Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de cardiología
versión impresa ISSN 0188-2198
Rev. Mex. Cardiol vol.26 no.2 México abr./jun. 2015
Review
The vascular endothelium: a review series
I. Basic aspects of the vascular endothelium
El endotelio vascular: una serie de repaso
I. Aspectos básicos del endotelio vascular
Guillermo Ceballos,* Gabriela Gutiérrez-Salmeán,* Eduardo Meaney*
* Laboratorio de Investigación Integral Cardiometabólica
Escuela Superior de Medicina, Instituto Politécnico Nacional
Correspondence to:
Guillermo Ceballos MD, PhD
Laboratorio de Investigación Integral Cardiometabólica
Escuela Superior de Medicina,
Instituto Politécnico Nacional.
Plan de San Luis y Diaz Mirón s/n
Col. Santo Tomás
México D.F. 11340
Tel 5557296300 ext.62820
E-mail: gceballosr@ipn.mx
ABSTRACT
The vascular endothelium is a key regulator of blood flow thus blood pressure. Endothelial cells play a major role in vascular biology by modulating both vasodilation and vasoconstriction through autocrine, paracrine, and hormonal-like mechanisms and molecules such as nitric oxide, prostacyclin, endothelin, and thromboxane. Whenever these fail, endothelial dysfunction presents and may further associated with the development and evolution of a number of cardiovascular pathologies.
Key words: Endothelial cell, endothelial dysfunction, vascular endothelium, vascular system, vasoreactivity
RESUMEN
El endotelio vascular es un regulador clave del flujo sanguíneo y, por tanto, de la presión arterial. La célula endotelial juega un papel de suma importancia en la biología vascular, ya que media tanto la vasodilatación como la vasocontricción, a través de mecanismos autocrinos, paracrinos y endocrinos que involucran moléculas tales como óxido nítrico, endotelina, prostaciclina y tromboxano. Cuando alguno de dichos mecanismos falla, aparece la disfunción endotelial, misma que puede vincularse -en un futuro- al desarrollo y progresión de numerosas patologías cardiovasculares.
Palabras clave: Célula endotelial, disfunción endotelial, endotelio vascular, sistema vascular, vasorreactividad
This is the first of a series of short reviews on endothelium physiology and pathophysiology, covering the majority of issues regarding the function and dysfunction of these extraordinary and complex cells, perhaps the most important cell type in human organisms, not only because it is the most abundant cell type (excluding blood cells) in the body, but because it is the only one that "touches" every parenchymal cell in the economy. The importance of the adequate endothelial functionality and all the abundant function pathways present in this cell type makes very difficult to screen all the possibilities in this kind of short reviews so, we apologize in advance if we do not review extensively all the pathophysiological pathways reported to date.
For many years, both in Medicine and Physiology, the endothelial cell was referred to be an inert sac whose function was simply to separate the blood from the tissues. However, following the discovery of Furchgottt and Zawadzki1 in 1980, showing that the vasodilator action of acetylcholine in the aorta of rabbit is dependent on the presence of an intact endothelium, a new scenario arose in which it is very clear that the endothelium is an extremely active tissue that plays an intimate role in many physiological processes. Also we know that many substances readily available in the blood may act on the smooth muscle in the vessel wall via the endothelium, through the release of several vasoactive agents.
A normal blood vessel function known as vasoreactivity, is essential for survival. Blood vessels must be able to integrate diverse signals, i.e. variations of pH, temperature, mechanical forces or neuro-signals, to quickly respond and regulate in turn the amount of blood flow of different organs in response to their needs, for the collective "wellbeing" of the organism.
The vascular system includes different types of blood vessels such as arteries and veins. The walls of vessels (except capillaries) are formed of three distinct layers (intima, media and adventitia) whose organization and relative thickness are characteristic of a particular type of vessel. In veins, these three strata are in contact while they are separated by the internal and the external elastic lamina in arteries. The middle layer, the tunica media composed of smooth muscle cells and elastic fibers (mainly collagen and elastin), is responsible for the vasomotor activity (vasoconstriction or vasodilation). Locally, vasoreactivity is regulated by molecules released from the nerves terminating in the adventitia tunica (nerve terminals do not touch endothelial cells). These molecules must diffuse to smooth muscle cells or endothelium to act. Endothelial cells contain specific receptors at the plasmalemmal surface through which circulating vasoactive agents stimulate or inhibit the endothelial production of vasorelaxing or vasoconstricting agents, for its consequent interaction with vascular smooth muscle.2
Besides neuro-signals, the blood flow induces mechanical strains and shear that participate in the regulation of vasoreactivity, through mechanoceptors at the surface of endothelial cells.
The vascular endothelium is the inner layer of cells that line the blood vessels of the circulatory and lymphatic fluids. As key regulators of blood flow, endothelial cells play a major role in vascular biology via autocrine, paracrine, and hormonal-like mechanisms involving control of endothelial-derived relaxing factors (EDRF) as nitric oxide (NO) and prostacyclin (PGI2) and the vasoconstrictors; endothelin (ET-1) and thromboxane (TXA2).3
In addition to control of vasodilation and vasoconstriction, endothelial cells are major regulators of vascular homeostasis through the maintenance of vascular tone, prevention of vascular smooth muscle proliferation; reduction in leukocyte adhesion and activation, inhibition of platelet aggregation, and thrombus formation, among others.3
Endothelial dysfunction, a condition characterized by abnormalities of these mechanisms, is implicated in the development and evolution of a number of pathologies. Main cardiovascular diseases (CVD), as stroke, coronary artery disease, heart failure, and cardiac arrest, are the predominant causes of morbility and mortality in Western world. CVDs cause ~35% of all deaths in subjects 65 years of age or older. Furthermore, the prevalence of CVDs increases progressively, from ~5.5% in people aged 25-44 years to ~41% in people 65 years of age or older. Thus, in many instances, CVDs can be considered true diseases of aging. Besides, atherosclerosis and hypertension are in the beginning vascular diseases with potential capacities of causing parenchymal disasters (stroke, myocardial infarction and heart failure, among many others). Arterial lesions in both conditions start with the classical pathogenic trial of nitroxidation, inflammation and endothelial dysfunction. Age related alterations in arteries are thought to lead to a dysfunctional phenotype that precedes CVDs.4,5
The dysfunctional endothelial phenotype is common to humans and non-human primates as well as rodents and other mammals. The consequences of the altered endothelial function contribute to a number of hemodynamic changes, including augmented tone in large and resistance arteries, induction of greater shear and oscillatory perpendicular stress, and elevated large artery stiffness, concurrent phenomena that lead to increase systemic arterial blood pressure, and promote atherogenesis.4
The endothelium tissue is composed of endothelial cells anchored on a basal lamina and a sub-endothelial layer, which is an extracellular matrix (ECM) allowing mechanical anchoring. The estimated number of individual cells constituting the endothelium is somewhere in the order of 1-6 x 1013. An important property of endothelial cells is quiescence. In adults, in normal conditions, the average endothelial cell divides in around twice in a lifetime. Nevertheless, if it is required, endothelial proliferation can be very rapid, for example, in wound healing angiogenesis, when it is quickly initiated, being this a process exquisitely regulated since it is switched off quickly also.2
Several vascular diseases are just restricted to specific types of vessels. For example, vasculitis shows marked predilection for specific arteries, veins or capillaries, while tumor cells may metastasize to selective vascular beds and atherosclerosis is restricted to large and middle-size arteries. There is no such thing as a homogeneous endothelium, since an extensive endothelial heterogeneity is the rule. In fact, there is high diversity and regional specificity of the endothelial cells at different sites in the vasculature and the specific endothelial phenotype depends strongly of the related parenchymal cell type (neurons, myocytes, nephrons, etc.).6
The heterogeneity of endothelium may include: 1) structural organization; 2) biochemical composition and content of specific receptors; 3) functional and pharmacological properties; 4) differential developmental ages related to cellular renewal and 5) properties related to specific location at the cardiovascular system and given vascular bed.
Variations in the structure of capillary endothelium allowed its morphologic classification into: continuous, fenestrated and discontinuous. The continuous type characterized by occluding tight junctions, is the most prevalent and is found in arterioles, capillaries and venules of skeletal, smooth and cardiac muscle, mesentery, skin, connective tissue, lung, brain, eye and conduit vessels. Fenestrated (small windows from 50 to 80 nm in diameter) endothelium is found in vessels of secretory and excretory organs (endocrine and exocrine glands), gastric mucosa, at kidney glomerular and peritubular capillaries, synovia and choroid plexus, where some of these capillaries possess membranous diaphragm-like structures. Discontinuous endothelium is a special form of fenestrated endothelium which is found in sinusoids of liver, spleen and bone marrow. In this type the membranous diaphragm-like structures do not exist.7
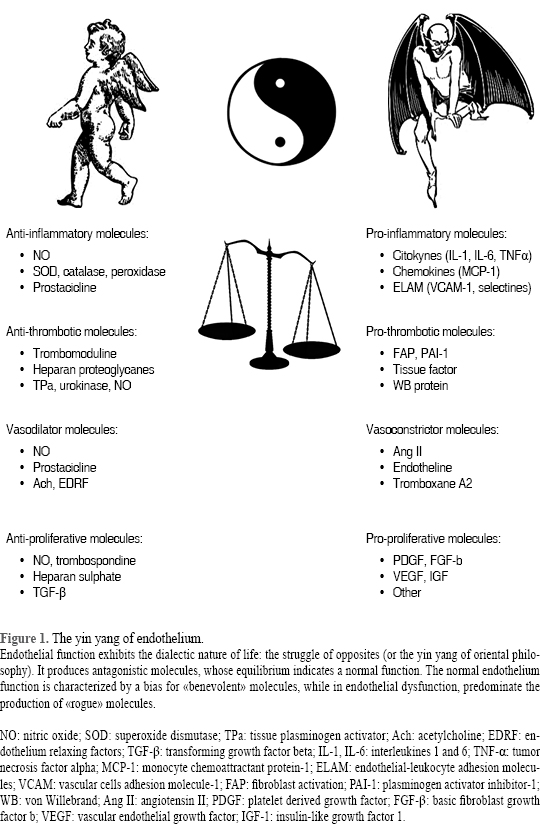
From the functional point of view, arterial endothelium is extremely dynamic and performs many vital functions that vary from one segment of the arterial tree to another, as well as, from one organ system to another. The vascular endothelium releases molecules that act in autocrine and/or paracrine manner that help to regulate the function and health of the vascular network including; the maintenance of blood in a fluid state; exchange of fluid and molecules between the blood and surrounding tissues; creation of new vascular networks; participation and facilitation of the immune response; and the control of vascular resistance in response to changes in blood flow by regulating arterial tone in resistance arteries. Endothelial function is a clear-cut example of a dialectic diagram, in which the action of different molecules playing opposite roles, yield to a dynamic equilibrium. A healthy vascular endothelium has a tightly regulated balance between pro- and antioxidants, vasodilators and vasoconstrictors, pro- and anti-inflammatory molecules, pro and anti-proliferative factors, and pro- and anti-thrombotic signals and substances. Alterations in these balances induced changes leading to a diseased or dysfunctional endothelium that has lost its tightly regulated balances and displays pro-oxidant, vasoconstrictor, pro-inflammatory and pro-thrombotic properties. One hallmark of vascular endothelial dysfunction is the impaired endothelial dependent dilation that strongly depends on its capability to synthesize nitric oxide, which is predictive of future CVD events.8
The production of NO by the endothelium is mediated by the activity of endothelial nitric oxide synthase (eNOS) enzyme which catalyzes NO and L-citrulline production from L-arginine. eNOS is initially located at the plasma membrane associated with a protein called caveolin-1 in the inner surface of the caveolae. After sensing an adequate stimuli (ie, acetylcholine, 5- hydroxytriptamine, substance P, arginine-vasopressin, angiotensin II, histamine, calcitonin gen-related peptide, neuropeptide Y, atrial natriuretic peptide, vasointestinal polypeptide, endothelin-1, thromboxane-2, prostacyclin I2, bradykinin, estradiol interaction with the proper acceptor molecule, and/or changes in shear, gaseous thiols, etc.) it is activated, initiating its coordinated activity.9
The ability of eNOS to generate NO relies on a multi-step activation process. The first stage is calcium dependent; the entry of calcium (Ca2+) and an interaction with calmodulin (CaM) on specific motifs allows eNOS to detach from the membrane and be targeted to the cytoplasm. Then, the association with the heat shock protein 90 (hsp90) promotes eNOS dimerization and protects eNOS against proteosomal degradation. In addition to protecting eNOS, hsp90 also participates in the recruitment of kinase phosphatases involved in further activation of eNOS. The phospho-inositide 3-kinase (PI3K)/Akt pathway can phosphorylate eNOS, mainly on serine-1177 to increase NO production, representing a Ca2+-independent regulatory mechanism for activation of eNOS. A greater activity can be reach by the calcineurin-dependent dephosphorylation of threonine-495, which enhances Ca2+/CaM binding to eNOS. Although Ser-1177 and Thr-495 are the main phosphorylation sites described in the literature, other residues such as Ser-617 and Ser-635 also increase eNOS activity when phosphorylated, while phosphorylation of Ser-116 decreases it. Another pathway involving the protein kinase A (PKA) has also been described to phosphorylate and activate eNOS in response to mechanical stimuli. Moreover, if the stimulus is prolonged, eNOS mRNA can be transcribed and stabilized. This underlines the complexity of eNOS activation when considered in a multifactorial and physiological context, because the eNOS enzyme is activated in different ways in endothelial cells.3,6,9
On the other hand, the endothelium can also generate a series of vascular smooth muscle contracting agents, known collectively as endothelium-derived contracting factors (EDCF), which include endothelin-1, vasoconstricting prostanoids, isoprostanes and reactive oxygen species (ROS) whose production and pathology-associated characteristics will be covered in future reviews.
In summary, a sufficient and efficient production of EDRFs is vital to maintaining relaxation, blood pressure and blood flow. The actions of endothelial cells, however, extend beyond relaxation to include other "prohealthy" activities such as; inhibition of platelet and leukocyte adhesion and cell growth. An assessment of endothelium-derived relaxing action gives an indication of overall endothelial function, which can be extrapolated to indicate overall vascular function.
Reduced bioavailability of EDRFs has been shown to predict overall cardiovascular risk and it is recognized that alterations in endothelial cells functions are central to the development and progression of most types of vascular disorders.
To finish and even when a detailed revision will be presented in the near future, the increased prevalence of obesity deserves special mention as it is closely associated with the rising incidence of cardiovascular diseases and type 2 diabetes mellitus. Diabetes creates an environment adverse to vascular function through a wide variety of metabolic alterations and it is linked to macro- and microvasculopathy. Macrovascular complications include coronary artery disease, stroke and peripheral vascular disease. Microvascular consequences include retinopathy, nephropathy, and peripheral neuropathy, the first two regarded as major causes of blindness and end-stage renal failure. Obesity-related insulin resistance and type 2 diabetes are associated with progression of endothelial impairment.4
Then, endothelial dysfunction is a key event in the pathogenesis of diabetic micro- and macrovasculopathy. The contributing factors underlying impaired endothelial function in diabetes are varied and may include; metabolic abnormalities such as hyperglycemia, excess liberation of free fatty acids (FFA) and insulin resistance.
Although normal insulin signaling provides protection from glucotoxicity in endothelial cells, hyperinsulinemia further exacerbates hyperglycemia induced endothelial injury. Insulin resistance leads to enhanced FFA production, which inhibits insulin signaling and accelerates vascular insulin resistance. Thus, glucotoxicity, lipotoxicity, insulin resistance and a mutual interaction between these factors occur to promote the development and progression of endothelial dysfunction in type 2 diabetes. Conventional therapies to reduce hyperglycemia, dyslipidemia and insulin resistance represent important clinical options to improve endothelial function and delay the progression of vascular complications.
In conclusion; any effort to maintain/improve the correct functionality of the vascular endothelium deserves special attention since this cell type dysfunction is present in the majority of the chronic degenerative diseases that humanity is actually suffering.10
REFERENCIAS
1. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980; 288 (5789): 373-376. [ Links ]
2. Hall JE. Guyton and Hall: medical physiology. 12th ed. USA: Elsevier; 2012. [ Links ]
3. Triggle CR, Samuel SM, Ravishankar S, Marei I, Arunachalam G, Ding H. The endothelium: influencing vascular smooth muscle in many ways. Can J Physiol Pharmacol. 2012; 90 (6): 713-738. [ Links ]
4. Puddu P, Puddu GM, Cravero E, Vizioli L, Muscari A. Relationships among hyperuricemia, endothelial dysfunction and cardiovascular disease: molecular mechanisms and clinical implications. J Cardiol. 2012; 59 (3): 235-242. [ Links ]
5. Bhatnagar P, Wickramasinghe K, Williams J, Rayner M, Townsend N. The epidemiology of cardiovascular disease in the UK 2014. Heart. 2015; pii: heartjnl-2015-307516. http://dx.doi.org/10.1136/heartjnl-2015-307516. [Epub ahead of print] [ Links ]
6. Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998; 91 (10): 3527-3561. [ Links ]
7. Pugsley MK, Tabrizchi R. The vascular system. An overview of structure and function. J Pharmacol Toxicol Methods. 2000; 44 (2): 333-340. [ Links ]
8. Vita JA. Endothelial function and clinical outcome. Heart. 2005; 91 (10): 1278-1289. [ Links ]
9. Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012; 10 (1): 4-18. [ Links ]
10. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014; 2014: 943162. [ Links ]
Nota
Este artículo puede ser consultado en versión completa en: http://www.medigraphic.com/revmexcardiol














