 17 (5): 483-489. [ Links ]
28. Rodriguez Y, Giri M, Feyen E, Christophe AB. Effect of metformin vs. placebo treatment on serum fatty acids in non-diabetic obese insulin resistant individuals. Prostaglandins Leukot Essent Fatty Acids. 2004; 71 (6): 391-397. [ Links ]
29. Fendri S, Debussche X, Puy H, Vincent O, Marcelli JM, Dubreuil A et al. Metformin effects on peripheral sensitivity to insulin in non diabetic obese subjects. Diabetes Metab. 1993; 19 (2): 245-249. [ Links ]
30. Pedroza-Tobias A, Trejo-Valdivia B, Sanchez-Romero LM, Barquera S. Classification of metabolic syndrome according to lipid alterations: analysis from the Mexican National Health and Nutrition Survey 2006. BMC Public Health. 2014; 14: 1056. [ Links ]
31. Aguilar-Salinas CA, Olaiz G, Valles V, Torres JM, Gomez PFJ, Rull JA et al. High prevalence of low HDL cholesterol concentrations and mixed hyperlipidemia in a Mexican nationwide survey. J Lipid Res. 2001; 42 (8): 1298-1307. [ Links ]
32. Quintero-Castillo D, Luz-Araujo H, Guerra-Velazquez M, Reyna-Villasmil E, Santos Bolivar J, Torres-Cepeda D et al. Lipid profile in obese and non-obese women with polycystic ovary syndrome treated with metformin. Endocrinol Nutr. 2010; 57 (6): 262-267. [ Links ]
33. Giugliano D, De Rosa N, Di Maro G, Marfella R, Acampora R, Buoninconti R et al. Metformin improves glucose, lipid metabolism, and reduces blood pressure in hypertensive, obese women. Diabetes Care. 1993; 16 (10): 1387-1390. [ Links ]
34. Aghahosseini M, Aleyaseen A, Safdarian L, Moddaress-Hashemi S, Mofid B, Kashani L. Metformin 2,500 mg/day in the treatment of obese women with polycystic ovary syndrome and its effect on weight, hormones, and lipid profile. Arch Gynecol Obstet. 2010; 282 (6): 691-694. [ Links ]
35. O'Donnell CJ, Elosua R. Cardiovascular risk factors. Insights from Framingham Heart Study. Rev Esp Cardiol. 2008; 61 (3): 299-310. [ Links ]
36. Pereira MA, Weggemans RM, Jacobs DR, Jr., Hannan PJ, Zock PL, Ordovas JM et al. Within-person variation in serum lipids: implications for clinical trials. Int J Epidemiol. 2004; 33 (3): 534-541. [ Links ]
37. Marcovina SM, Gaur VP, Albers JJ. Biological variability of cholesterol, triglyceride, low- and high-density lipoprotein cholesterol, lipoprotein(a), and apolipoproteins A-I and B. Clin Chem. 1994; 40 (4): 574-578. [ Links ]
38. Millan J, Pinto X, Munoz A, Zuniga M, Rubies-Prat J, Pallardo LF et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009; 5: 757-765. [ Links ]
39. Gaziano JM, Hennekens CH, O'Donnell CJ, Breslow JL, Buring JE. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation. 1997; 96 (8): 2520-2525. [ Links ]
40. Stampfer MJ, Sacks FM, Salvini S, Willett WC, Hennekens CH. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med. 1991; 325 (6): 373-381. [ Links ]
41. Quispe R, Manalac RJ, Faridi KF, Blaha MJ, Toth PP, Kulkarni KR et al. Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: The very large database of lipids-4 (VLDL-4) study. Atherosclerosis. 2015; 242 (1): 243-250. [ Links ]
42. Weiler MCS, Wollinger LM, Marin D, Genro JP, Contini V, Morelo Dal Bosco S. Waist-to-height ratio (WHtR) and triglyceride to HDL-C ratio (TG/HDL-c) as predictors of cardiometabolic risk. Nutr Hosp. 2015; 31 (5): 2115-2121. [ Links ]
43. Maturu A, DeWitt P, Kern PA, Rasouli N. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of beta-cell function in African American women. Metabolism. 2015; 64 (5): 561-565. [ Links ]
Nota
Este artículo puede ser consultado en versión completa en: http://www.medigraphic.com/revmexcardiol














