Introduction
In teaching sessions when the following question is raised: “Which air mass is heavier: a dry one or one containing water vapor?”, more than half of the class, give the wrong answer and around 20% guess the right answer. Perhaps based on their own experience, they assumed that a moistened material increases its weight.
In textbooks and manuals, estimating both relative and absolute humidity uses the molecular water-air ratio (ε=0.622); however, obtaining the correct answer using those equations may not be straight forward. In this work, the laws of Avogadro’s, Dalton’s and Amagat’s are used with the aim to simplify the computation of humidity; provide an approach to help to identify the influence of water content in the density and to increase its precision.
The set of equations presented here were compared with the known ones and can be used in teaching this subject to both professionals and the public.
Objectives
To provide a new perspective to students that might assist to infer the effect of humidity in estimating the moist air density; and to present a set of equivalent equations that can be employed in teaching this topic.
Method
In a gas mixture, the physical properties of each constituent contribute to the bulk composition. For instance, according to Dalton’s law, the partial pressure of each compound contributes to the total pressure of the mixture. Based on that, the dry average molecular weight (Md) of a gas mixture of n compounds can be obtained from:
Where M i and y i are the molecular weight and the mol fraction of the constituent i respectively. Moreover, the mole fraction can be obtained from the ratio of the compound with respect to the mixture:
Where: Pi, Vi and ni are the pressure, volume and moles of compound i, respectively; and Pt, Vt and nt are the total pressure, volume and moles of the gas mixture. Thus, for the mixture we have ∑ y i = 1
As for the case of dry air, the mole fraction of oxygen (O2), nitrogen (N2) and argon (Ar) are yO2=0.209, yN2=0.780 and yAr=0.009 respectively (Wallace & Hobbs, 2006). Using the molecular weight of each one, O2 = 32 g/mol, for N2 = 28 g /mol and for Ar = 40 g /mol, the dry air molecular weight can be obtained using equation 1:
It can be noticed that the molecular weight of dry air (Md) is between the molecular weights of oxygen and argon. Since nitrogen is more abundant, the average molecular weight Md is close to it rather than to the other air components.
From the principle of Avogadro, the wet fraction (f H) can be defined as the ratio of water molecules and wet air molecules. In addition, we can also use the ratio of water vapor volume and humid air volume, and the ratio of the partial pressure of water vapor and the total pressure:
Where f H is the wet fraction, nH2O, VH2O PH2O are the moles of water vapor, volume and pressure respectively and nair, Vair, Pair - are the dry air moles number, volume and pressure respectively. The values of f H ranges from 0 for dry air and 1 value for pure water vapor.
Using a similar approach, the molecular weight of wet air (Ma), which is a mixture of water (Mw) and dry air (Md) molecular weights can be estimated as follows:
Where Mw is the water molecular weight of water (18 g/gmol) and Md is the dry air molecular weight of dry air (28.9 g / gmol).
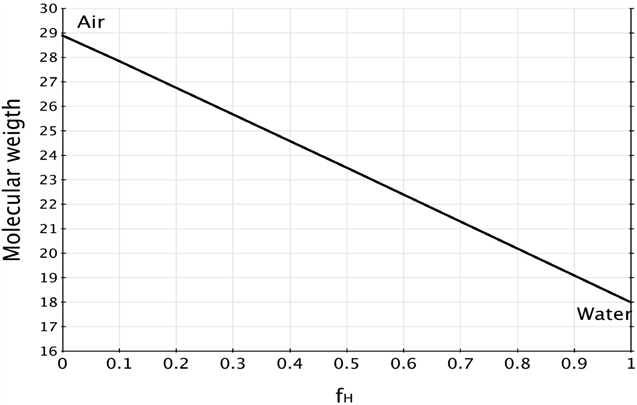
We define an air parcel as an air volume that has a specific pressure, temperature and fixed composition conditions. Using equation (4) it is possible to calculate and plot the values of Ma for dry air (f H =0) and for water vapor (f H =1) Figure 1 presents the possible Ma values. It is observed that when water vapor content increases, the air parcel molecular weight goes from the molecular weight of the dry air (Md = 29 g/mol) to the molecular weight of the water (Mw = 18 g/mol).
Nevertheless, as environmental conditions, the general equation of the gases is satisfied and the gas density can be obtained with the following relation:
Where P-pressure (atm), V- volume (L), M - Mass of the compound (g) molecular weight (g / mol), R gas constant (L atm K-1 mol-1) and T temperature (K).
Density (ρ) can be obtained from Equation 5:
Where ρ is the gas density (g/L). From the equation 6 it can be observed that the density of a gaseous mixture with the same pressure and temperature conditions is directly proportional to the gas molecular weight. Therefore, in an air parcel that contains water vapor, its density will be lower than that of dry air at the same conditions of T and P. Hence, wet air is lighter than dry air.
Absolute humidity
Absolute humidity is the concentration of water vapor in the air. It refers to the mass of water vapor in a unit of air volume (Stull, 2015), and corresponds to the partial density:
Using equation 6 in 7 the following expression is obtained:
From the above equation, you can identify the partial pressure of the water is Pw = fHP.
Specific humidity
The specific humidity (q) is the ratio of water vapor mass with respect to the total mass of humid air (Jacobson, 1999):
Substituting water (eq. 8) and wet air (using eq. 4 in eq. 6) densities in eq. 9 the following relation is obtained:
Equation 10 shows an explicit relation between dry and water molecular weights in the specific humidity estimation.
Mass Mixing Ratio
The mass mixing ratio (r) is the water vapor mass (m) with respect to the dry air mass (Stull, 2015):
Substituting equation 6 for dry air and water vapor in equation 11, and simplifying, is obtained:
Comparison between equations
The following equations are commonly used in text books (Jacobson, 1999; Perry et al., 1997; Stull, 2015) for computing different quantities using the ratio water-air molecular weight (ε=0.622) in the case of the mass mixing ratio the equation used is:
Where Pw is water vapor partial pressure, Pd is the dry air partial pressure.
The specific humidity is calculated by:
And the moist air molecular weight:
Using the known equations can have an error with respect to the proposed equations of up to 0.21%. For r and q, using ambient water content Pw=10 hPa, Pd=1013 hPa this is mainly because ε has only 3 significant figures even though equations 10 and 14 are equivalent algebraically (see appendix for demonstration). In atmospheric sciences the water content varies from 0 to 5% (Wallace & Hobbs, 2006), however in stack emissions the moist content varies from 10% (in boilers) to 100% (reactor exhaust).
Results
Equations 4, 10 and 12 can be used to provide additional insight in the calculations of different properties of the moist air since they show the molecular weight of water explicit and have more precision (Table 1).
Discussion
We commonly observe that for materials that are moistened with liquid water, it is possible that the air contained in them can be displaced and therefore a gas is replaced by a liquid making its weight to increase. An example is observed in the wet fabric or in a wet sponge; however, that does not happen in gases since most of the air molecules have a higher molecular weight than water. Thus, by including water vapor in the air, the contribution of the vapor of water makes the density of the wet air mass to decrease. This can be seen in equation 4 and with Figure 1. Moreover, calculating the density of that air mass using equation 6 it can be identified that moisten air has a lower density. In nature this can be found when water vapor rises until it reaches a condensation temperature, starting the formation of clouds.
In our teaching experience, we observe that this set of equations help the students to identify the contribution of water vapor in calculating the molecular weight, density and humidity of the air.
Conclusion
The new procedure presented in this study for estimating humidity has several advantages for students. By using Avogadro’s and general gas’ law, the equations become algebraically equivalent to the traditional equations, but the use of the symbol “ε” is avoided. This new method offers a different perspective for students to understand the properties of moist air and can be used as a complement to the traditional approach in lessons related to this topic. These equations can help students to better understand the concepts of humidity and gas laws, providing a more comprehensive understanding of the topic. Overall, this fresh approach offers a useful and effective tool for educators to teach and explain humidity to students concisely and helping to engage students and enhance their learning experience.











 nueva página del texto (beta)
nueva página del texto (beta)