Introduction
In recent decades, green chemistry (Anastas, P., 2010) has become a necessary topic for the education of future chemists and chemical engineers (Kennedy, S. A., 2016; Barcena, H. 2017; Timmer, B. J. J., 2018; Bouldin, R. M., 2019; Franco Moreno, R. A., 2020 and references cited; Rosales Martínez, A., 2021). In this context, it is commented on how a simple enantiomer of a drug can be more active than a racemic mixture, or even how one enantiomer of a drug can be harmful compared to its other enantiomer, giving as an example the well-known teratogenic effects of the S-enantiomer of thalidomide (Tokunaga, E., 2018). Because of the negative impact on health caused by the S-enantiomer of thalidomide, the pharmaceutical industry began to implement the need to synthesize and test stereochemically pure drugs according to the dictates of green chemistry.
While the concept of green chemistry began to be developed, the scientific community observed how the exchange of a hydrogen atom from a deuterium atom in a compound can modify its chemical, physical, and biological characteristics, and activities (Englander, S.W., 1996 and references cited).
However, the potential and applications of this simple isotopic exchange, unlike the concept of green chemistry, have not been widely introduced as an inherent component of the chemistry curriculum (Gehman, C. A., 2021; Nichols, M. A., 2010).
This manuscript seeks to emphasize deuteration as a valuable tool to introduce in the chemistry curriculum and that can help students and chemistry educators in several ways: (1) introduce the concept of isotope (deuterium) and kinetic isotope effects, (2) describe the main methods of deuterium labelling, and (3) applications of the deuteration.
Concept of deuterium and kinetic isotope effects.
Deuterium, also known as heavy hydrogen, is a stable, nonradioactive isotope of hydrogen described by H. Urey in 1932 (Urey, H. C., 1932). Deuterium can be found on Earth with a natural abundance of 0.015% (Rosman, K. J. R., 1997).
Deuterium compounds can exhibit important kinetic isotope (KIE) effects, that is, the change in the reaction rate of a chemical transformation when one of the atoms in the reactions is replaced by one of its isotopes. Formally, KIE is the ratio of rate constant for the reaction involving the light isotopically substituted reactant from the rate constant for the reaction involving the heavy isotopically substituted reactant.
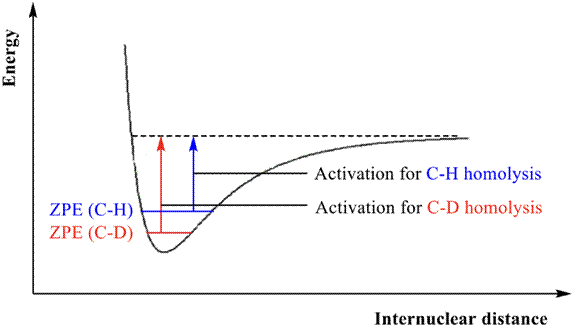
The primary KIE (Altman, L. J., 1978) is attributed to a bond breaking event at the C-H/C-D bond, which in turn depends upon the zero point for vibrational frequency of both isotopic compounds. The C-D bond has a lower vibrational frequency and a lower zero- point energy (ZPE) (Figure 1). This implies a greater energetic input needed to reach the transition state, and consequently a slower reaction rate.
The secondary KIE (Hansen, P.E., 2015) is the effect assigned to rehybridization or arises from isotopic substitution that is remote from the bonds undergoing reaction. The secondary KIE tends to be much smaller than the primary KIE.
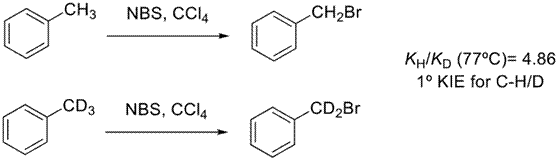
An illustrative example of the primary KIE is the halogenation of toluene using N-bromosuccinimide (NBS) as the brominating agent (Wilberg, K. B., 1958) (Figure 2). In this case a benzylic hydrogen undergoes radical substitution and was found that PhCH3 brominates 4.86 faster than PhCD3.
Principal methods of deuterium labeling
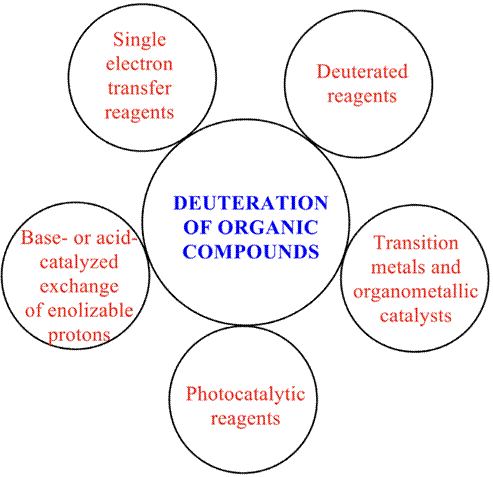
To develop efficient methodologies for the deuteration of organic compounds, several modern reactions have been reported (Atzrodt, J. 2007) (Figure 3). Traditional reactions for deuterium incorporation were the base-or acid-catalyzed exchange of enolizable protons for deuteration. However, this procedure is not valid for the incorporation of deuterium at positions not enolizable. Also, to obtain high isotopic purities, multiple treatments of the enolizable compound with deuterium oxide are required (Murray, A. 1958). Another important tool for the deuteration of molecules was the reduction of functional groups using deuterated reagents (Nagaoka, M., 1999). However, the highly flammable nature of these reagents and their high cost constitute a significant disadvantage. Then, the use of transition metals and organometallic catalysts represented attractive procedures for the deuteration of mainly organic compounds. Therefore, palladium metal and heavy water are used for H/D exchange in benzylic and aliphatic C-H bonds (Sajiki, H., 2005). On the other hand, palladium complexes are used for ortho-selective deuteration of arenes (Ma, S., 2014). Ruthenium complexes are useful for α-deuteration of amines and alcohols (Takahashi, M., 2005), and iridium complexes can catalyze H/D exchanged vinyl groups, arenes, and cyclic alkenes (Yung, C., 2004; Skaddan, M. B., 2004; Zhou, J., 2008). More recently, single electron transfer systems such as SmI2/D2O (Szostak, M., 2014), Cp2TiIIICl/D2O (Rosales Martínez, A., 2016; Rosales Martínez, A., 2021) and titanocene(III) (Henriques, D. S. G., 2021) have been postulated as excellent systems for the deuteration of organic compounds. The first of them mediated the chemoselective synthesis of α, α-dideuterio alcohols from carboxylic acid, and the Cp2TiCl complex is a cheap and sustainable green reagent that mediates the deuteration of organic compounds from epoxides, activated halides, alkenes, alkynes, carbonyl compounds, and ozonides (Rosales Martínez, A., 2016; Rosales Martínez, A., 2017, Rosales Martínez, A., 2021). Chiral titanocene(III) catalysts (Gansäuer, A., 2006) represent promising reagents for the enantioselective deuteration of organic compounds. Finally, due to interest in green chemistry, different reactions involving both UV and visible light have been described (Dong, Y., 2019; Liu, C., 2018; Zhou, R., 2019; Loh, Y. Y., 2017; Amin, H. I. M., 2019; Shi, S., 2020). These procedures benefit from conditions that do not require strong bases or acids.
Applications of deuteration
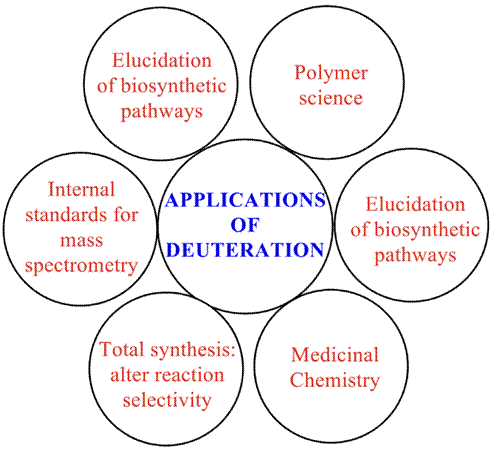
Multiple applications of deuteration of organic compounds have been reported (Figure 4).
In the context of organic and analytical chemistry, deuterated compounds are widely used in the study of reaction mechanism, the elucidation of biosynthetic pathways, the use of internal standards for mass spectrometry, modification of reaction selectivity in total synthesis, and in the pharmaceutical industry. Therefore, in reactions mechanism and metabolic pathways, deuterium (De Feyter, H. M., 2021 and references cited) can be distinguished from ordinary hydrogen easily by its mass, using infrared spectrometry or mass spectrometry. Deuteration can be detected by infrared spectroscopy, since the mass difference affects the energy of the vibrations of molecules, being the vibration of the deuterium-carbon bond in spectral regions free of other signals. An illustrative example of this can be observed in the article reported by MacCarthy (McCarthy, P., 1985), where infrared spectra of deuterated compounds are illustrated. So, for 2-hydroxybenzyl alcohol the two O-H stretching bands at 3420 cm-1 and 3140 cm-1 in the hydrogen form of the alcohol have disappeared completely from the deuterated alcohol and the corresponding O-D stretching bands appeared at 2550 cm-1 and 2350 cm-1, respectively. These differences are caused because the heavier atoms will cause attached bonds to absorb at lower frequencies.
Deuterated internal standards (Grocholska, P., 2021) for mass spectrometry are widely used to correct for sample preparation variations and to compensate for variability in signal intensity due to ion-suppression caused by matrix components that can influence the efficiency of ionization.
In the field of polymer science (Li, L., 2021) deuteration has enabled polymer scientists to address several questions. Considering that the scattering lengths for hydrogen isotopes (deuterium and protium) result in differences in the scattering length densities for deuterated and protiated materials, neutron scattering as an analytical technique is an excellent tool for the study of polymeric structures. Also, in polymer science, deuteration is an important design factor in polymers. For example, in optoelectronic organic materials, the exchange of hydrogen for deuterium allows for more stable active layer materials because the C-D bond is stronger than the C-H bond. The main drawback in the production of polymeric materials lies in the cost and availability of starting materials (monomers) and the choice of synthetic route.
Finally, in the pharmaceutical field, although scientists started to incorporate deuterium into drugs in the 1960s (Belleau, B., 1961), it has been in the last decade this effort has undergone significant development (Pirali, T., 2019). Chemists introduce deuterium at strategic positions in the drugs to obtain deuterated drugs where the deuterium-carbon bonds are stronger than the hydrogen-carbon bonds. These tweaked molecules can better withstand increasing the drug´s half-life. However, deuterium does not only have positive effects on the drug. In some instances, deuterated compounds can behave in an unpredictable way. The generation of toxic metabolites as opposed to harmless ones is the principal inconvenient, being a necessary exhaustive investigation to predict how metabolic enzymes will process the drug. Another concern is the cost of incorporation of deuterium since many reactions require noble metals or deuterated solvents, both of which increase the cost of production.
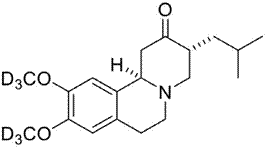
This article is not a comprehensive review of the applications of heavy drugs in medicinal chemistry. However, as an illustrative example, I will comment on the first deuterated drug approved. The first deuterated drug approved by the Food and Drug Administration was Austedo (deutetrabenazine, d6-tetrabenazine, or deuterated benzoquinoline) (Mullard, A., 2016) (Figure 5), from Teva Pharmaceuticals, for the treatment of Huntington´s disease caused by the reduction of serotonin and dopamine (2017). FDA Approves the First Deuterated Drug). The main effect of this deuterated drug is the decrease in metabolic breakdown by 69-87% (Sommer, A., 2016). This allows reducing the dose only twice a day, leading to a decrease in negative side effects such as anxiety, depression, and sleepiness. Pirali, T., 2019 and Kaur, S., 2017 reported an excellent review of other deuterated drugs and candidates.
Conclusions
The deuteration of organic compounds reveal a valuable concept that can be used as didactic material in the teaching of chemical, pharmaceutical, and material sciences as a process capable of showing to students’ important concepts such as isotopic effects, and applications of deuterated molecules. This article is highly adaptable to a second- year introductory organic chemistry students as part of their curriculum. In addition to the theoretical description, an appropriate way to include the concepts mentioned in the curriculum is by carrying a laboratory experiment that allows students to carry out hydrogen-deuterium (H-D) exchange reaction. A useful laboratory experiment to conduct H-D exchange was reported by Jung et al. (Giles, R., 2014).











 nueva página del texto (beta)
nueva página del texto (beta)