The color of transition metal complexes makes Coordination Chemistry one of the most appealing topics addressed in inorganic chemistry courses. The explanation of this property in coordination compounds lies in the so-called d-orbital splitting. This subject becomes even more interesting when the theoretical discussion is accompanied by laboratory experiments that allow students to corroborate the nondegeneracy of d-orbitals due to the coordination of certain ligands to the transition metals.(Warzecha et al., 2019) However, in an introductory course on coordination chemistry, we have noticed that it becomes difficult for students to visualize whether a homoleptic Werner-type coordination compound (i.e. an octahedral or a tetrahedral complex in which all ligands bound to the metal center are identical) has a high- or a lowspin configuration; this problem arises from the fact that d-orbital splitting depends on several factors such as geometry, nature of ligand, oxidation state of the transition metal and the position of the metal in the periodic table (transition series). Moreover, when the coordination number (CN) is 4, it is also complicated for students to determine whether the favored geometry of the complex is tetrahedral or square planar.
Despite the fact that in the literature there is a large number of chemistry textbooks that explain outstandingly well the structure, nomenclature, molecular geometry, isomerism and bonding theories for coordination compounds,(Lawrance, 2009),(Housecroft, Catherine E.; Sharpe, 2005),(Miessler, Gary L.; Fischer, Paul J.; Tarr, 2014),(House, 2008) nowadays there is no tool to systematically determine spin states in octahedral or tetrahedral complexes. To facilitate this learning process, we have designed two flowcharts (Figures 1 and 2) that assist the students to correctly analyze this d-orbital splitting phenomenon. These diagrams have been implemented in our introductory courses of inorganic chemistry and, over six years, we have verified that these flowcharts allow students to acquire the skills to, afterwards, deal with more challenging cases such as coordination compounds bearing non-identical ligands (heteroleptic complexes), or ligands not included in the common spectrochemical series. In our experience, these diagrams have been an excellent tool to assign the high-spin or low-spin configurations in homoleptic Werner-type compounds.
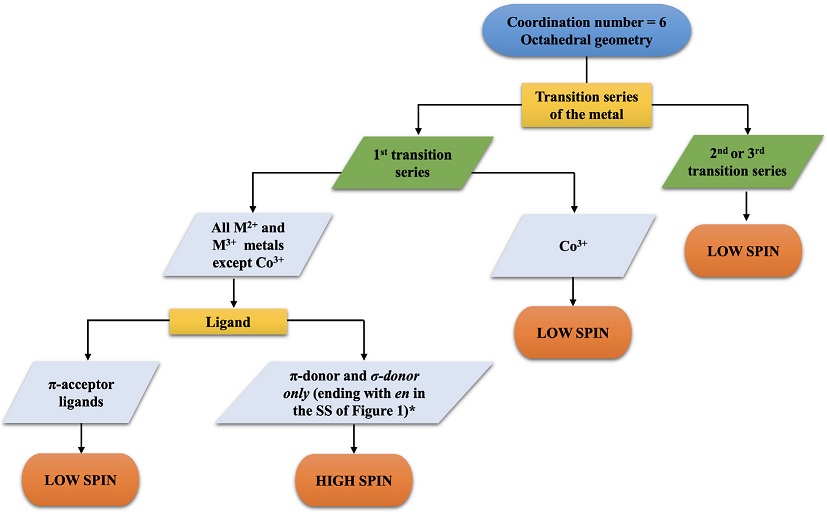
Figure 1 Flowchart of the high- vs. low-spin assignment for octahedral complexes (coordination number = 6). SS = Spectrochemical Series. *Exceptions have been observed: OS = +3 and σ-donor only chelate ligands = Low-spin state.
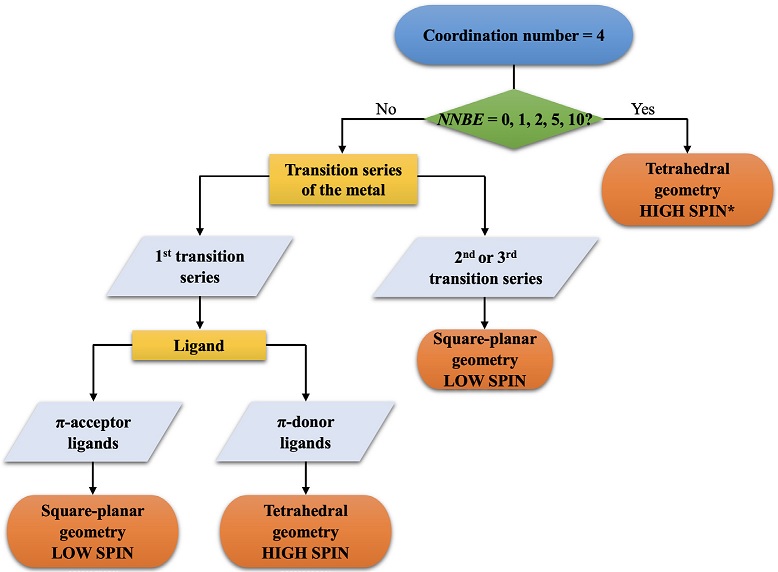
Figure 2 Flowchart of the high- vs. lowspin assignment for complexes with coordination number = 4. *Some exceptions have been observed: OS > +3 and absence of π-donor ligands = Low-spin state.
The description of the flowcharts is presented below, but first it is necessary to define important aspects related to the d-orbital splitting.
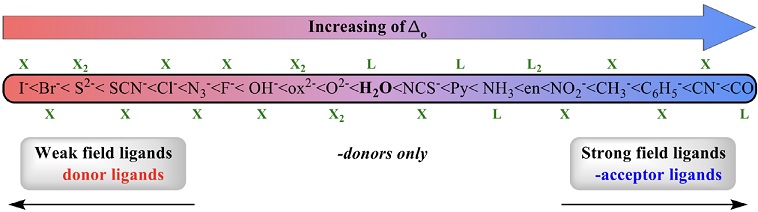
The flowcharts are introduced to students after the Ligand Field Theory (LFT)(Cotton, 1964),(Johnson & Graham, 2015) is discussed. According to this theory, in an octahedral geometry, ligands that generate large energy separation between d-orbitals (i.e., large Δo) are usually π-acceptors, which can perform back-donation especially with electronrich metals. The π-acceptor ligands have low-energy vacant orbitals (π*) where they can favorably receive the electronic density from the metal. Some of these ligands can be easily recognized when the donor atom A presents an unsaturated bond together with another neighboring atom B (for example, M−A=B). On the other hand, ligands that generate a small Δo are commonly π-donors, which are characterized by having at least one free electronpair on the atom directly bound to the metal (for example, M−Ä−B). These ligands can yield additional electronic density to the metallic center by forming a πbond. It is important to emphasize that not all ligands form π-interactions with the metals; for example, ammonia and amines, which are classified as σ-donors only. Some of these different ligands are organized in a spectrochemical series according to their ability to increase or decrease Δo (Figure 3). In our experience, the description of ligands given above is useful for students to classify a ligand that is not included in the spectrochemical series.
It is also convenient to classify the ligands as L- and X-type ligands according to the neutral convention. L-type ligands are molecules that form a covalent-coordinated bond with the metal center; these are neutral species that formally contribute with two electrons, from a lone-pair or an unsaturation (π-bond), to establish the interaction with the transition metal. Examples of L-type ligands are H2O, ROH, RNH2, NH3, CO, alkenes, and alkynes. The X-type ligands are anions that form a covalent bond with the metal, e.g., OH-, RO-, N3 -, CN-, and halide ions. If the anionic ligand forms more than one covalent interaction with the metal center, each interaction should be considered for the classification, for example, the oxo ligand (O2-, M=O) is classified as an X2 ligand. Meanwhile, it is useful to determine four parameters that generally describe a metal complex.(Astruc, 2007) The first parameter is the “Number of Valence Electrons, NVE” (Scheme 1, eq. 1), which indicates the number of total electrons around the metal center within a complex, i.e., valence electrons of the metal in its neutral state plus the electrons provided by the ligands and the electrons provided by coordination sphere charge (q) (one more electron for each negative charge and one less electron for each positive charge). In this method, the X- and L-type ligands yield 1 and 2 electrons, respectively. The second parameter is the “Number of Non-Bonding Electrons, NNBE” (Scheme 1, eq. 2), which determines the number of electrons that the metal has not used to form bonds. This parameter is important because it provides a quick visualization of the expected reactivity of a complex. For example, when a metal in a complex has NNBE = 0, it cannot be oxidized as it has no electrons to donate (nobody can give what they do not have!). In the literature, NNBE parameter is usually represented as d NNBE. The third parameter is the “Oxidation State, OS” (Scheme 1, eq. 3), which represents the hypothetical charge that a metal has if the M-X bonds are completely ionic (which is not true since the M-X bonds in coordination compounds are generally polar covalent); nonetheless, it is a useful parameter as a first idea about the lack or excess of electronic density on the metal. Finally, the fourth parameter is the “Coordination Number, CN” (Scheme 1, eq. 4), which is the number of occupied positions by the ligands around the metal. The main CNs are 2, 3, 4, 5 and 6, and the most common values are 4 and 6. In Table 1, we present two examples for the calculation of these four parameters.
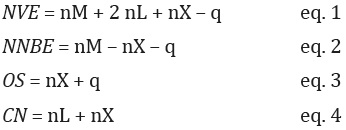
Scheme 1 Equations to obtain NVE, NNBE, OS and CN parameters. (nM = number of valence electrons of the metal in its ground state; nL = number of neutral L-type ligands; nX = number of anionic X-type ligands; q = coordination sphere charge).
Table 1 Determination of the four basic parameters in the description of two selected coordination complexes.
| [Fe(ox)3]3- | [Co(NH3)6]2+ |
| [Fe(X2)3]3- = [FeX6]3-
(ox2- = oxalate is X2-type ligand, meaning that oxalate is a bidentate ligand forming two Fe-O covalent bonds) |
[CoL6]2+ (NH3 is L-type ligand) |
| NVE = 8 + 2(0) + 6 - (-3) = 17 | NVE = 9 + (2)(6) + 0 - (+2) = 19 |
| NNBE = 8 - 6 - (-3) = 5 (d5 complex) | NNBE = 9 - 0 - (+2) = 7 (d7 complex) |
| OS = 6 + (- 3) = 3 | OS = 0 + 2 = 2 |
| CN = 0 + 6 = 6 | CN = 6 + 0 = 6 |
Having defined the NVE, NNBE, OS and CN parameters and the equations for their calculation, we can now review the flowcharts in Figures 1 and 2.
We start with the coordination number of six (CN = 6); the most common geometry in this case is octahedral, so only such geometry is considered. According to the flowchart for a complex with CN = 6 (Figure 1), the considerations to take into account for the assignment of the spin configuration are as follows:
If the metal belongs to the first transition series, then:
If it is Co3+ (d 6), a low-spin complex is formed.(Åkesson et al., 1994) In this exception, a great energy of stabilization of the crystalline field is generated when six electrons of Co3+ are placed in the t 2g sublevel compared to that obtained in a high-spin configuration.
If it is a M2+ or M3+ ion (except for Co3+) bound to π-donor or σ-donor only ligands (ending with ethylendiamine ligand, en, in the spectrochemical series of the Figure 3), a high-spin complex is formed). However, the combination of M3+ with chelate σ-donors only, generates low-spin complexes.(POON & ANDREW, 1979),(Comba, 1994),(Bhowmick et al., 2018),(Machida et al., 1986)a high-spin Ni(II This high field may be due to the combination of a high oxidation state and excellent sigma donor ligands.
If it is a M2+ or M3+ ion (except for Co3+) bound to π-acceptor ligands, a low-spin complex is formed.
If the metal belongs to the second or third series of transition metals, the complex will tend to have a low-spin configuration, regardless of its oxidation state and the nature of the ligands to which it is bound.
For example, for iron, which is a metal from the first transition series, hexaaqua complexes [Fe(H2O)6]2+ (NVE = 18; NNBE = 6; OS = +2; CN = 6) and [Fe(H2O)6]3+ (NVE = 17; NNBE = 5; OS = +3; CN = 6) are high-spin (4 and 5 unpaired electrons, respectively); likewise [FeBr6]3- (NVE = 17; NNBE = 5; OS = +3; CN = 6) is a high-spin complex(Jouini et al., 1983) because bromide is a π-donor ligand. Meanwhile, [Fe(NO2)6]3−, [Fe(CN)6]3- (NVE = 17; NNBE = 5; OS = +3; CN = 6) and [Fe(CN)6]4- (NVE = 18; NNBE = 6; OS = +2; CN = 6) are lowspin complexes(Clark et al., 1954),(Koubek & Elert, 1996) (1, 1 and 0 unpaired electrons, respectively) because NO2 - and CN- are π-acceptors.
With respect to σ-donor only ligands, they tend to form high-spin complexes. For example, [M(NH3)6]2+ (M = Cr, Fe, Mn, Co and Ni)(Redaktion, 2006) as well as [M(en)3]2+ (M = Mn, Co, Ni)(Cortijo et al., 2020) are high-spin complexes. Nevertheless, in the case of M3+ with amine chelate ligands, being excellent σ-donor ligands, they tend to generate low-spin complexes, as is the case of [Fe(en)3]3+. (Renovitch & Baker, 1968) A very illustrative example is found in the laboratory practice proposed by Morrow with 1,8-dinitro-3,6,10,13,16,19hexaazabicyclo-(6,6,6)eicosanelso ligand called dinitrosarcophagine or “dinosaur”;(Burns et al., 2016) this hexamine ligand forms a high-spin complex with Co2+, while the analogous complex with Co3+ is a low-spin complex.
For the second and third series of transition metals, low-spin complexes are usually observed even with π-donor ligands, for example [RuF6]3- (OS = +3),(Allen et al., 1973) or [Ru(H2O)6]3+ (OS = +3).(Bernhard et al., 1984)
The flowchart in Figure 1, facilitates the initial high- or low-spin configuration assignment in homoleptic compounds. However, we have observed that this first step allows the students to develop the ability to assign the spin state in a wide variety of Werner-type complexes. For example, the ethylenediaminetetraacetate (EDTA) ligand is not included in the spectrochemical series of Figure 3. For this hexadentate ligand, would we expect high- or low-spin complexes according to the flowchart in Figure 1? Bearing four acetate groups (π-donor ligands) and two amino groups (σ-donor only ligands) we would expect EDTA to form high-spin complexes with M2+ and M3+ metals of the first transition series. However, in the case of Co(III) we would expect a low-spin complex. For metals of the second and third transition series, we would expect complexes with only low-spin configuration. This assignment agrees well with the reports in the literature. Indeed, Co3+ forms a low-spin complex with EDTA(Douglas & Radanović, 1993) while Mn2+ and Fe3+ (from the first transition series) form high-spin complexes;(Hessler et al., 1994) on the other hand, both Ru2+ and Ru3+ (from the second transition series) form low-spin complexes.(Rocha et al., 2002)
Regarding the coordination number of four (CN = 4), there are several reports in the literature on the factors that influence the geometry and spin state preferred by a compound.(Cirera et al., 2004, 2008; Poli, 1996). For instance, an excellent analysis of tetrahedral geometry for homoleptic complexes was carried out by Alvarez et al. where they proposed the so-called “Magic Cube”. Taking these reports into account, we have extracted the information to generate a flowchart (Figure 2) to facilitate students a correct determination of geometry and spin states. According to the flowchart for a complex with CN = 4, the considerations to consider are as follows:
If NNBE = 0, 1, 2, 5 or 10, the complexes will adopt a tetrahedral geometry. In this geometry, the complexes are high spin regardless of the transition series of the metal, ligands, and oxidation state. However, some exceptions observing tetrahedral low-spin complexes have been found, especially when the metal oxidation state is ≥ +4, with no π-donor ligands and metals mainly from the second or third transition series.(Byrne & Theopold, 1989; Hay-Motherwell et al., 1991),(Jenkins et al., 2002).
If the complexes have NNBE ≠ 0, 1, 2, 5 or 10 and the metal belongs to the first transition series, then:
If the complex has NNBE ≠ 0, 1, 2, 5 or 10 and the metal belongs to the second and third transition series, the square-planar geometry is favored regardless of the ligands.
Although steric effects and chelate ligands have a great influence on geometry, the guide of Figure 2 is a great help for homoleptic coordination compounds. For example, the complexes [MnO4]-, [MnCl4]2- and [Ni(CO)4], which have NNBE of 0, 5, and 10, respectively, exhibit tetrahedral geometry regardless of the oxidation state of the metal (+7, +2 and 0, respectively) or whether the metal is bound to a π-donor (O2- and Cl-) or a π-acceptor ligand (CO).
Another example is found in [NiCl4]2- and [Ni(CN)4]2-.(Manch, 1961) Nickel belongs to the first series of transition metals and, in both compounds, NNBE = 8. Because chlorides are π-donor ligands, [NiCl4]2- has a tetrahedral geometry; on the contrary, cyanides are π-acceptor ligands, so [Ni(CN)4]2- has a square-planar geometry. In contrast, for the [PdCl4]2- and [Pd(CN)4]2- complexes, (Börgel et al., 2016) where palladium is an element of the second series of transition metals, both complexes are square-planar regardless of whether the metal is bound to a π donor (Cl-) or a π-acceptor (CN-) ligand.
Due to the simplicity of these flowcharts, we consider them a simple and practical guide for students in introductory courses on Coordination Chemistry. The use of these diagrams is suggested after reviewing of the concepts of the Ligand Field Theory. Finally, it is important to emphasize that although the use of these diagrams allows the correct analysis of a wide variety of complexes, their use should be handled with caution since there are exceptions (some of them have been commented in this work).











 nueva página del texto (beta)
nueva página del texto (beta)