Introduction
In 2011, the International Union of Pure and Applied Chemistry (IUPAC) published recommendations for the definition and determination of hydrogen bonds (H-bonds or H-bridges) (Arunan et al., 2011b). These recommendations were long overdue. For most of the twentieth century, both instructors and students of introductory chemistry courses have been left uncertain by the many concepts involved in hydrogen bonding, without a clear guide to organize these concepts.
One of the members of the IUPAC task group, Desiraju, recommends the term H-bridge be used to describe this interaction (Desiraju, 2002; Desiraju, 2011). There are several reasons to agree with this recommendation. Both H-bond and H-bridge were in common use until the 1930s (Huggins, 1936), at which point Pauling’s influence tipped the balance toward ‘bond’ (Quane, 1990). Today, the term ‘bridge’ is still extant in Spanish-speaking (puente de hidrógeno) and German-speaking (Wasserstoffbrücke) chemistry courses. ‘Bridges’ are also used frequently for interactions in biological structures (Jeffrey & Saenger, 1991). More importantly, for teaching purposes in English-speaking chemistry courses, the differentiation between ‘bond’, which most students associate with a covalent bond (Niaz, 2001), and the non-covalent ‘bridge’ is important to remove confusion due to polysemy. For these reasons, and to provide a twenty-first century update after 100 years of the first publication on the water dimer (Arunan, 2020; Scheiner, 2020), we will use the term ‘H-bridge’ throughout this article.
Perhaps the most clarifying proposal by IUPAC was the insistence that H-bridges exist between an H-bridge donor made up of X-H, where X is an abbreviation of an atom or group of atoms connected to a hydrogen atom, and an H-bridge acceptor, made up of Y-Z, where Y is the site of interaction with H and Z is an atom or group of atoms (Arunan et al., 2011a). The interaction between X-H and Y-Z forms a new species X-H•••Y-Z, where the three dots indicate the non-covalent interaction. Thus, the formation of an H-bridge requires that X-H and Y-Z (separated in space initially) approach each other to form X-H•••Y-Z; all the parts (X-H, H•••Y, Y-Z) are important to define the H-bridge (Desiraju, 2011).
For the definition of an H-bridge, the IUPAC task group provides several criteria. The main criteria are paraphrased below (Arunan et al., 2011b).
1) H-bridges are formed by inter-species forces, which might contain various components
2) X-H before H-bridge formation is a polar covalent bond, with a polarity indicated by δ- X-H δ+
3) The equilibrium geometry of X-H•••Y forms an angle of ~180°
4) The average bond length X-H increases as the H-bond forms X-H•••Y-Z
These recommendations both broadened the definition of an H-bridge as well as insisting on evidence before claiming its presence. Evidence can be experimental (e.g., Infrared spectra, Nuclear Magnetic Resonance spectra, neutron diffraction crystallography, determination of Gibbs energy of formation) or calculational, or (preferably) both (Arunan et al., 2011b). Importantly, the types of atoms involved in both the H-bridge donor and acceptor have been amplified. “The original examples of hydrogen bonding were found to involve the electronegative atoms F, O, or N. The current IUPAC definition given in the ‘Gold Book’ still specifically mentions these atoms, though it adds a caveat suggesting that the phenomenon is not limited to these atoms…Clearly, it has been realized that X may be any element having electronegativity larger than that of H (F, N, O, C, P, S, Cl, Se, Br, and I) and Y could be any of these elements and also π-electrons.”(Arunan et al., 2011a)
As a result of this new definition, the number of examples of H-bridges in chemistry has increased (Scheiner, 2018). For the introduction of H-bridging in general chemistry, there are more than 40 distinct combinations for X-H•••Y-Z, even after limiting the species to inorganic compounds where Z = H. The presentation and organization of these data in introductory chemistry is the topic of this article; it is directed to the instructors of general chemistry, in order to present better the topic of H-bridges. This article avoids trying to explain theoretically what exactly an H-bridge is (Oliverira, 2015) but rather focuses on how to teach students empirically to recognize and use the concept of H-bridges. This focus starts with the concept of H-bridges on the symbolic level (Ordenes, Arellano, Jara, & Merino, 2014); first, as the shorthand X-H•••Y-Z, and then as Lewis structures organized in different types. Later, the macroscopic and submicroscopic (Ordenes, Arellano, Jara, & Merino, 2014) consequences of H-bridges (physical properties) can be predicted. “Two significant challenges [are] faced by chemistry instructors: i) to graphically represent forces of attraction between molecules and ii) to develop the imagery that in the liquid state, orientation of molecules toward each other because of polarities is transitory.” (Bucat & Mocerino, 2009)
Grids have been shown to be very useful for the organization of inductive and resonance effects for Electrophilic Aromatic Substitution (Lamoureux & Arias-Álvarez, 2020) and Aromatic Acidity and Basicity (Lamoureux & Arias-Álvarez, 2021). The separation of chemistry data in two-dimensions, and the use of color to indicate trends within the data, provides a different perspective than memorization of a list. We show in this article that grids can be used for the introduction to H-bridges. More than 90% (Schultz, 2010) of important examples of the H-bridge interactions for simple compounds can be codified in five grids. Moreover, this format can continue to be expanded as a student advances into organic chemistry and beyond, incorporating more examples of less obvious types of H-bridges.
Examples of hydrogen bridging in second-row elements
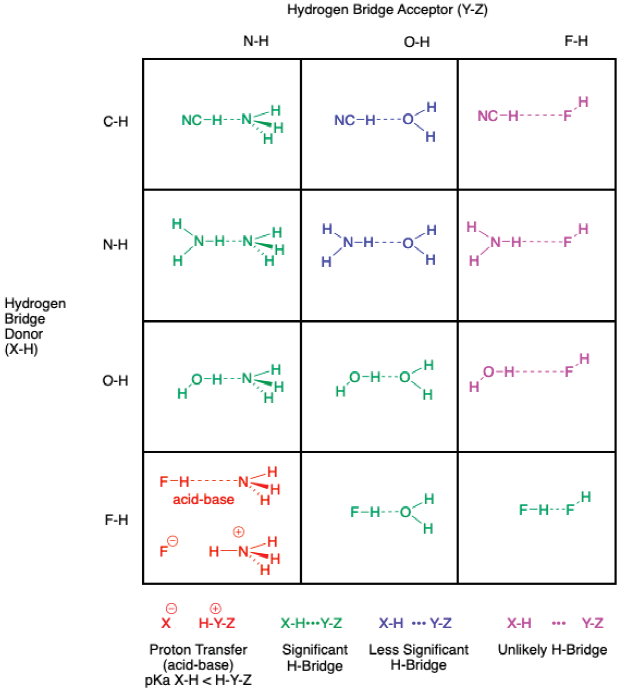
A basic grid is shown in Figure 1, where the x-axis corresponds to the Hydrogen Bridge Acceptor (Y-Z) and the y-axis to the Hydrogen Bridge Donor (X-H). Each box in the grid thus corresponds to an example of a X-H•••Y-Z species. The grid can be expanded by changing the identity of X on the y-axis and the identity of Y on the x-axis, following the row numbers of the main elements in a periodic table (only simple examples will be portrayed).
The first grid (Figure 2) for instructors in introductory chemistry involves the inorganic compounds (Z=H) incorporating the elements C to F for the atom X and N to F for Y. These elements are in the second-row and the series of P to Cl are the third-row elements. The corresponding species X-H•••Y-Z are the most commonly encountered and should be explicitly illustrated with Lewis structures - the H-bridge donor on the left and the H-bridge acceptor on the right - with the H-bridge shown as a dashed line.
In Figure 2, NC-H (hydrogen cyanide or hydrocyanic acid) is included as an example of an inorganic H-bridge donor with a C-H group. It is important to expand the H-donor possibilities beyond X = N, O, F and there is sufficient evidence that NC-H forms H-bridges to include it among known H-bridge donors (Karpfen, 2002). Further discussion of other C-H donors can be delayed until later classes in organic chemistry or biochemistry (Desiraju & Steiner, 2001).
As we were preparing this article, we realized that grids are useful to show that the interactions vary among the different combinations. The types are divided into unlikely, less significant and significant H-bridges, according to the importance for students to identify these types. Other H-bridges (Emsley, 1980) also exist, but examples of these types are best left to advanced chemistry courses. The significance of an H-bridge can be estimated by the acidity of X-H and by the basicity of Y-Z: “The pK a of X-H and pK b of Y-Z in a given solvent correlate strongly with the energy of the hydrogen bond formed between them.” (Arunan et al., 2011b)
The demarcation between types is not exact (Desiraju, 2002), thus the focus of the students should be on the linearity of the H-bridge and the recognition of common and uncommon types. In this article the focus is on the importance of the H-bridges to predict chemistry and not the exact energy: “It is clear that specifying an energy cutoff is arbitrary and does not help in identifying or excluding the possibility of a hydrogen bond being present. Directionality rather than energy is the discriminative attribute for a hydrogen bond.” (Arunan et al., 2011b) To show clearly the difference in types, the length of the dashed line in the structures in the grids has been varied according to the estimated interaction (longer dashed lines for unlikely interactions, not exactly to scale!) and have color-coded the structures into types (Figure 2).
Furthermore, in the presentation of all possible combinations of X-H and Y-H, a student should learn that an H-bridge in some cases is the initial step in the rupture of the covalent X-H bond to form the conjugate acid/base. “Hydrogen bonds are involved in proton-transfer reactions (X-H•••Y → X•••H-Y) and may be considered the partially activated precursors to such reactions.” (Arunan et al., 2011b) Where the pK a of X-H is well below the pK a of the conjugate acid of Y-H, the proton transfer between acids and bases is indicated by a red color and by the structures of the initial H-bridge followed by the ion-separated pair (Figure 2).
Only the simplest formation of dimers is shown in the grid, even though in several cases the conglomerate structure can be more complex. For example, ammonia has three X-H groups and one Y-H group and so can form not only a dimer but also a complex framework of H-bridges (Beu & Buck, 2001). Water has two X-H groups and can form stable dimers, trimers, tetramers, pentamers and hexamers (and perhaps larger) cage structures (Ludwig, 2001). Hydrogen fluoride can form extended chains (McLain, Benmore, Siewenie, Urquidi, & Turner, 2004). These larger cluster are of lesser importance to the first-year student attempting to learn the complexities of chemical concepts, yet there might be some exceptions beyond dimers to show interested students. Obviously, water as a bulk solvent has important implications for chemistry and the understanding of H-bridges formed between a solute and clusters of water as a donor and as an acceptor is the first step. Another possible concept is the formation of hydrates (S•xH2O), where the • indicates the non-covalent interactions - some combination of H-bridges and other interactions - between x number of water molecules and a solute (S). The fact that there is an equilibrium between S•xH2O and S + x H2O - in many cases simply heating gently a hydrate removes the water of hydration - highlights the differences between H-bridges and covalent bonds (e.g. bond strengths, reversibility, transitory orientation, physical versus chemical change).
Another topic that might be introduced is the solubility of non-polar solutes in bulk water, where entropy, not H-bridges, is the deciding factor (Alger, 1994). If a small non-polar solute, such as methane, is dissolved in water, there is the possibility of forming a cluster of organized H-bridging water molecules around the solute even if the solute does not H-bridge; this network is known as a clathrate or clathrate hydrate (Ludwig, 2001).
Some general observations can be made about these 12 distinct boxes shown in Figure 2, which incorporate all the distinct types of H-bridges. First, there are several interactions that appear possible (shown in magenta) yet we consider absent or unlikely to exist. These types occur with F as the H-bridge acceptor in the upper-right corner of the grid. The fluorine atom does have lone pairs to form potential interactions as Y-H, yet the base strength of F in F-H is so weak that we consider possibilities of H-bridges with acids of high pK a dubious. In the case of the F-H•••F-H dimer, there is strong evidence of H-bridge formation, due to the stronger Brønsted acidity of the F-H group as donor (McLain, Benmore, Siewenie, Urquidi, & Turner, 2004). One cannot use the oversimplified rule that states ‘H-F forms H-bridges’; whereas F-H•••OH is significant for hydrofluoric acid in water, for instance, O-H•••FH is not acceptable (Figure 2). The possibility of future experimental evidence for interactions with F-H as an acceptor cannot be ruled out, however it is safe to say that these potential H-bridges would be very weak (Leung, Marshall, Bozzi, Cohen, & Lam, 2011).
Second, among the common H-bridges in the center boxes of the grid, it is important to differentiate between O-H•••NH and N-H•••OH (Figure 3). These are interactions that occur, for example, when ammonia is dissolved in water. The former interaction is stronger than the latter; this difference can be justified by the stronger acidity (X-H) of water over ammonia and the stronger basicity (Y-H) of ammonia over water. Interestingly, the estimated values for N-H•••NH and O-H•••OH H-bridge strengths are intermediate between the O-H•••NH and N-H•••OH extremes (Steiner, 2002); in aqueous ammonia all these interactions should be important (Figure 3). What is not present in aqueous ammonia, however, is the presence of detectable amounts of proton transfer product composed of H4N+ -OH = ammonium hydroxide; more than 30 years after a published complaint, bottles from chemical companies still list this latter substance as an ingredient (Yoke, 1989)!
In the second row of a periodic table, F-H is the strongest acid of the H-bridge donors X-H. In the same row of a periodic table, N-H is the strongest base of the H-bridge acceptors Y-H. In this regard, the second-row elements can be relatively ranked in terms of acidity, X-H (F-H >> HO-H >> H2N-H) and basicity Y-H (NH3 > OH2 >> FH), where >> can be considered to indicate more than 10 pK a units. This knowledge of the order of acidity and basicity guide the student to estimate the H-bridge interactions.
Finally, the lower left-hand side region of the grid showing proton transfer as structures colored red can be understood by the trends in acidity as one passes from right to left in a row of a periodic table. Comparing the values of pKa of the donor (H-F = 3.20) and the conjugate acid of the acceptor (H4N+ = 9.25, all values of pKa and physical properties are from the CRC Handbook (Lide, Ed., 2006)), one can predict the thermodynamic product between HF and NH3 is not the H-bridged structure, but rather the proton transfer species.
Examples of hydrogen bonding in third-row elements
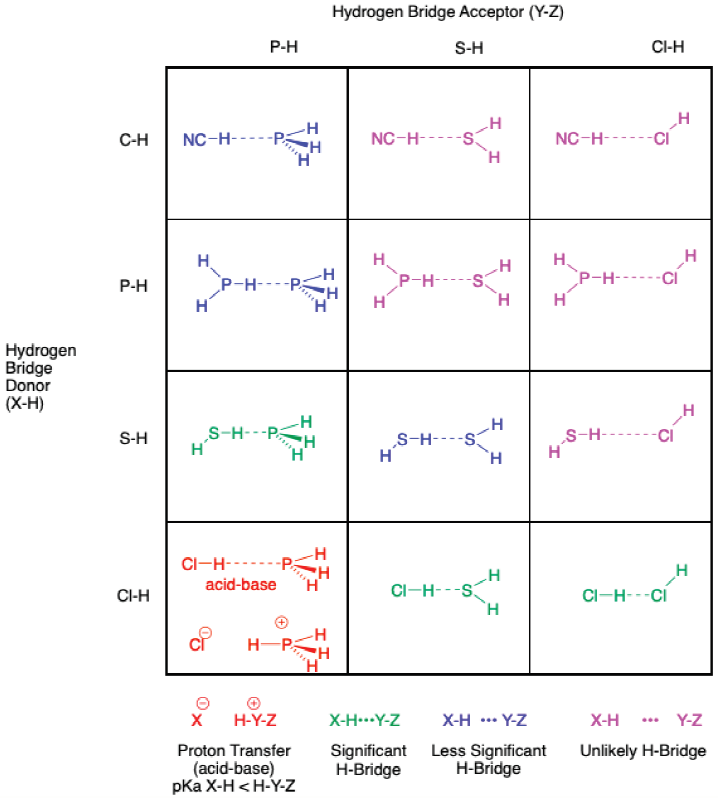
Moving downwards in a periodic table, one expects the same possibility of H-bridges between X-H and Y-H. These interactions can be organized in a grid similarly to the second-row elements (Figure 2) but there are several important differences (Figure 4). The most obvious difference is the propensity of less significant H-bridges, and the paucity of significant H-bridges, in this subset of elements. The color-coding clearly highlights the difference (Figure 4). These trends can be predicted by the competition between acidity and basicity. In terms of acidity of X-H, the lower the position in a column of a periodic table, the stronger the acid (effect of polarizability) and as such, the stronger the expected H-bridge. More influential, however, is the decrease in basicity of Y-H (Y = P, S, Cl) compared to Y-H (Y = N, O, F), which has been ascribed due to the pairing of a soft base (the softness increasing as one moves down a column) with a hard acid (X-H), weakening the putative H-bridge (Jeng & Ault, 1990). The H-bridges are not completely absent among third-row elements, only attenuated relative to the second-row elements (Sennikov, 1994).
Among the elements of the third-row, the order of acidity of X-H is Cl-H >> HS-H >> H2P-H. The values of acidity range over 30 units of pKa (from -6 to 7 to 29, respectively). The order of basicity of Y-H is PH3 > SH2 > ClH, where all three atoms are considered very weak bases. It can be seen in the grid (Figure 4) that H2S covers the entire range of H-bridges: “H2S is a model system in which the hydrogen bond goes from absent or very weak to structurally significant” (Arunan et al., 2011b).
It should be pointed out that we could not find any examples of Si-H as an H-bridge donor (i.e. with the polarity δ- Si-H δ+) so we have substituted C-H as an H-bridge donor in Figure 4. This lack of evidence is not necessarily evidence of the lack of H-bridges in Si-H complexes; in the future the range of H-bridges might increase to include this third-row element.
The general tendencies of the grids (Figures 2 and 4) should be pointed out. The upper-right side (magenta region) contains H-bridges of questionable existence. The lower-left corner (red structures) provides an example of proton transfer. The lower boxes contain the significant H-bridges.
In the case of hydrochloric acid (Cl-H) and the conjugate acid of phosphine (PH3) in the lower-left corner, the difference in pKa values, approximately -6 and -4 (Alyea & Song, 1996), respectively, indicates that the proton transfer is favorable.
How to use grids of H-bridges to predict properties
The importance of identifying, organizing and classifying H-bridges cannot be understated. The presence of an H-bridge has a large effect on the melting point, boiling point, and solubility in water of many compounds. Hence, once the interactions are organized and relatively quantified, a student in general chemistry can make predictions about the bulk properties of simple chemicals.
For example, which compound would one predict to have the higher melting point: H2O or H2S? Although there are several factors (e.g., other non-covalent forces such as dipole-dipole or dipole-induced dipole as well as entropic considerations) in play, the common focus uses the fact the H2O forms stronger H-bridges than H2S. These stronger interactions increase the enthalpy of fusion (ΔHfus). Since the ΔHfus varies directly with melting point (mp), water would be expected to have a higher melting point. This is collaborated by the experimental data: solid water has a mp of 0 °C, whereas for solid H2S the mp is -85 °C.
The same treatment can be applied to the boiling point (bp): which do you predict to be higher, the bp of NH3 or PH3? The same caveat that many factors might be involved should be invoked but, in this case, it is assumed that the enthalpy of vaporization (ΔHvap) increases with stronger H-bridges, augmenting the boiling point. The experimental values - ammonia (-33 °C) versus phosphine (-88 °C) - support this conclusion.
The solubility in water depends on the endothermic rupture of solute-solute interactions, the endothermic rupture of solvent-solvent interactions combined with the exothermic formation of solute-solvent interactions to provide an overall enthalpy of solution (ΔHsolv). Entropic effects during dissolution are probably favorable. Thus, for a solute to be soluble (ΔGsolv overall negative) in water, the exothermic solvent-solute attractions have to be optimized to provide enthalpic assistance. H-bridges between the solute and water are an entirely justifiable way to predict increased solubility. For example, which is more soluble in water, NH3 or PH3? For NH3 (solubility ~310 mg/mL at room temperature) the H-bridges with water, especially O-H•••NH, are predicted to be more significant than the water-PH3 interactions (solubility ~0.3 mg/mL at room temperature).
This latter example is an interesting case of extrapolation of the data presented in the grids; the H-bridges between water (second row element) and phosphine (third row element) are not directly shown in the grids and one must use ‘chemical intuition’ to predict the overall tendency. One way to compare H-bridges between the elements is to estimate whether the interaction increases or decreases on interaction of a third-row element with water (Figure 5). Generally, if a harder H-bridge with water is replaced with a softer element, the strength of the H-bridge donor and acceptor decreases (Rablen, Lockman, & Jorgensen, 1998). Thus, one can roughly estimate the interactions between the third-row elements and water based on the interactions between third-row elements.
When a student can predict properties and can justify their prediction reasonably, this pedagogy using grids has been successful. As Paiva wrote (Gil & Paiva, 2010): “Understanding in chemistry begins when facts and phenomena, observed and organized in a systematic manner and eventually correlated, are interpreted in terms of atoms and their associations.”
Hydrogen bonding of anions
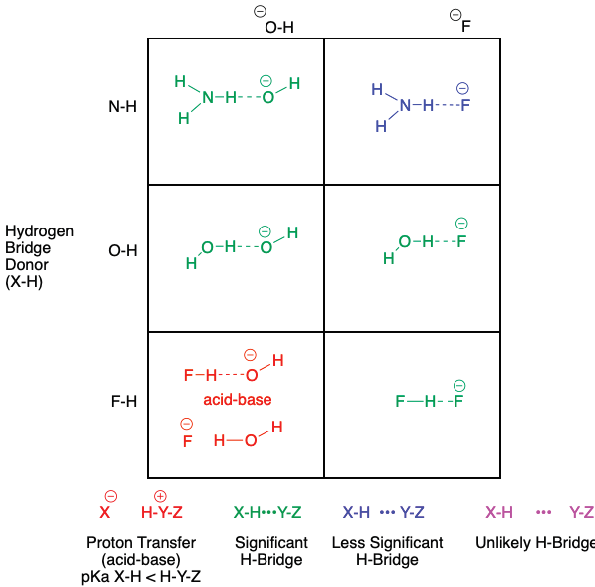
Apart from neutral species, it is known that anions can also be H-bridge acceptors. In fact, all other considerations equal, it is expected that the anionic species forms stronger H-bridges than the neutral species (i.e. -OH forms stronger H-bridges as an acceptor than OH2). As shown in Figure 6, all the combinations of H-bridges exist, with the proton transfer shown in red in the bottom-left corner creating a mismatch. It should be pointed out that the F-H•••F- species (bifluoride) in the bottom-right corner is considered the strongest type of all H-bridge interactions (Arunan et al., 2011b).
Fluoride ion is an especially strong H-bridge acceptor (Brammer, Bruton, & Sherwood, 2001). It is a small, negatively-charged anion with low polarizability and medium basicity, thus providing a strong attraction to H-bridge donors (note that there is no Z group for these anions, only a Y donor). In Figure 6, only the interaction of one donor and one acceptor are shown, yet anions can interact with multiple donors to form complexes. For example, there are three or four water molecules in the case of solvated fluoride (Vincent & Hillier, 2005).
We have limited the options in Figure 6 to anions that exist in aqueous solution; amide anion (-NH2) cannot exist without reaction in water. We have also not included the metal counterions (e.g. Na+, K+) for the anions as this non-covalent interaction with other species would be considered ionic, not H-bridges.
Comparing the second-row anions (Figure 6) with the anions as H-bridge acceptors from the third-row (Figure 7), one can see the same tendencies as seen above. The lower basicity of the third-row members results in weaker H-bridges. The chloride anion is a very weak base yet forms H-bridges with weak or strong acids (Aullón, Bellamy, Brammer, Bruton, & Orpen, 1998). Based on the interaction of water as a H-bridge donor, one could predict the salts of the anions Cl- or HS- to form hydrates and be soluble in water due to H-bridges, which is the case (Vázquez & Sindelar, 2018).
Hydrogen bonding of cations
In Figure 8 is presented the final grid for the most common H-bridges - protonated cations stable in water that act as H-bridge donors. It is expected that the cationic species forms stronger H-bridges than the neutral species (Arunan et al., 2011b), again comparing similar atoms, i.e. +NH4 forms stronger H-bridges as a donor than NH3. It has been estimated that the hydronium ion (H3O+) as a donor forms H-bridges almost double the strength of H-bridges of H2O in bulk water (Markovitch & Agmon, 2007).
As a general rule, one sees that F-H is not a good acceptor for H-bridges and that the strongest acid hydronium (H3O+) is the best donor of H-bridges in this grid (Figure 8). In fact, the hydronium ion is well solvated in aqueous solutions (Reed, 2013) and it should be emphasized that the Hydron (H+) should never be used to oversimplify acid/base aqueous chemistry. The order of acidity is H3O+ > F-H > +NH4; the difference between the pK a of hydronium (0.00) and ammonium ions (9.25) means that the acid-base proton transfer occurs when ammonia is dissolved in acidified water, as shown in red in the lower-left corner.
With the series of grids, one can use the organization of H-bridges to predict a wide variety of properties for both neutral and ionic species. The prediction of melting point, boiling point and solubility based on the structure and knowledge of H-bridges are the most basic aspects of chemistry. Once the simplest species can be identified and organized, the application of grids to organic molecules or biomolecules can be attempted in later courses with more confidence.
Conclusion
Five grids are presented to provide instructors in introductory chemistry organization of the interactions of H-bridge donors and acceptors based on the new IUPAC recommendations. By organizing the representations of H-bridges with Lewis structures in grids, a clearer relationship between the structure of H-bridges and pKa is revealed. The interactions are divided into proton transfer, significant H-bridges, less significant H-bridges and unlikely H-bridges and have color-coded these types to clearly show the difference among types. Third-row elements show a diminution in the ability to form H-bridges, yet these H-bridges can be important and should be highlighted. Prediction of physical properties of these systems is possible once the relative significance of the H-bridges is known. Anions and cations are special cases that also require their own discussion.











 nueva página del texto (beta)
nueva página del texto (beta)