Introduction
In recent years, it is possible to notice lack of interest in students who are learning chemistry, which makes it necessary to improve teaching practices and bring new ideas. In this sense, the innovation of general education is inevitable to adapt it to the better development in the knowledge community.
On the other hand, according to educational policies of the Ministry of Education, Culture, Science and Technology of Argentina, teaching should be designed to develop skills in students (Hanh, 2018). In this way, discourses related to educational competences often aim to promote and encourage students towards an integral development beyond the transmission of knowledge and skills. For this reason, the need to fulfill the application of knowledge, skills and attitudes which are expressed through the Know, the Know-How and the Knowing Self is pointed out (Martinez Rodriguez, Corona, & Ibáñez, 2015). With this in mind, a project to design essences is proposed. The topic "perfumes" is interesting because it's related to sensations and emotions. It is known that the art of perfumes started in ancient Egypt for religious purposes, so Egyptians became familiar with the art of floral extraction. At that time, the priests alone were in charge of perfumery and the greater part of all the aromatics brought into the country was stored in the temples. The use of perfumes for sacred rites was gradually adapted to private use and only the wealthy could afford such luxuries (Van de Pol, 2003). Today, a perfume is considered an essential accessory. So, this topic is presented as a way to enrich the teaching proposals towards the improvement of the students' experiences.
This work was designed relating the purposes of the Ministry of Education, Culture, Science and Technology of Argentina with the curriculum content through a topic of interest to students. The time devoted to the project had a term of fifteen hours: six of them were employed in the reading and analysis of bibliographical material, three hours in experimental work, three hours in the report and three hours in the evaluation. In addition, it represents a possibility to carry out a microenterprise in the near future giving an additional advantage related to the ability to specify a personal project.
According to this idea, the fundamental skills of the field of chemistry were addressed: orality, reading, writing, approach and resolution in problematic situations, critical and creative thinking, and collaborative work. From this perspective, it is understood that the capacities are associated with the social, affective and cognitive processes necessary for the integral formation of the person. In this sense, provide a basis to continue processing, incorporating and producing new knowledge.
In this way, the general objective relates to improving the teaching-learning process in chemistry. On the other hand, the specific objectives were:
Consider the teacher as a guide and facilitator of learning. These should guide students to integrate their knowledge, and think critically and creatively (Sesena & Tarhanb, 2010). In addition, this specific objective should enable the development of students' skills and equip them to analyze and solve multidisciplinary problems in a sustainable manner (Figueiredo, Neves, Gomes, & Vicente, 2016). Another is to arouse interest in students to find creative solutions to problems (Campos & Martínez, 2019). It is also pretended in this objective that students observe chemistry in a specific case: experimental work can implement intellectual, procedural and attitude skills to conduct research, teaching and personal training. This allows process actors to ask the reason to investigate and analyze the processes of daily life. (Karpudewan & Kulandaisamy, 2018). On the other hand, active learning maintains students' interest and focuses, and allows them to improve their ability to solve problems and retain information (Sivarajah, Curci, Johnson, Lam, Lee, & Richardson, 2019). Laboratory activities implement learning through interaction with materials for a better understanding of this curricular subject (Ibrahim Elzagheid, 2018). Finally, science research requires students to learn not only its content, but also the practices that characterize the construction of scientific knowledge (Mortimer & Araújo, 2014).
Stages in the development of the process
The lessons were divided in four stages which are summarized in Table 1.
Table 1 Activities schedule
| Stages | Activities |
|---|---|
| 1 | Read the material and answer the question guide |
| 2 | Perform experimental work in the laboratory |
| 3 | Carry out and hand in of a report |
| 4 | Return of the evaluation carried out by the teacher throughout the process |
Stage 1: The bibliographical material was offered to the students. It includes the following topics: formulation of perfumes, classification of perfumes according to their notes, chemical structure of aromas, essential oils (EO), and the phyto-ingredients. In addition, the extractive processes of EO and the regulation of cosmetics in Argentina were indicated. Students, in groups of two, should read one of the materials cited and answer a question guide. This stage is important to intensify oral skills, reading and writing.
Stage 2: This stage involves an experimental work. Through it, the use of laboratory material is increased. This stage is important to solve problematic situations and to learn to work collaboratively.
Stage 3: This stage is important to intensify writing skills. Students achieve a global understanding by synthesizing the main ideas and using critical thinking to obtain the respective conclusions. On the other hand, the writing of the report allows improving the ability to communicate.
Stage 4: In this stage the evaluation process is carried out.
Stage 1: Bibliographical material
Perfume formulation
The perfume is a mixture of essential oils, alcohol and a fixing agent, and is used to provide a pleasant and lasting aroma to different objects, mainly to the human body. It can be defined as a liquid solution of aromatic components in an appropriate solvent. For its use the surrounding space is sprayed, being perceived by the people who are in the nearby area (Teixeira, Rodríguez, Gomes, Mata, & Rodrigues, 2013). Depending on their origin, perfumes can be classified as natural or synthetic. Plant organisms can synthesize a large number of volatile compounds. While some attract pollinators in the form of floral aromas, others discourage animals that would otherwise feed on them. In addition, many volatile plants have antimicrobial activity and, therefore, can protect their vital parts.
The components of the aromas of the plants tend to be oily in nature at room temperature. The characteristic that originated the name is that they constitute the essence of the aroma. The aroma produced is a complex mixture of several hundred volatile compounds that evaporate in the air (Séquin, 2017).
Classification of perfumes according to notes
Perfumes can also be classified according to the type of note they provide. A note defines the olfactory impression of a single smell. There are different notes, among others floral, citrus, fruit, woody, oriental, spicy, animal, and leather. They are classified into three different types and are responsible for the persistence of the aroma (Chisvert, López-nogueroles, Miralles, & Salvador, 2018).
Type 1: The main notes are the most volatile fragrances, which are perceived right after the application and can last several minutes (Teixeira, Rodríguez, Gomes, Mata, & Rodrigues, 2013). These compounds are polar and soluble in water depending on the physical properties of the system, such as pressure, temperature and chemical potential (Masango, 2005).
Type 2: The average notes are less volatile and, therefore, are perceived with greater force after the main ones fade and therefore last for a few hours.
Type 3: The base notes are the least volatile, they are perceived with greater intensity after the averages have disappeared and last for many hours or even for days (Teixeira, Rodríguez, Gomes, Mata, & Rodrigues, 2013).
Aromas. Chemical structure
The volatile molecules of the EO have similar chemical characteristics despite their structural diversity. They are organic molecules of relatively small sizes (with up to 15 carbon atoms). They are mainly hydrocarbons, non-polar and insoluble or only slightly soluble in water. Therefore, low boiling points and relatively high evaporation rates are observed, particularly at high temperatures (Séquin, 2017).
Among the complex mixtures of compounds that constitute a perfume, fragrances can be classified through different families according to their chemical structure (Chisvert, López-Nogueroles, Miralles, & Salvador, 2018). One of them is terpenes. Terpenes are hydrocarbons that consist of isoprene (2-methylbuta-1,3-diene) units. The isoprene unit is shown in Figure 1 (Hamid, Aiyelaagbe, & Usman, 2011).
A wide range of chemical substances present in plant substrates belong to this family, since they are secondary metabolites whose role is related to the mechanisms of protection, pollination and growth (De Melo, Silvestre, & Silva, 2014).
Simple aliphatic alcohols and aldehydes are part of the floral fragrances that humans perceive as a pleasant smell. Among them, cis-3-hexenol possesses a green grass aroma, on the other hand octanal has the smell of fruit that occurs naturally in citrus EO (Séquin, 2017), in turn, benzaldehyde is known as "bitter almond oil", the geranial (or Citral A) (3,7-dimethyl-2,6-octadienal) has an intense smell of lemon, and it is present in the skin thereof; and vanillin (4-hydroxy-3-methoxybenzaldehyde) is the main responsible for vanilla odor. In addition, in mature fruits, esters can be found, for example ethyl 2-methylbutyrate in apples (Séquin, 2017). These compounds are indicated in Figure 2.

Figure 2 Structural representation of a) cis-3-hexenol, b) benzaldehyde, c) geranial, d) vanillin, e) ethyl 2-methylbutyrate.
The chemical structure of a perfume can be represented as a pyramid. In the upper part of it, are the lightest aromas of rapid evaporation (high notes), which constitute 15-25% of the perfume. Below that, there are the middle notes, which constitute 30-40% of the body of the same and in the lower part are the base notes, which contribute between 45-55% (Teixeira, Rodríguez, Mata, & Rodrigues, 2009).
Essential Oils
Essential oils are also called etheric or volatile oils (Siddique, Rahman, & Hossain, 2012). They correspond to a liquid mixture of volatile compounds, which are obtained from aromatic plants (Amorati, Foti, & Valgimigli, 2013). These complex mixtures are characterized by having two or three main components in relative high concentrations in addition to other components present in this mixtures (Bakkali, Averbeck, Averbeck, & Idaomar, 2008). The EO are composed of a mixture of terpenes, terpenoids (mainly monoterpenes and sesquiterpenes, in the form of hydrocarbons, alcohols, aldehydes, esters, ethers, ketones, oxides and phenols), which determine the characteristic aroma of the plant species (Ali, Al-wabel, Shams, Ahamad, Khan, & Anwar, 2015). They are formed in the chloroplast of the leaf or by hydrolysis of certain glycosides and can be found in different parts of the plant.
Phyto-ingredients and Extractives processes
The phyto-ingredient is established as any vegetable raw material that has been processed conveniently to be included in cosmetic formulations. It can come from fresh or dried plants, secretions, oils, or it can be a product isolated from them by using special methodologies. It is generally heterogeneous in its composition. Some of the phyto-ingredients used in cosmetic formulations are: dyes, extracts, essential oils, aromatic waters, resins, gums and fixed oils (Ferraro, Martino, Bandoni, & Nadinic, 2012).
In obtaining phyto-ingredients, the quality of the vegetable raw material and the extraction process used are very important. The physical state of the extraction must secure the physical and chemical stability of the product in its commercial life.
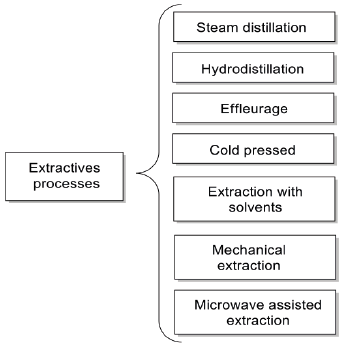
The extractive process is a unitary technique of mass transfer by diffusion of an original phase (plant material) to another that will be part of the extract. Figure 3 summarizes some of the most used extractive process. The Effleurage process is applicable to flowers, for example jasmine, which have low essential oil content and are so delicate that heating would destroy them before releasing the oils. The petals of the flowers are placed in plates of animal or vegetable fat without odor that will absorb the essential oil of the flowers. After a few hours, the fat has absorbed as much EO as possible, the exhausted petals are removed and replaced by new ones. This procedure continues until the fat or oil becomes saturated with the EO. The addition of alcohol helps to separate the essential oil from the fatty substances. Then, the alcohol evaporates through an evaporator, leaving only the EO (Hamid, Aiyelaagbe, & Usman, 2011).
Regulation on cosmetics in Argentina
The provision number 345/2006 defines the products of degree 1 as "Personal hygiene products, cosmetics and perfumes, which are characterized by having basic or elementary properties, whose verification is not necessary initially and do not require detailed information on their use and its use restrictions". This category includes cologne water, perfumed water, perfume and aromatic extract. In turn, it defines grade 2 products as "Personal Hygiene Products, Cosmetics and Perfumes that have specific indications whose characteristics require safety and/or efficacy verification, information, care, use and use restrictions". This category includes the children's colony (ANMAT, 2006).
In the first article of the provision number 1112/99 the list of prohibited substances for use in personal hygiene products, cosmetics and perfumes is indicated. It includes: hydrogen cyanide and its salts, antibiotics, antimony and derivatives, arsenic and derivative compounds, benzene, elemental bromine, cells, tissues and products of human, bovine, ovine or caprine origin, chlorine, chloroform, chromium, chromic acid and its salts, estrogens, narcotics, nicotine and its salts, chlorofluorocarbon propellants, vaccines, toxins, serums and iodine (ANMAT, 2006).
Taking into account the theoretical material detailed above the students had to answer the following questions guide:
Identify the functional groups in the structural representation of Figure 2.
For the compounds mentioned, explain the relationship between the presence and position of the functional groups in the carbon chain and the fragrance of the compounds. Propose an example.
Which are the raw materials used to obtain essential oils?
-
In relation to the formulation of a perfume:
a) Which is the main grade or grade in a greater proportion?
b) Which note is the first to be perceived?
Suppose you have a perfume and cologne of the same brand, which one will last the longest after being placed? Justify.
Explain the characteristics of the "Effleurage" extraction method.
Why the development of perfume should follow a regulation?
Stage 2: Experimental work in a laboratory
The method of Effleurage was implemented to obtain rose's essential oil. It consisted in applying an animal butter to the roses and let stand for two days. So, the essential oil were dispersed through the butter, during two days, acquiring a large part of the fragrant particles of the raw material. The fat that covered the flowers and absorbed their essences was washed with alcohol to remove the absorbed essences. Then, alcohol was evaporated and produced concentrated EO. The rest of the EO were obtained commercially.
Preparation of perfume
The ingredients used in the preparation of the perfume were: cereal alcohol, and different EO (mango and passion fruit, jasmine, coconut, vanilla, roses, sweet lemon, funny orange, cinnamon). For its part, the materials used were: pipettes, beakers, and pro-pipettes.
Before starting with the work in the laboratory, a review of the laboratory materials to be used, their uses and hygiene and safety standards were made. Each student designed and produced their perfume based on the following methodology.
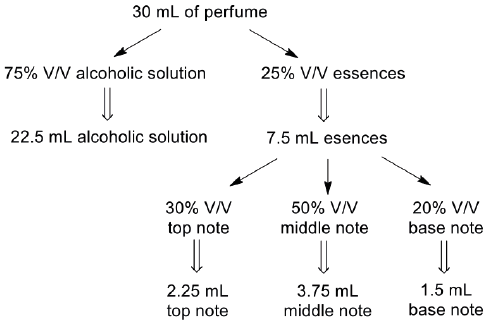
The final volume of each elaborated perfume was 30 mL. It consists of 22.5 mL of cereal alcohol and 7.5 mL of essences. The latter are in the following proportions: 30% V/V high notes (sweet lemon, orange fun), 30% V/V medium notes (coconut vanilla, mango and passion fruit, jasmine, roses) and 20% V/V of base notes (cedar). It is possible to use a combination of top notes. For this, it was necessary to determine the proportion in which each note should be added. The notes must be added in the following order: top, medium and base. It is important not to alter the order. Next, an 80% V/V alcohol solution (prepared with cereal alcohol and water) is added.
Once the perfume has been prepared, it is packaged in 30 mL plastic containers and stored in the refrigerator for two days so that the notes are transformed into a single fragrance. Then, it is stored at room temperature for two months, protected from the light to avoid oxidation reactions. This Storage time is to increase the fixation and durability of the fragrance.
The amounts used in the preparation of the perfume are indicated in Scheme 1.
Stage 3: Report
The writing of the report was necessary to indicate that the experiment was carried out, analyzed and understood. In order to offer the reader a clear and complete idea of the activities and the experiments carried out and the conclusions, it was necessary to organize the ideas and the data.
The report included the following topics:
a) On the first page, the following had to be entered: name of the institution, working title, personal name, teacher's name, date.
b) Objectives: It was considered of a crucial importance to be clear about the objectives of the work.
c) Theoretical framework: It was indicated according to the resolution of the question guide. The search for extra information, writing and coherence was valued.
d) Calculations of the volume of essences: The design of the perfume was taken into account. Here, the students had to be clear about the concept of concentration of solutions and their respective calculations.
e) Experimental procedure: The steps developed for the preparation of the perfume had to be explained. Besides, it was important to detail the laboratory material used, and the health and safety standards considered.
f) Conclusions: The conclusions about the obtained results had to be made explicit. The writing and clarity of ideas were valued.
g) Bibliography: The bibliography consulted had to be detailed according to the APA standard.
Stage 4: Evaluation
Previously to beginning with the project, teacher gave the students the evaluation criteria which are shown in Table 2.
Table 2 Evaluation criteria.
| Evaluation criteria | Student score |
|---|---|
| WORK PERFORMED IN CLASSROOM | |
| Consultation of extra material | |
| Classes attendance | |
| Complete answers | |
| Depth in the answers | |
| WORK PERFORMED IN THE LABORATORY | |
| Work organization and participation | |
| Skill in the use of laboratory equipment | |
| Appliance of security standards | |
| Usage of volumetric transfer techniques | |
| Be cooperative and respectful with classmates | |
| Usage and operation of pipettes | |
| DIGITAL PRESENTATION OF EXPERIMENTAL WORK | |
| Usage of recommended bibliography | |
| Depth in the analysis | |
| Presentation on time | |
| Clarity and precision in its content | |
| Correct writing and orthography |
After the correction, the teacher explained to each student individually the observations made and the assigned score.
Results and Discussion
Stage 1
The students showed interest in the subject. They understood the concepts: essential oils, secondary metabolites, phyto-ingredients and extractive processes. In addition, they returned to bear in mind the ideas: solubility, volatile compounds and boiling point. Besides, they learned the classification of perfumes. On the other hand, through this work, students applied concepts already studied in previous courses, such us solution concentration, health and safety standards.
They were able to identify the organic compound characteristic of the different aromas presented in Figure 2. In this way, they were able to recognize that the structure corresponding to figure 2a represent an alcohol, the one corresponding to Figure 2b, 2c and 2d are an aldehyde and the one corresponding to Figure 2e is an ester.
Respect to the relationship between the functional groups and fragrance, students concluded that functional groups affect the fragrances of compounds. For example, carvacrol, which has an -OH group near to -CH3 smells like oregano, while thymol, which has a -OH group near to -CH(CH)3 smells like thyme.
Besides, students were capable of identify: a) different aromatic plants in their own garden that could be used to extract their essential oil, such us lavender, rosemary, cedar, mint; b) different citrus trees, such us lemon and orange; and c) different plants like jasmine and roses for the same objective.
Related to question 5, they generally responded that the fragrance that will persist for longer is the perfume due to its high content of essential oils (around of 40%), instead, the colony contains less than 5% essential oils and leaves a very light essence on the skin.
The students took into account that there is a regulation on cosmetics in Argentina and the importance of it. So, the production of perfumes must follow the regulations to avoid the use of dangerous or prohibited substances such as benzene, antibiotics, and arsenic, among others, to use appropriate preservatives or dyes and with the objective of standardizing production.
It is noteworthy that most of the reports presented additional information, which indicates a good predisposition on the part of the students to increase their knowledge.
Stage 2
In this stage, students had the opportunity to work in a laboratory. They obtained EO of jasmine by the Effleurage method. The yield in EO was approximately 22% (Equation 1).
Then, based on the theoretical material and available essences, each student developed their perfume. Taking into account the number of participating students, various perfumes were obtained. In Table 3 only eight of the combinations performed are shown.
Table 3 Perfumes realized by eight students
| Student | Top note | Middle note | Base note |
|---|---|---|---|
| 1 | Sweet lemon 2,25 mL | Coconut vanilla 3,75 mL | Cedar 1,5 mL |
| 2 | Orange fun 1 mL Sweet lemon 1,25 mL |
Mango and passion fruit 3,75 mL | Cedar 1,5 mL |
| 3 | Fun orange 2,25 mL | Jasmine 3,75 mL | Cedar 1,5 mL |
| 4 | Fun orange 2,25 mL | Rose 3,75 mL | Cedar 1,5 mL |
| 5 | Sweet lemon 1 mL Fun orange 1,25 mL |
Coconut vanilla 1 mL Mango and passion fruit 2,75 mL |
Cedar 1,5 mL |
| 6 | Sweet lemon 2,25 mL | Mango and passion fruit 3,75 mL | Cedar 1,5 mL |
| 7 | Sweet lemon 0,5 mL Fun orange 1,75 mL |
Jasmine 2,75 mL Rose 1 mL |
Cedar 1,5 mL |
| 8 | Orange fun 0,5 mL Sweet lemon 1,75 mL |
Coconut vanilla 3,75 mL | Cedar 1,5 mL |
Results of the reports presented
The students prepared and handed in the report in the set time. The reports were completed according to the requested content and following the topics proposed. The works were made in digital format and sent by e-mail. The formats were appropriate and creative, some of they included photos of the working process in the laboratory and developed a set of safety rules. Most reports showed a very good and detailed redaction, and included specific vocabulary. They were capable of explaining the calculations which correspond to the volumes of each essence used according to each students' design.
Evaluation
The design of the evaluation instrument could be considered in a positive sense. In this respect, the fact of anticipate the criteria involved in the evaluation process let students be clear about the importance of going from the theory to the practice in the working process. The students were able to communicate with each other and explain their results. This work allowed students to see that the teaching-learning process take a considerable time and requires different instances, and that it is important to know the theory prior to the developing of the experimental work.
Conclusions
Currently, project-based learning is a teaching approach that is increasing in education. The results achieved could show an interesting development of the work in the laboratory and the knowledge achieved by the students involves an improvement in their perception of chemistry. The implementation of this submission is relevant for the teaching-learning process.
The students showed a high level of participation, agreement and motivation. They accepted and improved the suggestions given by the teacher during the preparation of the report.
This project allowed to lead the students to the study of chemistry and could be an opportunity for them to generate microenterprises in the near future. In general, the process is a way of linking theory with practice, promoting integral projects.











 nueva página del texto (beta)
nueva página del texto (beta)