Introduction
Electrophilic aromatic substitution (SEAr or EAS) is undoubtedly an important part of organic chemistry. Many new concepts are introduced during the presentation of this reaction, typically over two or three chapters in a textbook; this article relates only to the concept involving how substituents affect aromatic reactivity. We consider that there are four essential abilities that the students should master at this point before continuing in their program.
1) Use of the terms 'activated' and 'deactivated' to describe the more rapid reaction, or less rapid, respectively, of an electrophile with a substituted aromatic ring in comparison with benzene in an SE Ar reaction.
2) Prediction of activation or deactivation in an SEAr reaction using only the Lewis structure of the substituted aromatic ring substrate. The 'reading' of a structure to infer chemical properties is an important skill for undergraduate students (Graulich & Schween, 2018).
3) Examining any substituent and separating the inductive and resonance effects responsible for the activation or deactivation. Knowing how the substituents affect reactivity is the first step in predicting structure/reactivity relationships.
4) Application of these same effects subsequently in the course (e.g. Diels-Alder, carbonyl chemistry or acid/base chemistry) to predict reactivity that do not involve SEAr mechanisms.
These four abilities contain the basic concepts of resonance, delocalization of electrons, electronegativity, π-systems, electrophiles, and nucleophiles. For students interested in materials science (Stover et al., 1991), organic synthesis (Zanger et al., 1993), organometallic ligands (Palopoli et al., 2019), and pharmaceutical/medicinal chemistry (Dalvie et al., 2010) the knowledge of electronic effects based on substituents is important to relate structure to properties.
How this information is presented to students is, however, still based on textbooks of the past. Most modern textbooks provide the mechanism of SEAr for benzene, and continue with substituted benzene. The substituents are generally presented as a hierarchical list based on their reactivity and regioselectivity, instead of separate categories. Several articles (Ault, 1966) and textbooks (Bruice, 2016) conflate the substituent reactivity with the selectivity (i.e. an activated substituent somehow directs ortho or para), increasing the complexity and encouraging the students to memorize the information. In this article, we present a logical and organized presentation of substituted aromatic systems to clarify the reactivity in an SEAr.
Effect of substituents on reactivity of SEAr
The rate of substitution of a proton on the aromatic ring with an electrophile is directly affected by the substituents on the ring. The textbook reason is that the rate-determining step of most SEAr occurs where the electrophile attacks the π-system to form a Wheland intermediate. To assess a substituted aromatic ring as activated or deactivated depends on the kinetic reactivity in the first step. The rate of the reaction should be dependent on the stability of the Wheland intermediate, yet the absolute rates determined are complicated by several effects (Tomberg et al., 2018). However, the prediction of relative reactivity can usually be correlated with the structure of the substrate: "How do we know whether a substituent functions as a donor or acceptor? In electrophilic aromatic substitution, the answer is simple. Because the attacking species is an electrophile, the more electron rich the arene, the faster the reaction. Conversely, the more electron poor the arene, the slower the reaction. Hence, electron donors activate the ring, whereas electron acceptors deactivate. (Vollhardt & Schore, 2014)"
To attract an electrophile at a rate more rapid than for benzene, increased electron density is required. Only a substituent that donates (releases) electron density greater relative to a hydrogen provides this activated ring, and hence a greater rate. A substituent that relatively withdraws (extracts) electron density to an extent greater than a hydrogen provides a deactivated ring, and hence a lesser rate. One criterion for prediction is the relative donation or withdrawal of electron density in the substrate. The determination whether a ring is activated or deactivated should not, however, be presented as a list to be memorized since it removes the nuanced influences of the substituents (i.e. inductive and resonance effects).
Inductive and resonance effects
The relative donation/withdrawal of electron density has traditionally been presented based a great gathering of experimental data during the twentieth century. We refer, of course, to Hammett's success in determining a constants and the derivation by Swain and Lupton of F and R values (Hansch et al., 1991). These experimental data points are based on the separation of effects according to the 'English School of Chemists' into the inductive (or field) and resonance (or mesomeric) components. Whereas the actual quantitative data for each substituent might transcend the requirements for students of introductory organic chemistry to learn, their relative values are important to predict reactivity. The experimental data determined from investigating the Hammett parameters should not be presented as the explanation of the SEAr reactivity. There is a correlation between the Hammett parameters and the reactivity, but correlation is not causation.
In this article we use the
Based on these inductive or resonance quantitative data, the tendency has been to adapt a Lewis representation of the two main effects (Figure 1). It should be noted that this relative reactivity can be determined by direct observation of the structure of the substrate, with a little inductive reasoning. Generally, substituents that donate to the aromatic ring by induction contain electropositive elements; substituents that withdrawal by induction contain electronegative elements. A third category typically not mentioned is that some substituents connected to the aromatic ring have a neutral inductive effect. Donation by resonance occurs if the substituent contains a π-system (lone pair, electron-rich double or triple bond) directly connected to and co-planar with the π-system of the aromatic ring. Withdrawal by resonance is predictable when a π-system (empty p-orbital, electron-poor double or triple bond) is directly connected to and co-planar with the π-system of the aromatic ring (Figure 1). A third category is a Lewis structure without a π-system or lone pair on the substituent, in which case the resonance should be categorized as neutral.
To compare the kinetic reactivity of benzene with a substituted aromatic ring, one must ask if the substituted ring is more, equally, or less reactive than benzene. The problem arises how to predict the relative reactivity in most common cases. To provide a quantum-chemical basis for the observed reactivity, the molecular electrostatic potential (MEP) is known to become an effective descriptor of the effects of the substituents. The MEP is a colored plot mapped onto isosurfaces of electron density, which can be experimentally measured with X-ray diffraction and easily calculated with modern software. Politzer (Politzer et al., 1984 ; Politzer et al., 1985), and others (Galabov et al., 2013), (Remya & Suresh, 2016), have shown that the MEP of substituted arene substrates have predictive power in SEAr.
The MEP is color-coded to match a probe of attraction to the pi-system: red reflects a large electron density (negative potential) where yellow, green or blue reflects a depleted π-system (positive potential). Although these potentials do not necessarily indicate local changes in the π-electron distribution (Shusterman & Hoistad, 2001), they suffice to provide a visual guide to the ground state (Wheeler, 2013).
We propose using molview.org (Bergwerf, 2015), a free Internet page, so that the students can gain dynamic feedback between the Lewis structures and MEP. Using molview, a student can rapidly draw the Lewis structure and directly compute a MEP surface (Figure 2). A variation in the substituent in the Lewis structure causes a visual variation in the MEP. As a warning, molview indicates "Calculation results can be inaccurate or wrong", since it uses the software Jmol (Herráez & Hanson, 2017) for the rapid (not ab initio) calculations. The purpose is to compare relative surfaces, not to provide highly reliable calculations nor to compare subtle electronic effects, and for these purposes molview is sufficient (e.g. free, rapid, dynamic feedback and allows overlay and rotation of the three-dimensional structure and surface).

Figure 2 Lewis structure and corresponding Molecular Electrostatic Potential (MEP) from molview.org. The color scale related to partial charge shown in all figures is based on a JavaScript from https://chemagic.org
More or less reactive than benzene? Activated or deactivated?
We suggest dividing inductive and resonance components into a grid for each substituent, instead of the single parameter "electron donation" or "electron withdrawal". In this system, the student must decide where to place the substituted aromatic ring in a 3x3 grid. According to such a grid scheme, one can predict the relative rates of reactivity based on the position in the grid. For example (Figure 3), the substituents that donate by induction or resonance (or both), indicated with green horizontal lines in the grid below, are considered to be activated relative to benzene. The substituents that withdraw by induction or resonance (or both), indicated with red diagonal lines, are considered to be deactivated relative to benzene. Those substituents in the upper-left and lower-right hand corners, indicated with a blue cross-hatched pattern, must be evaluated separately as there are competing effects. These positions should show the students that there is not merely one way to organize the groups as activated or deactivated.
We initiate the grid placement with the archetypical aromatic ring: benzene. In this case, hydrogen is the substituent, considered to be the simplest substituent. It is known that the quadrupole moment of benzene provides evidence that the electronic density flows from hydrogen to the carbon (Anslyn & Dougherty, 2006), yet we are interested in the relative difference in electron density among a series of substituted aromatic compounds. In Figure 2, the MEP of benzene shows a large electron density (red) in the ring. Where in the grid should benzene be placed? (Figure 4).
In this case, one can quickly determine that it is convenient to standarize inductive
and resonance effects for hydrogen as a substituent (
What is the case for nitrobenzene, with a nitro group attached to the aromatic ring?
A electron-density withdrawing group such as nitro is readily placed in the upper
right of the grid (Figure 5) as the
quantitative data

Figure 6 MEP generated for nitrobenzene and the corresponding Lewis representations of inductive and resonance effects
Next, for a substituent such as -CCl3, where should we place this group? A
student should view this group as lacking any π-system or lone pairs directly
connected to the ring, and hence might predict CCl3 to have a neutral
resonance effect, but the chlorine atoms provide a strong inductive withdrawal. The
Lewis representation appears in Figure 8. The
best answer would be to place -CCl3 in the middle right square (Figure 7) and to consider it a deactivating
substituent. This prediction would seem to be confirmed with the electronic
parameters
The curious case of alkyl substituents
When one compares the reactivity of an alkylated aromatic ring, for example toluene, to benzene, the answer should be clear. Toluene reacts 17 times as rapidly as benzene in a nitration reaction (Hornback, 2006); other electrophilic additions are also accelerated. The MEP shows subtly greater electron density than for benzene (Figure 9) and also provides evidence that alkyl aromatics are activated.

Figure 9 MEP generated for toluene and the corresponding Lewis representation of various inductive and resonance possibilities
A question arises in a confusing variance in models; how to classify the alkyl group
depends on the source of the classification. Several recent textbooks (Roos & Roos, 2015), (Hornback, 2006), (Chaloner,
2014) consider the Lewis structure as the best guide and classify the
group as an inductive donor only (Figure 10,
A). Based on the quantitative data (for methyl

Figure 10 Various possibilities for the organization of inductive and resonance effects for methyl substituent (and other alkyl substituents): A=donate by induction, neutral by resonance, B=neutral by induction, donate by resonance, C=withdraw by induction, donate by resonance, D=donate by induction and resonance
In a recent article Salvatella(Salvatella, 2017) concluded that alkyl groups are -I +R (i.e. withdraw by induction, donate by resonance) with the caveat that resonance is stronger than induction in this case. This argument is represented in Figure 10, C. Finally, Vollhardt indicates the combination of both effects: "Thus, simple alkyl groups, such as methyl, are donating by virtue of their inductive and hyperconjugating frame." (Vollhardt & Schore, 2014) This combination is shown in Figure 10, D. The possibility of withdrawal by resonance (hyperconjugation) can be ignored as the overlap has little effect on this system (Nguyen et al., 2007).
What are we to make of these various positions, each supported by references in the chemical literature? The first step is to acknowledge their existence and teach students 'multiplistic' or 'relativistic' models (Finster, 1989). The induction/resonance model is useful, yet there might be cases where the model breaks down (such as the alkyl group classification). Until IUPAC provides a consensus model, or a new standard model is proposed that becomes incorporated into the organic textbooks, we should admit the limitations and provide flexibility in these cases. Science should be presented as an evolving process of classification, where multiple viewpoints show that there is much more to understand.
Competing effects
As mentioned in all textbooks of organic chemistry, the two main effects sometimes
run in opposite directions. We can classify the substituents with competing effects
in the lower right quadrant and separate these into three groups (donation >
withdrawal, donation ~ withdrawal and withdrawal > donation). For a methoxy
substituent, there is a strong donation according to resonance coupled to a
withdrawal according to induction. The Lewis representations show these competing
effects (Figure 11). Thus, OMe becomes an
activating substituent

Figure 11 MEP generated for anisole and the corresponding Lewis representation of the inductive and resonance effects
In the category of balanced effects, the most important example to show the students
is fluorobenzene, for which the qualitative data

Figure 12 MEP generated for fluorobenzene and the corresponding Lewis representation of the inductive and resonance effects
Fluorobenzene has been considered to possess almost the same reactivity of benzene,
whereas chlorobenzene is considered deactivated. How should we represent the
differences? The chloro substituent can be dissected into its stronger inductive and
weaker resonance contributions

Figure 13 MEP generated for chlorobenzene and the corresponding Lewis representation of the inductive and resonance effects
As Gould noted "when the I (inductive) and R (resonance) effects of a substituent are in opposite directions we cannot predict, in the absence of further information, whether that substituent will activate or deactivate the ring" (Gould, 1959). To facilitate this prediction, the substituents with competing effects might be classified in extended version of the effects (Figure 14). One might further divide the lower right-hand corner into three subsets: those that donate greater than their withdrawal, those that withdraw greater than their donation and those for which induction and resonance are almost balanced.
Another interesting series comprises the oxygen analogues for which substitution on O
alters the reactivity pattern. Beginning with phenol, one finds that the -OH
substituent strongly activates the aromatic ring. This reactivity is predicted as
the reactivity parameters
However, after a acetylation on the oxygen, the acyl group (Ac) here acts in
competition with the aromatic ring to increase slightly the inductive effect but to
decrease greatly the resonance effect

Figure 16 MEP generated for phenyl acetate and the corresponding Lewis representation of the resonance effects
If one sulfonates the oxygen to form the methanesulfonate group (mesylate, Ms), the
donative resonance effects are further decreased below the withdrawing inductive
effects
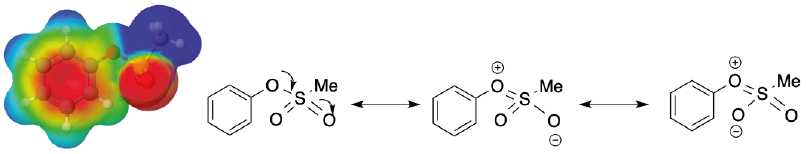
Figure 17 MEP generated for phenyl mesylate and the corresponding Lewis representation of the resonance effects
To place these effects in perspective, we apply the extended grid to arrange the oxygen derivatives (Figure 18). The same trend is predictable for nitrogen derivatives (e.g. -NH2, -NHAc, -NHMs). This fine control of reactivity that depends on the substituents is important in a synthesis. For example, if a phenol is too reactive for a selective SEAr, the oxygen can be derivatized as an ester before another, and more selective, transformation. In a subsequent step, hydrolysis of the ester provides the phenol.

Figure 18 Extended grid showing placement of oxygen derivatives with donation and withdrawal in competition. Ac=acetyl, Ms=methanesulfonyl
Interestingly, the 'chemical space' wherein a substituent withdraws by induction and donates by resonance (i.e. the lower right-hand quadrant of the grid) completely overwhelms the known possibilities in which donation according to induction and withdrawal according to resonance occur (i.e. the upper left-hand quadrant of the grid). We could only find one example of the latter substituent type. Highlighting these rarities might prove useful to motivate students to investigate reactivity on their own to fill the grid completely (Figure 19). Activated substituents (approximately centered in the lower left-hand quadrant of grid) and deactivated substituents (approximately centered in the upper right-hand quadrant of grid) are clearly separated.
By spatially dividing the electronic interactions in two-dimensions (Figure 20), students can practice the four essential abilities listed in the Introduction. This approach may be more challenging compared to traditional and simpler memorization approach but it provides more information and perhaps a better presentation of the concepts.
Bingo-type games to practice the abilities
Presenting the induction and resonance information in a grid organizes the concept; it allows also an opportunity to play games so that the student can master the abilities required.
Games are an important part of learning and have the advantage of portraying learning as fun (Samide & Wilson, 2014), (Tuomisto, 2016). To be educational, the game must have a learning objective (in this case, the abilities of EAS), pre-requisites (basic knowledge of EAS), structure (a 3x3 grid, games last 15-30 min), pedagogy (varied levels of difficulty, thinking visible through discussion) and social activity (working in groups, all students involved) (Tuomisto, 2016).
Several games have been previously invoked to teach concepts in EAS. The orientation ofsubstitution has been predicted with created bull's eyes and "zeroing-in" on the regioisomer (Forbes et al., 2007). Synthesis of multi-substituted benzene can be designed using the "Aromatic Substitution Game" (Zanger et al., 1993). In the laboratory, puzzles have illustrated the experimental results of EAS (McGowens & Silversmith, 1998).
In this case, the 3x3 grid allows facile modification to play games with the students. One variation on the assignment is to provide a blank grid and three cards or stamps that correspond to substituted aromatic rings to a group of 2 to 3 students. The rules are to place the substituents in the proper categories while forming a row (vertical, horizontal or diagonal). It is important that the student play the game with a pen or pencil to write the Lewis structures with the appropriate effects, or with access to a computer to check the MEP. The students should be provided with sufficient time to discuss among themselves and to obtain feedback.
After the structures are placed in the correct positions, the students should be able to predict whether the aromatic rings are activated or deactivated or have the same reactivity as benzene; Figures 21 and 22 show some possibilities. After each game, there should be arranged a discussion to provide further information about the assignments.

Figure 21 Grid showing the placement for three substituents in a vertical row (red = deactivated, green = activated)

Figure 22 Grid showing the placement for three substituents in a diagonal row (red = deactivated, green = activated)
In another variation, provide each student with an empty grid and announce the substituent group on cards or balls (akin to a reverse-BINGO game). The rules are to show substituents in a series to the students, who try to place correctly three substituents in a row (vertical, horizontal or diagonal) and yell BINGO! If a student can explain his or her choice of the effects to the other students, they win the game. For the more daring, a blackout or cover-all game would require the winner to cover the entire grid.
Conclusion
The amount of information involved in the topic of aromatic chemistry can be overwhelming. To practice the abilities required for the section of SEAr (i.e. use of the terms activated and deactivated, prediction of activation or deactivation, explanation of the reactivity, subsequent application of these effects), it is important that the student first organize the information. Using grids, we provide a method for separating the effects (the position in the grid) and the ability to predict the effects for new substrates. This method is an alternative to memorization of a list and emphasizes the useful ability of separation of induction and resonance for the students to learn.
Associated content
Supporting Information
Supporting Information is available: Data for substituted aromatic rings











 nueva página del texto (beta)
nueva página del texto (beta)












