Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de cardiología
versión impresa ISSN 0188-2198
Rev. Mex. Cardiol vol.26 no.4 México oct./dic. 2015
Original Research
Prognostic factors of protein-losing enteropathy in a Mexican cohort of patients with congenital heart disease, surgically corrected with Fontan procedure
Factores pronósticos de enteropatía perdedora de proteínas en una cohorte mexicana de pacientes con cardiopatía congénita, corregidas quirúrgicamente con cirugía de Fontan
Horacio Márquez-González,* Lucelli Yáñez-Gutiérrez,* Diana López-Gallegos,* Christopher O Camargo-Zetina,* Irais C Ortiz-Vázquez,** Moisés Jiménez-Santos,*** Jaime Alfonso Santiago-Hernández,**** Homero Alberto Ramírez-Reyes,**** Carlos Riera-Kinkel*****
* Servicio de Cardiopatías Congénitas.
** Servicio Gabinetes.
*** Servicio de Tomografía.
**** Servicio de Hemodinamia.
***** División de Cirugía Cardiotorácica.
UMAE Hospital de Cardiología Centro Médico Nacional Siglo XXI.
Correspondence to:
Horacio Márquez González MD
UMAE Hospital de Cardiología
Av. Cuauhtémoc Núm. 330,
Col. Doctores, 06720,
Del. Cuauhtémoc, México, D.F.
Tel. 56276900. Local 2203
E-mail: horacioinvestigacion@hotmail.com
ABSTRACT
Background: Protein-losing enteropathy (PLE) is a known postoperative complication affecting about 10% of patients surgically managed with Fontan procedure. The mortality rate associated with this complication increases to 50%. Objective: To determine the risk factors associated to the development of PLE in patients surgically managed with Fontan procedure. Methods: This was a case-cohort study, and the universe of the trial comprised all patients treated with univentricular surgery. We included male and female patients with congenital heart disease that conditioned a single ventricle syndrome. Those patients with previous intestinal disease causing protein loss, were excluded, cow's milk protein allergy, intestinal resection (previous or after heart surgery), use of cyclic parenteral nutrition or Fontan's dismantlement. Follow-up began immediately after hospital discharge from Fontan procedure. Outcome variable was the development of PLE; independent variables were some before and after surgery hemodynamic and echocardiographic variables, infections and treatment. Statistical analysis: We used measures of statistical dispersion and central tendency. Risk was calculated for each variable estimating the hazard ratio (HR), adjusted for confounding factors; and Kaplan-Meier estimator was used for survival analysis. Results: Eleven (26%) out from patients 42 developed PLE. The median of time between Fontan procedure and the development of this complication was five years. The prognostic variables were: systolic pressure of pulmonary artery between 12-15 mmHg, > 3 years between Glenn and Fontan procedures, aggravated chronic malnutrition, direct bilirubin values > 1.5 mg/dL, pulmonary resistances (APR) between 3-3.5 Wood units, previous hepatomegaly and pleural effusion > 6 day-period. The probability of dying from PLE was 63% in a 10-year period. Conclusions: The prognostic factors associated with PLE are previous hepatic damage and borderlines values of venous pressure.
Key words: Fontan procedure, protein-losing enteropathy, congenital heart disease, prognostic value.
RESUMEN
Antecedentes: La enteropatía perdedora de proteínas (EPP) es una conocida complicación que afecta alrededor del 10% de los sujetos operados con el procedimiento de Fontan. La mortalidad asociada a esta complicación se eleva al 50%. Objetivo: Determinar los factores de riesgo asociados al desarrollo de EPP en pacientes postoperados de procedimiento de Fontan. Métodos: Este es un estudio de caso-cohorte y el universo comprendió a todos los pacientes corregidos con cirugía univentricular. Se incluyeron pacientes de ambos géneros, con cardiopatías que condicionaran síndrome de ventrículo único. Se excluyeron aquellos con enfermedad previa intestinal causante de pérdida de proteínas, alergia a las proteínas de la leche de vaca, resección intestinal (previa o después de la cirugía cardiaca), aquellos con nutrición parenteral cíclica o desmantelamiento del Fontan. El inicio de seguimiento comenzó inmediatamente después del egreso de la cirugía de Fontan. La variable de desenlace fue el desarrollo de enteropatía perdedora de proteínas. Las variables independientes estudiadas fueron algunas variables hemodinámicas y ecocardiográficas pre- y postquirúrgicas, infecciones y tratamiento. Análisis estadístico: Se usaron medidas de dispersión y tendencia central. Se calculó el riesgo por cada variable, estimando el cociente de riesgo (Hazard Ratio, HR en inglés), ajustándose a variables de confusión. Se utilizó el estimador de Kaplan-Meier para el análisis de supervivencia. Resultados: Once de 42 pacientes (26%) desarrollaron EPP. La mediana de tiempo entre la cirugía de Fontan y el desarrollo de esta complicación fue de cinco años. Las variables pronósticas fueron presión sistólica de la arteria pulmonar entre 12-15 mmHg, el tiempo > 3 años entre las intervenciones de Glenn y Fontan, la desnutrición crónica agudizada, una cifra de bilirrubina directa > 1.5 mg/dL, URP entre 3 y 3.5 Unidades Wood, hepatomegalia previa y derrame > 6 días. La probabilidad de mortalidad al desarrollar EPP a 10 años fue de 63%. Conclusiones: Los factores pronósticos asociados a EPP son el daño hepático previo y las variables limítrofes de presión venosa.
Palabras clave: Procedimiento de Fontan, enteropatía perdedora de proteínas, defectos cardiacos congénitos, valor pronóstico.
INTRODUCTION
Fontan's operation is a procedure used for congenital heart disease (CGD) with a single functional ventricle syndrome (SVS).The technique was designed to exclude systemic venous return to a univentricular heart, deviating it directly in the pulmonary artery.1
Candidates for Fontan procedure must fulfill certain requirements such as a systolic pressure of the pulmonary artery (SPPA) less than 12 mmHg; arterial pulmonary resistances (APR) less than 3.5 Wood units; absence of atrioventricular valve regurgitation; preserved function of the single ventricle; sinus rhythm; Nakata index > 200 and MacGoon ratio < 1.5.2,3
The protein-losing enteropathy (PLE) is a complication that can affect about 10% of patients who underwent a Fontan procedure. It is the consequence of the retrograde overload of venous flow towards the hepatic and splanchnic circulation, originating intestinal epithelial lesion and inability to retain proteins. When PLE presents, 10-year mortality rises to almost 50%.4
In our center, the Hospital de Cardiología of Centro Médico Nacional Siglo XXI, the incidence of this complication is higher than the one reported worldwide; so we performed a cohort study to determine the possible variables associated with this outcome in a 10-year period.
METHODS
We set-up a cohort of patients that underwent Fontan procedure in the Congenital Heart Disease Service of the Hospital de Cardiologia, Centro Médico Nacional Siglo XXI, from 2005 to 2015; choosing the case-cohort study design. The protocol was approved by the Local Investigation Ethics Committee.
We included patients of both genders with classic tricuspid atresia, pulmonary atresia with intact ventricular septum, severe Ebstein anomaly or any other congenital heart disease that conditioned SVS corrected by univentricular surgery, who had complete medical records before and after heart surgery, and who were closely followed-up in the outpatient clinic of our Service. Were excluded patients with previous diagnosis of PLE, intestinal resection, cirrhosis, acute viral hepatitis, major abdominal surgery or autoimmune diseases such as chronic ulcerative colitis or Crohn's disease. Patients that required abdominal surgery (for small and/or large intestine) or had signs of liver disease during follow-up were also excluded.
PLE was the variable of interest, requiring confirmation from the Gastroenterology or Pediatric Gastroenterology Service, based on clinical and laboratory criteria.
Follow-up consultations after Fontan procedure were conducted every fourth months in the outpatient clinic. If there was a suspicion of PLE, a gastroenterological consultation was requested to confirm diagnosis.
The selected prognostic variables were mean pressure of the pulmonary artery estimated through echocardiography, pulmonary resistance units (PRU), inspiratory collapse of inferior cava vein over 50%, liver function before surgery, type of congenital heart disease, time between the Glenn and Fontan procedures, time of cardiopulmonary by-pass, and existence and duration of pleural effusion. The possible confounding variables were intestinal allergy and heart standstill after surgery.
A case was defined when a subject developed PLE that during follow-up, and the cohort were the rest of patients.
The calculation of the sample size was not performed because all patients with eligibility criteria of the Congenital Heart Disease Clinic were included.
Statistical analysis: For descriptive statistics, we used frequencies and percentages for qualitative variables; for quantitative variables, median (for central tendency measure) and interquartile ranges (for dispersion measure). For statistical inference, a chi-squared (c2) test was performed or a Fisher exact test if assumptions were not fulfilled. Risk was calculated estimating the Hazard Ratio (HR), followed by a stratified analysis by confounders. The statistically significant variables were assessed through survival analysis using the Kaplan-Meier estimator, and significance was calculated by Log-Rank method. The statistical software used was SPSS version 20 for Mac.
RESULTS
The analysis comprised 42 patients; the actual median age was 15 (7-25) years; 22 (58%) of the subjects were males.
Tricuspid atresia was the most common congenital heart disease in 15 (39%). Eleven patients (26%) developed PLE, and the median time between Fontan procedure and the development of PLE was 5 years. The rest of characteristics are displayed on table I.

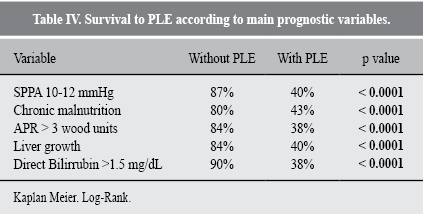
On the bivariate analysis to calculate the HR to develop PLE, the statistically significant variables were (Table II): SPPA between 10-12 mmHg; PRU between 3-3.5 Wood units; time between the Glenn and Fontan procedures; aggravated chronic malnutrition; hepatomegaly before surgery, and the development of pleural effusion on the immediate post-surgery period.
On table III, we display the stratified analysis of the 2 main confounding variables (previous intestinal allergy and asystole on immediate post-op period). A greater risk for PLE was documented in patients with intestinal allergy, SPPA between 12-15 mmHg (HR 2.6, p = 0.01) and aggravated chronic malnutrition (HR 1.7, p = 0.03).
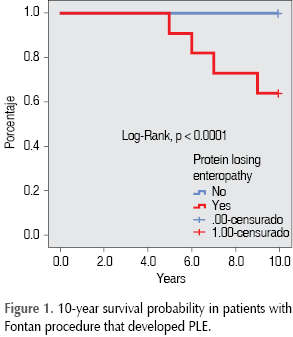
The statistically significant variables were submitted to univariate analysis of survival rate, showing that the presence of those decrease the probability of being PLE-free in a 10-year period, as shown in table IV. The probability of death after the development of PLE is 63%, as shown in Figure 1.
DISCUSSION
In our cohort of patients that underwent surgery for univentricular derivation with a 10-year follow-up, a frequency of 26% of PLE was documented. Most series report an incidence of 2.5-10%; however, the majority of these studies included only small groups with short follow-up periods.5
As it is known, the main cause of PLE is the retrograde increase of portal circulation secondary to the raise of pulmonary pressure.6 Because of this, some technical strategies had been developed to modified the Fontan procedure, as to avoid the closure of intracardiac shunts or, the mostly use of a fenestrated tube aimed to achieve venous decompression in case of pressure increase.7 In our case, all patients by protocol have a fenestrated tube.
The statistically significant variables associated with PLE were directly related to the increase of venous pressure (mean pressure of pulmonary artery), raised PRU and the persistence of pleural effusion on the immediate period after surgery. The relevance of these variables are according with the worldwide recommendations established previous to Fontan procedure, with prognostic implications.8
A variable directly associated to the development of PLE is the time from Fontan procedure to the diagnosis of PLE. The longer the duration of elevated systemic venous pressure secondary to univentricular derivation, the greater the chance to develop intestinal lesion.9 In our study, we found that a period greater than fourth years between Glenn and Fontan procedures increases the risk of PLE, a fact that had not been previously reported. We explain this phenomenon by the fact that despite the superior vena cava flow is derived to the pulmonary circulation, there is a greater time of exposition to intracardiac pressures.
Kiesewetter et al.9 described liver changes thorough time in a cohort of patients with Fontan procedure that developed secondary cirrhosis, finding abnormalities of liver function tests such as bilirubins, coagulation times, albumin and hepatic enzymes (AST/ALT). In our study, increased levels of direct bilirubin and previous hepatomegaly were associated with worst prognosis for the development of PLE; thus establishing the possibility that pre-surgery liver alterations could condition part of the risk for PLE.
Other evidence found in this study is the relationship between nutritional status and the poor prognosis for PLE. We know that CHD itself is a cause of malnutrition. Even worse, the sudden deterioration of the nutritional status can overshadow the clinical course, maybe because energy storages are insufficient to maintain requirements demanded by surgical stress.
It is a known fact that the liver is affected by the natural development of malnutrition, with implications in glycogenolysis, gluconeogenesis, glycolysis and protein formation. The increase of energy demands and the consequent failure of a deficient nutrition to satisfy it, could condition some of the damage caused by PLE.
The stratified analysis by confounders showed that those patients with previous intestinal allergy and borderline pressure values (systolic pressure of pulmonary artery) had greater risk to develop PLE, according with the findings of other studies that have showed a greater incidence of PLE in atopic patients, explained by the premature damage to intestinal epithelium. Undoubtedly, the pre-surgery factors shown in this study as risk variables worsen the prognosis to develop PLE.10,11
In our cohort, mortality was greater in patients with PLE. Around the world, when PLE is present, mortality increases to 50% in a 10-year follow-up period. In our case, mortality was smaller, because our follow-up period was shorter.
Because our sample size was insufficient to adjust confusion in a multivariate model, we decided to perform a stratified analysis for two confounders: atopic background and after-surgery asystole, finding the previously shown differences. Other authors have reported the time of cardiopulmonary by-pass as a significant variable, but we do not find this fact in our study. The importance of our study lays on the strict follow-up performed in the included patients, and the frequency of variables associated to the final outcome. We admit that there are methodological weaknesses such as the relatively small sample size, the fact that memory bias was not controlled, and an improper classification of some prognostic variables. Also are other well-known, before and after surgery factors, which were not considered in our analysis. New research questions arise from our study that justify the setting-up of other clinical studies aimed to answer them properly.
CONCLUSION
PLE in post-operative univentricular derivation patients presents more frequently in our hospital compared with other health facilities. In this cohort, the following variables were considered as worsening prognosis: venous limit pressures for Fontan procedure protocol, previous liver disease and atopic background.
REFERENCIAS
1. Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971; 6: 240-248. [ Links ]
2. Calderón-Colmenero J, Ramírez R, Viesca R, Ramírez L, Casanova M, García-Montes JA et al. Cirugía de Fontan. Factores de riesgo a corto y mediano plazo. Arch Cardiol Mex. 2005; 75: 425-434. [ Links ]
3. Cazzaniga M, Pineda LF, Villagrá F, Pérez de León L, Gómez R, Sánchez P et al. Operación modificada de Fontan o variantes efectuadas en un solo tiempo quirúrgico. Determinantes de la mortalidad. Rev Esp Cardiol. 2002; 55: 391-412. [ Links ]
4. Davidson JD, Waldman TA, Goodman DS, Gordon RS. Protein-losing gastroentero pathy in congestive heart failure. The Lancet. 1961; 1: 899-992. [ Links ]
5. Chakrabarti S, Keeton BR, Salmon AP, Vettukattil JJ. Acquired combined immunodeficiency associated with protein losing enteropathy complicating Fontan operation. Heart. 2003; 89: 1130-1131. [ Links ]
6. Laks H, Pearl JM, Haas GS, Drinkwater D, Milgater E, Jarmakani JM et al. Partial Fontan: advantages of an adjustable inter atrial communication. Ann Thorac Surg. 1991; 52: 1084-1095. [ Links ]
7. Senzaki H, Isoda T, Ishizawa A, Ishi T. Reconsideration of criteria for the Fontan operation. Influence of pulmonary size on postoperative hemodynamics of the Fontan operation. Circulation. 1994; 89: 1196-1102. [ Links ]
8. Rychik J, Gui-Yang S. Relation of mesenteric vascular resistance after Fontan operation and protein-losing enteropathy. Am J Cardiol. 2002; 90: 672-674. [ Links ]
9. Kiesewetter C, Sheron N, Vettukattill J, Hacking N, Stedman B, Millward-Sadler H. Hepatic changes in the failing Fontan circulation. Heart. 2007; 93: 579-584. [ Links ]
10. Orsi M, Fernández A, Follett F, Marchisone S, Saieg G, Busoni V et al. Alergia a la proteína de la leche de vaca. Propuesta de Guía para el manejo de los niños con alergia a la proteína de la leche de vaca. Arch Argent Pediatr. 2009; 107: 459-467. [ Links ]
11. Alberino F, Gatta A, Amodio P, Merkel C, Di Pascoli L,Boffo G, Caregaro L. Nutrition and survival in patients with liver cirrosis. Nutrition. 2001; 17: 445-450. [ Links ]
Nota
Este artículo puede ser consultado en versión completa en: http://www.medigraphic.com/revmexcardiol














