Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de cardiología
versión impresa ISSN 0188-2198
Rev. Mex. Cardiol vol.26 no.3 México jul./sep. 2015
Clinical case and Review
Effusive-constrictive pericarditis: a review
Pericarditis efusivo-constrictiva: revisión a propósito de un caso
Adalberto Arceo-Navarro,* Carlos Harrison-Gómez,** Francisco Sánchez-Lezama,*** Luis Gerardo Domínguez-Carrillo,**** Víctor Arredondo-Arzola*****
* Interventional Cardiologist. Cardiology Department, Hospital Ángeles León.
** Cardiologist. Cardiology Department, Hospital Ángeles León.
*** Cardiologist. Echocardiography Department, Hospital Ángeles León.
**** Rehabilitation Specialist. Professor of Medicine School. University of Guanajuato, México.
***** Cardiologist. Cardiology Department. T21 IMSS Hospital, León, Gto.
Correspondence to:
Adalberto Arceo-Navarro, MD
Hospital Ángeles León.
Av. Cerro Gordo Num. 311-550,
Col. Lomas del Campestre, León, Gto. México.
Tel. y fax +52.477.788.5655
E-mail: adalarce@yahoo.com
Recibido: 20/07/2015
Aceptado: 22/08/2015
ABSTRACT
Background: Effusive-constrictive pericarditis is an uncommon clinical hemodynamic syndrome in which constriction of the heart by the visceral pericardium occurs in the presence of tense effusion in a free pericardial space. This variety of constrictive pericarditis was observed and characterized by Hancock in 1971. The hallmark of effusive-constrictive pericarditis is the persistence of elevated right atrial pressure after intrapericardial pressure has been reduced to normal levels by removal of pericardial fluid. The causes are diverse and its course may be reversible or more frequently requiring extensive pericardiectomy. Clinical case: 35 year old male without an important medical history, with dyspnea and chest pain secondary to airway infection, in whom a diagnosis of pericardial effusion was made, handled with colchicine and NSAIDs, he presented decreased of pericardial effusion but worsening hemodynamic alterations corroborated by echocardiography. Diagnosed as an effusive-constrictive pericarditis a pericardiectomy was performed with excellent evolution. After multiple diagnostic tests the disease was catalogued like an idiopathic form. Conclusions: Effusive-constrictive pericarditis is a rare syndrome and it should be considered in the evolution of patients with pericardial effusion.
Key words: Effusive-constrictive pericarditis.
RESUMEN
Antecedentes: La pericarditis efusivo-constrictiva es un síndrome hemodinámico poco frecuente, en el cual el pericardio visceral constriñe al corazón con la presencia de líquido libre en el espacio pericárdico; fue descrito y caracterizado por Hancock en 1971. Se caracteriza por la presencia de presión elevada en la aurícula derecha persistente después de pericardiocentesis del derrame pericárdico; su etiología es diversa y su curso incierto, llegando a ser reversible o más frecuentemente requerir pericardiectomía extensa. Caso clínico: Masculino de 35 años sin antecedentes de importancia, con evolución de disnea progresiva y dolor torácico secundarios a infección de vías aéreas, en quien se efectuó diagnóstico de derrame pericárdico, manejado con colchicina y AINEs, evolucionando con disminución del derrame pero empeorando las alteraciones hemodinámicas, corroboradas por ecocardiografía. Con diagnóstico de pericarditis efusivo-constrictiva fue sometido a pericardiectomía con excelente evolución, clasificando el cuadro como idiopático después de múltiples pruebas diagnósticas en busca de la etiología. Conclusiones: La pericarditis efusivo-constrictiva es un síndrome poco frecuente que debe tenerse presente en la evolución de los pacientes con derrame pericárdico.
Palabras clave: Pericarditis efusivo-constrictiva.
Introduction
Constrictive-effusive pericarditis was initially described by Hancock1 in 1971. In the majority of patients with constrictive pericarditis it is not possible to establish the etiologic diagnosis even with pathologic specimens and histologic study of the pericardium, it is also known that the longer the evolution of the pericarditis is, the probability of making etiologic diagnosis diminishes, classifying them finally as idiopathic.2 It is possible that at least some of these patients initially had a non-diagnosed viral or tuberculosis pericarditis. Prospective studies of patients after a first bout of acute pericarditis shows that evolution to chronic constrictive pericarditis is very unusual, in fact, after a viral pericarditis, transition to chronic constrictive forms occurs approximately in 1% of cases.3 On the other side, transition to chronic constrictive pericarditis is very common after tuberculosis pericarditis and can occur in 40-50% of cases,4 or after purulent bacterial pericarditis in 30-40%.5,6 In these cases, the constriction can occur in an acute or subacute form, and they will require pericardiectomy in the following months after the acute episode. This natural history or expression of the disease permits us establish a very variable concept of continuum in the syndromes of cardiac compression; some patients have hemodynamic data exclusively of tamponade, in others tamponade predominate but with a component of constriction; others, mixed tamponade and constriction in what we call effusive-constrictive pericarditis; we also see patients where constriction predominates but with mild pericardial effusion, and finally, some patients have pure constriction.7 We present the case of a patient with the diagnosis of idiopathic effusive-constrictive pericarditis that needed pericardiectomy, and make a literature review of the topic.
CLINICAL CASE
Male, 35 years old, with a medical history of asthma in his childhood, and lumbar surgery at 28. In June 2014 he complained of non-productive cough and retrosternal chest pain, worse during inspiration, pleuritic quality, he was diagnosed at another medical facility with pneumonia and treated with non specified antibiotics, with no improvement; in July 2014 he was referred to a pulmonologist that prescribed a cephalosporin and bronchodilators, the cough improved and chest pain disappeared for 15 days. Then after, he began with mild to moderate effort dyspnea, dizziness on orthostatic stress, diaphoresis and abdominal distension.
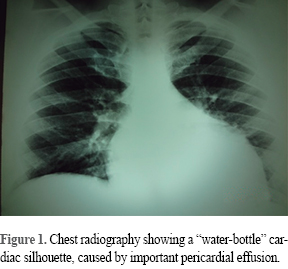
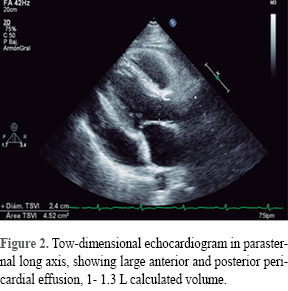
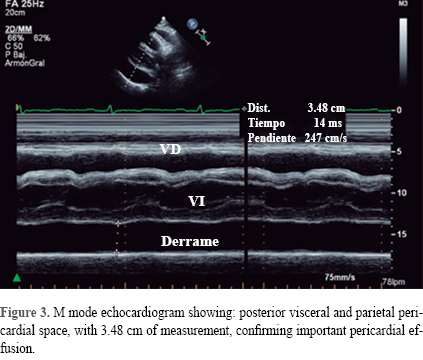
In a first cardiologic evaluation on August 4, 2014 a physical examination disclosed blood pressure 116/74 mmHg, heart rate 95 bpm, temperature 36.7 oC, a protodiastolic sound, and bilateral leg edema. ECG with sinus rhythm, poor R wave progression of R waves in right precordial leads and asymmetric T wave inversion in inferior and lateral leads; a thorax X-ray with a water bottle shaped heart, and conspicuous absence of pulmonary congestion (Figure 1). The initial echocardiogram showed (Figures 2 and 3) a large pericardial effusion quantified in 1-1.3 liters with evidence of tamponade physiology: 38% relative inspiratory augmentation of transtricuspid flow and 21% decrease in inspiratory flow across the mitral valve, also there was evidence of systemic venous hypertension with a dilated inferior cava vein (27 mm) that not collapse on inspiration; there was right ventricle compression, normal left atrium, and a left ventricle ejection fraction of 79%. Laboratory test with mild anemia, Hb 11.9 g/dL, urine test with granular casts, and mild proteinuria, eritrosedimentation rate, rheumatoid factor, antinuclear, anti DNA, anti smooth muscle and HIV where negative. With the diagnosis of pericarditis we began treatment with colchicine 1 mg tid, ibuprofen 600 mg tid and pantoprazole 20 mg sid.
A second echocardiogram on August 20, 2014 revealed a hiper-reflectant parietal pericardium with still large posterior and lateral pericardial effusion, quantified in 950 mL, abdominal ultrasound with liver esteatosis grade II. His dyspnea had improved, he complained of nausea, emesis and abdominal distension. Blood pressure 88/56, heart rate 108 bpm, yugular venous distention 3/4, precordial auscultation with a fixed split second sound, and abdominal pain on palpation in epigastrium. ECG without changes.
The third echocardiogram on September 10, 2014 disclosed less pericardial effusion, now quantified in 500 mL, still with diastolic collapse of right ventricular free wall. One week after this third echo, the patient decided to interrupt colchicine because of gastric symptoms, and one month later, September 18, 2014 he returned with severe weight increase related to anasarca, dizziness on orthostatism, severe dyspnea on minimal effort, non-productive cough, and data of systemic venous hypertension, related to the constrictive process, the ECG with the same right precordial abnormalities previously described. On the fourth echo done October 6, 2014 the ejection fraction was 73%, less pericardial effusion, quantified 350-400 mL.
With the diagnosis of constrictive-effusive pericarditis, a pericardiectomy was done October 16, 2014 (Figure 4), the surgical findings disclosed dark bloody pericardial effusion with a very thickened, granulomatous like and adherent visceral pericardium, suggestive of tuberculosis or carcinomatosis. Histopathology study reported: sclerosant fibrinous pericarditis, C reactive protein (CRP) and Ziehl-Nielsen in pericardial fluid and surgical specimens where negative for tuberculosis, also Mantoux test was negative, so, he did not receive antituberculosis treatment.

He had a good postsurgical outcome, only with persistent high pleural fluid output through right pleurocentesis that improved with anti-inflammatory drugs for several months. The syndrome of systemic venous hypertension disappeared completely, and by October 24, 2014 he had already lost 18 kg.
Finally, in the last echocardiogram on March 21, 2015 he had a left ventricle ejection fraction 74%, mildly dilated left atrium, dilated right heart cavities with normal right ventricle function, central venous pressure 10 cmH2O, and a systolic pulmonary pressure 37 mmHg. In an outpatient office visit April 21, 2015 he relates no symptoms, functional class I, jogs 4 km three times per week, the thorax X-Rays shows an exposed heart, enlarged pulmonary artery arc and clear lungs (Figure 5).

DISCUSSION
Physiopathogenesis of pericardial effusion
The pericardium sac normally contains between 15 to 50 mL of fluid, this produced mainly in the visceral pericardium and it is an ultrafiltrate of plasma. The pericardial functions8 are: a) diminish diastolic pressure, being responsible for the low pressure in the right heart cavities, b) distribute the forces and stress throughout the heart, it assists and assures a uniform myocardial contraction; because of the physiologic right heart filling during inspiration, the pericardium prevents left cavities dilation. The abnormal pericardial fluid production depends basically in the etiology9 (Table I) and the fluid characteristics10 can be: transudate, occurring with lymphatic duct drainage obstruction; and, exudate related to inflammatory, infectious, autoimmune or malignant disease; more than 60% of cases are related to a medical coexistent condition.11 The pericardium is elastic and compliant and accumulate a small to moderate quantity of fluid without significant increase in intrapericardial pressure, but once the volume of intrapericardial fluid continue to increase, there is a change in pressure-volume slope towards the left with increased intrapericardial pressure that restricts right ventricular filling. If the accumulation of pericardial is a slow process, this permits an increased parietal pericardium compliance and accumulation of larger quantities of pericardial fluid with minimal changes in intrapericardial pressure until the pressure-volume curve changes to its steep portion and restrictive right heart hemodynamics appear.12 This situation occurred with our patient, who probably had a slow accumulation of pericardial fluid that permitted and tolerated a 1.3 lts of pericardial effusion. So, the hemodynamic repercussion of pericardial effusion is directly related to the time it takes to accumulate.
Epidemiology
Pericardial effusion is seen frequently in autopsies even if clinically unnoticed (Table I). The prevalence is 3-6% in the general population, and 0.1% in referral hospitals.13,14 Acute pericarditis is the most frequent etiology of pericardial effusion,15 associated with myocarditis in 15% of cases. Cardiologic centers report that infectious disease represent 27% of cases of pericardial effusion, neoplasm 23%, radiation therapy 14%, and autoimmune disease 12%. One study showed that of 14,275 admissions, the prevalence of pericardial effusion that needed pericardiocentesis was 1.1%, with a rate of 11 pericardial effusions per 1,000 admissions/year.16 Pericardial effusions does not have predilections related to ethnicity or gender, it can occur at any age, more frequent in the fourth decade, relatively earlier in male;17 other differences include that in males the most common etiology is idiopathic (31%); neoplastic (24%), and infectious (16.7%). In females neoplastic disease is a more common etiology occurring in 29%, idiopathic 25%, and rheumatic/autoimmune in 14%, this last etiology being much more common in women than man with a rate 4.5/1 favoring women.
Symptoms, signs and diagnosis
Typically, patients with pericarditis complain of dyspnea and chest pain in 80% of cases,18,19 however, pleuritic quality chest pain is the cardinal symptom of pericarditis, worse during inspiration, when lying flat, and it may be relieved by leaning forward while seated, it may be referred to the trapezius ridge, neck, or back. Sometimes the patient may complain of dizziness, palpitations or syncope; 68% of patients refer cough, others voice hoarseness, anxiety and hiccups. Our patient had moderate effort dyspnea, and the moderate oppressive retrosternal pain.
Physical findings include:20 low pitched heart sounds 73% of patients, tachycardia 60%, elevated jugular venous pressure 58%, tachypnea 54%, and less often pulsus paradoxus and hypotension in 25%. On auscultation 10% have a pericardial knock, and pericardial rub in 33 to 85%.21 Our patient had several of the physical findings described. The electrocardiographic abnormalities22,23 have 4 stages:24 1st-PR interval depression and diffuse concave upward ST elevation of ST segment except aVR; 2nd normalization of abnormalities of 1st stage; 3rd diffuse T wave inversion, and 4th normalization of T wave abnormalities. Electrical alternant in voltage and direction of QRS waves usually in a 2:1 ratio is related to the heart movement into the fluid filled pericardial space; large pericardial fluid collections might disclose low QRS voltage25 < 0.5 mV in limb and less 1 mV in precordial derivations.26 In our case, the electrocardiogram revealed low voltage with tachycardia, inferolateral T wave inversion, and a "pseudoinfarct" pattern in anteroseptal wall, as described in the 3rd stage of electrocardiographic abnormalities.
The echocardiogram is the most valuable study in the evaluation of patients with pericardial effusion,27 images appear as an echo free space between visceral and parietal pericardium; the effusion accumulates more often along the posterior wall and expand in posterolateral direction; the echo free space is less than 10 mm in pericardium effusions less than 500 mL, between 10 and 20 mm in moderate sized effusions of 500-900 mL, and above 20 mm in large effusions over 1,000 mL; fluid next to the right atrium is a sign of pericardial effusion,28 and its collapse together with right ventricular diastolic collapse are echocardiographic signs of tamponade.29 In M-mode echo Horowitz criteria are useful.30 Sometimes, the echo can disclose a blood clot related to aortic dissection or heart perforation during invasive catheter procedures, also pericardial neoplasms sometimes can be seen in pericardium. The echocardiogram may have false positive results related to unspecific increased pericardial thickness, epicardial fat, atelectasia, pericardial cyst, and mediastinal lesions. Our patient initially had only important pericardial effusion, followed by development of a marked thick visceral pericardium with evidence of right ventricular hemodynamic collapse, all this associated with data of systemic venous hypertension with a dilated with no inspiratory collapse of inferior cava vein. Even the decrease of pericardial effusion there was a gradual progression to constrictive pericarditis.
Other imaging studies such as chest X-Ray radiography31 are useful to detect pericardial effusion larger than 250 mL, or rule out other lung or mediastinum disease. When there is a large amount of pericardial fluid we can see a typical "water bottle" heart similar to what we described in our patient thorax X-Ray, it is not uncommon to see a small left pleural effusion. CT scan32 can detect a smaller fluid collections such as 50 mm, and with MRI as little as 30 mL. Using different MRI pulses sequences and relaxation times the fluid characteristics can be obtained, and with myocardial imaging we can know if there is heart muscle damage and even according to late gadolinium enhancement pattern the type and etiology of myocardial pathology.
Examination of pericardial fluid acquired by pericardiocentesis is invaluable for establishing etiologic diagnosis,33 chronic inflammatory liquid is reported in approximately 40% of cases, elevated WBC > 10,000 cells with neutrophils predominance is suggestive of infection or rheumatic etiology; Gram stain and bacterial culture can be very specific; Light criteria measuring protein and LDH levels are useful to distinguish between exudate and transudate; specific gravity > 1.015 and total protein > 3.0 mg/dL indicate an exudate effusion; cytopathology is necessary to rule out neoplasia;35 sometimes and according with clinical data we might need special tests as virus culture, polymerase chain reaction, adenosine deaminase test and acid-alcohol resistant bacteria culture that can define tuberculosis infection;36 carcinoembrionary antigen in the pericardial liquid is highly suggestive of neoplasm; It has reported that the pericardial fluid cytopathology has a moderate sensitivity (54%) and high specificity (95%) for the diagnosis of neoplasia.37 In lung cancer the prevalence of PE is 37%; breast cancer 22%; leukemia/lymphoma 17%; and 5% in melanoma. Pericardial biopsy is particularly useful for diagnosing neoplastic disease. In our patient, the pericardial fluid during surgery had dark, hemorrhagic aspect, there was no microscopic evidence of bacteria culture, including mycobacterial infections. The surgical specimen of pericardium had several layers, 5-6 mm each one, of hard consistency and with pearly granulomatous dark aspect, very adherent to adyacent epicardium. Histopathological studies were done by two different pathologists, both diagnosed sclerosing fibrinous pericarditis, specific studies for acid-alcohol resistant bacteria, including CRP, on tissues and liquid were negative.
Treatment
Acute pericarditis treatment consist in colchicine treatment in combination with standard therapy with non steroid anti-inflammatory drugs,38 this is supported on the results of the CORP-2 study,39 recurrence at 18 months are much less with this combination. Using steroids as first line therapy in acute pericarditis is associated with increased relapse when the drug is withdrawn;40 its application should be considered in patients with recurrent pericarditis unresponsive or nontolerant to treatment with NSAIDs and colchicine. The European Cardiology Guidelines recommend that steroids should be restricted to patients with pericarditis associated with connective tissue disease, uremic,41 and autoreactive origin.42 Intrapericardial steroids appears to be effective in acute pericarditis,43 lessening the risk of recurrent pericarditis as when the steroid is used by systemic application. Pericardiocentesis is diagnostic and therapeutic; ideally it must be performed under echocardiography guidance; it is indicated in: management of hemodynamic compromise, suspected infection, neoplastic etiology and uncertain etiology; the procedure has 98% of success with 0.3 to 1.2% of mayor complications and 3.5 to 6.3% of minor complication.34 It has been reported that the slow drainage procedure (no longer than 48 hours to prevent infection), reduces the number of recurrences.44 Pericardioscopy45 increases the diagnostic sensitivity for undiagnosed PE, allowing biopsies of the pericardium; CT-guided pericardiocentesis is useful in those cases when ultrasound has not helped in differentiating other structures adjacent to the PE. Thoracotomy is reserved for those patients in whom other measures have failed, and it allows the creation of a pleuropericardial window. Sternotomy and pericardiectomy is indicated in patients with constrictive pericarditis, this procedure has a mortality rate of 5 to 15%. Our patient initially treated with NSAIDs and colchicine, improved, however, medical treatment was limited by gastric side effects. Later, he developed a constrictive pericardial physiology requiring pericardiectomy with good results.
Prognosis
Morbidity and mortality in pericardial effusion depends on etiology and comorbid conditions. The PE of slow onset is well tolerated in most patients; up to 50% of patients with a large PE with slow onset of more than six months might remain asymptomatic at first diagnoses. Cancer related PE has a high mortality (86% in a few months). Survival of patients with symptomatic HIV and PE is 36% at 6 months and 19% at one year.
Effusive-constrictive pericarditis46 is a rare entity, with a unknown prevalence; it has both a abnormal physiology including tamponade related to the compressive force over the heart related to the pericardial effusion, with elevated jugular venous pressure with X collapse and pulsus paradoxus, and constrictive physiology that sometimes becomes obvious only after pericardial fluid drainage by pericardiocentesis, when we see persistence of data of systemic venous hypertension, but now with a clear constrictive physiology, with a prominent jugular vein Y wave collapse. The diagnosis of effusive constrictive pericarditis47 can be suspected by the correct clinical interpretation of these physical signs, aided by Doppler echocardiography.48 Study and intrapericardial and intracavitary pressure measurements before and after of pericardiocentesis. Invasive study discloses an elevated intrapericardial pressure and elevation and equalization of diastolic pressures in both ventricles, after pericardiocentesis, intrapericardial pressure goes down to subatmospheric levels, but intracavitary pressures remain elevated. Furthermore, the patient does not get symptomatic improvement and cardiac output does not significantly increase; this is because in these patients the hemodynamic disorder is caused primarily by the constriction component produced by the thickened visceral pericardium (constrictive pericarditis). A study of 13 patients, with a maximum of 16 months from the onset of the pericardial disease, all 13 of them required pericardiectomy;49 another study of pericardial disease in 1,184 patients with diverse etiologies, effusive-constrictive pericardial disease was diagnosed in 15 patients; they have been symptomatic for less than three months; the etiology of pericarditis in these 15 patients was idiopathic in 7 cases, neoplastic in 4, post radiotherapy in two cases, tuberculosis in 1 case, and post surgical in 1 patient;50 parietal pericardial thickening (3 to 6 mm) and the visceral pericardium (5 to 6 mm) was found in all of them;51 the resection of visceral pericardium was difficult and laborious.
Therefore, a concept of a continuum in cardiac compression syndromes can be established, with some patients having an acute tamponade; others have predominance of pericardial effusion but with a component of constriction; others, a mixed situation of effusion related heart compression and constriction matching the description of the effusive-constrictive pericarditis;52 while others have essentially constriction but with presence of large pericardial effusion, and finally other patients have a frank constriction pericarditis.
CONCLUSION
Effusive-constrictive pericarditis is an uncommon and underdiagnosed clinical entity, we have to suspect it and make an early diagnosis in all patients with pericardial effusion, because it has important treatment implications with unfavorable prognosis if it not correctly diagnosed and treated.
REFERENCIAS
1. Hancock EW. Subacute effusive-constrictive pericarditis. Circulation. 1971; 43: 183-192. [ Links ]
2. Santa Cruz RJ, Sahagún SG, González CD, Sánchez GN. Análisis de las características clínicas, ecocardiográficas, microbiológicas y citopatológicas de derrames pericárdicos en un hospital de tercer nivel de atención. Arch Cardiol Mex. 2014; 84: 86-91. [ Links ]
3. Sagrista SA, Merce J, Permanyer MG et al. Clinical clues to the causes of large pericardial effusions. Am J Med. 2000; 109: 95-101. [ Links ]
4. Aguilar J, Summerson C, González E et al. Pericarditis tuberculosa Experiencia de 10 años. Arch Cardiol Mex. 2007; 77: 209-216. [ Links ]
5. Lind A, Reinsch N, Neuhaus K et al. Pericardial effusion of HIV-infected patients? Results of a prospective multicenter cohort study in the era of antiretroviral therapy. Eur J Med Res. 2011; 16: 480-483. [ Links ]
6. Pankuweit S, Ristic AD, Seferovic PM, Maisch B. Bacterial pericarditis: diagnosis and management. Am J Cardiovasc Drugs. 2005; 5: 103-112. [ Links ]
7. Ling LH, Oh JK, Schaff HV et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999; 100: 1380-1386. [ Links ]
8. Natanzon A, Kronzon I. Pericardial and pleural effusions in congestive heart failure-anatomical, pathophysiologic, and clinical considerations. Am J Med Sci. 2009; 338: 211-216. [ Links ]
9. Corey G, Campbell P, van Trigt P et al. Etiology of large pericardial effusions. Am J Med. 1993; 95: 209-213. [ Links ]
10. Ben-Horin S, Bank I, Shinfeld A, Kachel E, Guetta V, Livneh A. Diagnostic value of the biochemical composition of pericardial effusions in patients undergoing pericardiocentesis. Am J Cardiol. 2007; 99: 1294-1297. [ Links ]
11. Maisch B, Seferovic P, Ristic A. Guidelines on the diagnosis and management of pericardial diseases executive summary: The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2004; 25:587. [ Links ]
12. Hoit BD. Pericardial disease and pericardial tamponade. Crit Care Med. 2007; 35 Suppl: S355-364. [ Links ]
13. Permanyer-Miralda G, Sagristá-Sauleda J, Soler-Soler J. Primary acute pericardial disease: a prospective series of 231 consecutive patients. Am J Cardiol. 1985; 56: 623-630. [ Links ]
14. Nugue O, Millaire A, Porte H et al. Pericardioscopy in the etiologic diagnosis of pericardial effusion in 141 consecutive patients. Circulation. 1996; 94: 1635-1641. [ Links ]
15. Sagrista-Sauleda J, Angel J, Permanyer-Miralda G. Long-term follow-up of idiopathic chronic pericardial effusion. N Engl J Med. 1999; 341: 2054-2059. [ Links ]
16. Tsang T, Enriquez-Sarano M, Freeman W et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: Clinical profile, practice patterns, and outcomes spanning 21 years. May Clin Proc. 2002; 77: 429-436. [ Links ]
17. Ramírez F, Sarmiento M, Orjuela T et al. Características clínicas y ecocardiográficas de los derrames pericárdicos en pacientes del Hospital Universitario de San Vicente de Paúl. IATREIA. 2002; 15: 135-142. [ Links ]
18. Beck C. Two cardiac compression triads. JAMA. 1935; 104: 714-716. [ Links ]
19. Cheema M, Ghalib M, Shatoor A et al. Pattern of pericardial disease in the Asir Region of Saudi Arabia. Ann Saudi Med. 2007; 19: 171-173. [ Links ]
20. Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Outcomes of clinically significant idiopathic pericardial effusion requiring intervention. Am J Cardiol. 2003; 91: 704-707. [ Links ]
21. Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet. 2004; 363: 717-727. [ Links ]
22. Little WC, Freeman GL. Pericardial disease. Circulation. 2006; 113: 1622-1632. [ Links ]
23. Meyers DG, Bagin RG, Levene JF. Electrocardiographic changes in pericardial effusion. Chest. 1993; 104: 1422-1426. [ Links ]
24. Eisenberg MJ, de Romeral LM, Heidenreich PA. The diagnosis of pericardial effusion and cardiac tamponade by 12-lead ECG. A technology assessment. Chest. 1996; 110: 318-324. [ Links ]
25. Bruch C, Schmermund A, Dagres N et al. Changes in QRS voltage in cardiac tamponade and pericardial effusion: reversibility after pericardiocentesis and after anti-inflammatory drug treatment. J Am Coll Cardiol. 2001; 38: 219-226. [ Links ]
26. Spodick DH. Acute cardiac tamponade. N Engl J Med. 2003; 349: 684-690. [ Links ]
27. Pepi M, Muratori M. Echocardiography in the diagnosis and management of pericardial disease. J Cardiovasc Med. 2006; 7: 533-544. [ Links ]
28. Gandhi S, Schneider A, Mohiuddin S et al. Has the clinical presentation and clinician's index of suspicion of cardiac tamponade changed over the past decade? Echocardiography. 2008; 25: 237-241. [ Links ]
29. Naqvi TZ, Huynh HK. A new window of opportunity in echocardiography. J Am Soc Echocardiogr. 2006; 19: 569-577. [ Links ]
30. Horowitz MS, Schultz CS, Stinson EB et al. Sensitivity and specificity of echocardiographic diagnosis of pericardial effusion. Circulation. 1974; 50: 239-247. [ Links ]
31. Karia DH, Xing YQ, Kuvin JT, Nesser HJ, Pandian NG. Recent role of imaging in the diagnosis of pericardial disease. Curr Cardiol Rep. 2002; 4: 33-40. [ Links ]
32. Restrepo CS, Lemos DF, Lemos JA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007; 27: 1595-1610. [ Links ]
33. Eichler K, Zangos S, Thalhammer A et al. CT-guided pericardiocenteses: clinical profile, practice patterns and clinical outcome. Eur J Radiol. 2010; 75: 28-31. [ Links ]
34. Ben-Horin S, Bank I, Shinfeld A et al. Diagnostic value of the biochemical composition of pericardial effusions in patients undergoing pericardiocentesis. Am J Cardiol. 2007; 99: 1294-1297. [ Links ]
35. Kim SH, Kwak MH, Park S et al. Clinical characteristics of malignant pericardial effusion associated with recurrence and survival. Cancer Res Treat. 2010; 42: 210-216. [ Links ]
36. Aguilar JA, Summerson C, González ME et al. Pericarditis tuberculosa. Experiencia de 10 años Arch Cardiol Mex. 2007; 77: 209-216. [ Links ]
37. Refaat MM, Katz WE. Neoplastic pericardial effusion. Clin Cardiol. 2011; 34: 593-598. [ Links ]
38. Meurin P, Tabet JY, Thabut G et al. Nonsteroidal anti-inflammatory drug treatment for postoperative pericardial effusion: a multicenter randomized, double-blind trial. Ann Intern Med. 2010; 152: 137-143. [ Links ]
39. Imazio M, Belli R, Brucato A et al. Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP 2): a multicentre, double-blind, placebo-controlled, randomized trial. Lancet. 2014; DOI:10.1016/S0140-6736(13)62709-9. [ Links ]
40. Imazio M, Brucato A, Cumetti D et al. Corticosteroids for recurrent pericarditis: high versus low doses: a nonrandomized observation. Circulation. 2008; 118 (6): 667-671. [ Links ]
41. Rosenbaum E, Krebs E, Cohen M et al. The spectrum of clinical manifestations, outcome and treatment of pericardial tamponade in patients with systemic lupus erythematosus: a retrospective study and literature review. Lupus. 2009; 18: 608-612. [ Links ]
42. Maisch B, Ristic AD, Pankuweit S. Intrapericardial treatment of autoreactive pericardial effusion with triamcinolone; the way to avoid side effects of systemic corticosteroid therapy. Eur Heart J. 2002; 23: 1503-1508. [ Links ]
43. Artom G, Koren-Morag N, Spodick DH et al. Pretreatment with corticosteroids attenuates the efficacy of colchicine in preventing recurrent pericarditis: a multi-centre all-case analysis. Eur Heart J. 2005; 26: 23-727. [ Links ]
44. Allen KB, Faber LP, Warren WH. Pericardial effusion: subxiphoid pericardiostomy versus percutaneous catheter drainage. Ann Thorac Surg. 1999; 67: 437-440. [ Links ]
45. Nugue O, Millaire A, Porte H et al. Pericardioscopy in the etiologic diagnosis of pericardial effusion in 141 consecutive patients. Circulation. 1996; 94: 1635-1641. [ Links ]
46. Spodick DH, Kumar S. Subacute constrictive pericarditis with cardiac tamponade. Dis Chest. 1968; 54: 62-66. [ Links ]
47. D'Cruz IA, Pallas CW, Heck A. Echocardiographic diagnosis of effusive-constrictive pericarditis due to staphylococcal pericarditis after cardiac surgery. South Med J. 1991; 84: 1375-1377. [ Links ]
48. Castañón GA, AmézquitaLJ, Velasco OE, Deseano EJ, León GM. Pericarditis constrictiva: historia de un corazón oprimido Cir Cir. 2010; 78: 342-346. [ Links ]
49. Walsh TJ, Baughman KL, Gardner TJ, Bulkley BH. Constrictive epicarditis as a cause of delayed or absent response to pericardiectomy: a clinicopathological study. J Thorac Cardiovasc Surg. 1982; 83: 126-132. [ Links ]
50. Cameron J, Oesterle SN, Baldwin JC, Hancock EW. The etiologic spectrum of constrictive pericarditis. Am Heart J. 1987; 113: 354-360. [ Links ]
51. Watanabe A, Hara Y, Hamada M et al. A case of effusive-constructive pericarditis: an efficacy of GD-DTPA enhanced magnetic resonance imaging to detect a pericardial thickening. Magn Reson Imaging. 1998; 16: 347-350. [ Links ]
52. Sagrista SJ, Permanyer MG, Candell RJ, Angel J et al. Transient cardiac constriction: an unrecognized pattern of evolution in effusive acute idiopathic pericarditis. Am J Cardiol. 1987; 59: 961-966. [ Links ]
Nota
Este artículo puede ser consultado en versión completa en: http://www.medigraphic.com/revmexcardiol














