Instructor notes
The leaching experiment was carried out by groups of 15 students, divided into 5 teams. Three laboratory sessions, total of 5 hours, were devoted to the exercise:
I. First session (2 h)
a) Pretreatment of the piece of jewelry
To the approximately 0.40 g of gold jewelry (14 karat), the students add 30 mL of concentrated nitric acid (HNO3) and stir this mixture under hood for approximately 1.5 h at 50 °C.1,2 Once the piece has been reduced to dust and the release of nitrogen dioxide (NO2) is complete, the students stop the stirring.3,4 Subsequently, the solution (Solution A) is decanted, and the remaining solid (Solid A) is washed three times with 10 mL of distilled water.5
b) Preparation of the leaching solution
In a 10 mL volumetric flask, the students add 0.83 g (2.5 mmol) of K3[Fe(CN)6] and 1.17 g of NaCl (20.0 mmol); the salts are dissolved with a pH = 12 solution of NaOH to reach a final volume of 10 mL.
c) Leaching reaction
In a 100-mL flask, the students place Solid A and 10 mL of the leaching solution; the mixture is stirred at room temperature for 24 h (Figure 1a-c). 6
II. Second session (2 h)
d) Isolation of M[Au(CN) 2 ] (M = Na, K)
Students add 40 mL of water to the leaching reaction; then, the students add 0.57 g (3.75 mmol) of FeSO4. Immediately, the resulting solution develops an intense blue color (Figure 1d). Next, students add 5 mL of a NaOH solution of pH = 12;7 the precipitate is centrifuged at 2000 rpm for 5 min (Note: [Au(CN)2]- is in the solution).8 Subsequently, students decant the solution and evaporate the water by heating. 9
e) Isolation of [Ag 2 O]
Optionally, silver can be recovered. For this purpose, 10 mL of a saturated solution of NaCl should be gradually added to Solution A. Immediately, a white solid (AgCl) precipitates; this solid is separated by filtration and washed 3 times with 5 mL of distilled water. Then, 10 mL of a 5 M solution of NaOH is added to the obtained solid, and the mixture is heated for 15 min at 50 °C. Finally, the brown solid (Ag2O) obtained is washed with distilled water.
III. Third session (2 h)
f) Extraction and characterization of [Au(CN)2]-
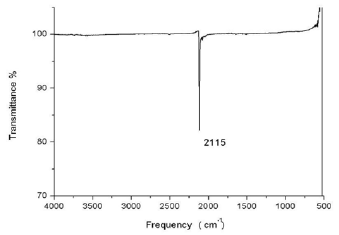
Students extract M[Au(CN)2] (M = Na, K) with anhydrous methanol (3 X 5 mL). Finally, the solvent is removed, leaving M[Au(CN)2] (M = Na, K) as a white solid (Figure 1e). The students characterize it by IR and UV spectroscopy. To obtain a UV spectrum with good resolution, it is necessary to prepare a solution between 0.4 mM to 0.7 mM of the final white solid; a sweep of 200 to 300 nm is made (Figure 2). The white solid is also analyzed in an IR spectrophotometer over a range of 400 to 4000 cm-1 (Figure 3).

Figure 1 Reaction of leaching at a) t = 0, b) t = 10 min, c) t = 24 h, d) Precipitation of KFe[Fe(CN)6] (Turnbull complex), e) M[Au(CN)2] (M = Na, K).
A summary of the procedure is presented in the following diagram:
Diagram of the experiment
According to the Molecular Orbital Theory, in a linear geometry complex, the dz2 orbital interacts with the two ligands along the z-axis (σ interactions); the resulting antibonding molecular orbital, with high contribution from the dz2 orbital, is higher in energy than the original dz2 orbital; the four remaining d orbitals do not interact (Figure 2). Additionally, CN- is a π acceptor ligand, with empty π* orbitals that can interact with gold dxz and dyz orbitals in a π fashion. The resulting bonding molecular orbitals with high contribution from the dxz and dyz orbitals are lower in energy than the original dxz and dyz orbitals. Therefore, the coordination of the two cyanide, generates the doubling of the five d orbitals in three sub-levels, 2πg(dxz, dyz), Δg(dxy,dx2-y2) and 2σg+(dz2), from lowest to highest energy, respectively (Mason, 1973). In the ion [Au(CN)2]-, the five d orbitals are full of electrons (d10 complex); thus, d to d electronic transitions are not possible. However, transitions between electrons in d orbitals of the metal and anti-bonding orbitals of cyanide (π*) are possible. These electronic transitions are known as metal to ligand charge transfers (MLCT) (Mason, 1976). For [Au(CN)2]-, four of these transitions are observed in the region between 200 to 500 nm. These transitions have been assigned to electronic transitions [2sg+®2pu], [2sg+®3pg], [Dg®2pu], and [Dg®3pg]; the students corroborate the identity of [Au(CN)2]- by observing four bands at 240, 230, 211 and 204 nm, respectively (Figure 2).
Recovery of gold contained in a piece of jewelry
Objectives
Carry out the leaching of the gold contained in a piece of damaged jewelry, using the leaching system K3[Fe(CN)6]/NaCl/NaOH.
Characterize by UV and IR spectroscopy, the dicyanoaurate M[Au(CN)2] (M = Na, K) obtained.
Analyze the splitting of the “d” orbitals in a complex of linear symmetry.
Theory
Gold is considered a precious metal because of its high stability against corrosive agents, conductive properties, and unique appearance. It has been used as currency throughout history, in jewelry, and as coating to prevent corrosion of metal surfaces, etc. Gold is found in nature almost exclusively as native gold (Au0) contained in sulfur minerals. The most common methods for extracting gold have involved the use of toxic reagents, for example, forming Hg-Au amalgams where gold oxidation is not required to extract it. Currently, one of the most widely used processes for industrial extraction is “leaching”. This process consists of the oxidation and solubilization of Au0 (s) in water. The two key factors in a leaching process are the oxidizing agent and the complexing agent, whose function is to stabilize the metal in the aqueous medium. For more than a hundred years, sodium cyanide (NaCN) has been considered the best leaching agent of gold and silver at an industrial level. In this system, the oxidizing agent is oxygen in the environment; the diluted solutions of cyanide dissolve gold according to the following equation:
This process is risky for operators due to the formation of hydrocyanic acid (HCN), which is a highly toxic gas that forms at pH <10 and is volatile at 25 °C. The method presented in this lab for gold leaching, uses potassium ferricyanide [Fe(CN)6]3-, which functions as an oxidizing agent and donor of cyanide ions to form the dicyanoaurate [Au(CN)2]- complex according to the following reaction:
The safety benefit of this method is that the only source of cyanide is [Fe(CN)6]3-, which together with other heavy metals such as Ag, Au, Hg and Pt, are able to form a covalent bond sufficiently strong enough to prevent the formation of HCN. The conditions of this system allow the carbon-iron bonds to be selectively broken, thanks to the presence of a strong electrolyte such as NaCl and the use of a basic pH, which facilitates the transport of electrons and the formation of the [Au(CN)2]-.
At the end of leaching, the amount of gold in the solution is significantly lower than the amount of [Fe(CN)6]3- that remains unreacted, which interferes with the characterization of the [Au(CN)2]- complex. This excess is eliminated by adding ferrous sulfate (FeSO4), producing the precipitation of Turnbull blue (ferric ferrocyanide) according to the following reaction:
Due to the electronic configuration of the complex, it is possible to identify it by means of UV and IR spectroscopies.
| Materials | Reagents |
|---|---|
| Volumetric flask (10 mL) | Gold jewelry piece (approximately 0.4 g) |
| Beaker (100 mL) | HNO3 |
| Centrifuge/Tubes for centrifuge | K3[Fe(CN)6] |
| UV and IR spectrophotometer | NaCl |
| Stirring plate | NaOH |
| Magnetic stirrer | FeSO4 |
| Rotary evaporator (optional) | Anhydrous methanol |
Process
a) Pretreatment of the piece of jewelry
To approximately 0.40 g of gold jewelry, add 30 mL of concentrated nitric acid (HNO3); keep this stirring under the hood for approximately 1.5 h. Once the piece has been reduced to dust and the release of nitrogen dioxide (NO2) is complete, stop the stirring. Subsequently, decant the solution (Solution A), and wash the remaining solid (Solid A) three times with 10 mL of distilled water.
b) Preparation of the leaching solution
A 10-mL volumetric flask is charged with 0.83 g (2.5 mmol) of K3[Fe(CN)6] and 1.17 g (20.0 mmol) of NaCl. Finally, add a pH = 12 solution of NaOH to reach a final volume of 10 mL.
c) Lixiviation and Purification
The leaching solution is added to a beaker (100 mL) with the gold solid; keep this stirring for 24 h at room temperature. Afterwards, 40 mL of water and 0.57 g (3.75 mmol) of FeSO4. Keep the reaction under stirring for 5 min, and then add 5 mL of a NaOH solution at pH = 12. The solution is to be divided in equal parts in centrifuge tubes, and the precipitate is compacted by centrifuging at 2000 rpm for 5 min. Decant the remaining solution, which should be colorless, in a beaker, and remove the solvent by heating (optionally a rotary-evaporator can be used). If possible, keep under vacuum for 12 h to ensure the complete removal of water. Extract the [Au(CN)2]- with anhydrous methanol (3 X 5 mL); combine the fractions and evaporate the solvent to dryness to obtain a white solid.
d) Characterization
UV spectroscopy: prepare a solution between 4X10-4 to 7X10-4 mol/L and scan from 200 to 300 nm. Compare the spectrum obtained with the spectrum reported in the reference.
IR spectroscopy: the white solid is analyzed in a range of 400 to 4000 cm-1.
Pre-lab Questionnaire
What is a leaching process?
What advantages does the leaching system used in this experiment offer with respect to the traditional process using NaCN?
What are the toxicity indices of K3[Fe(CN)6] and NaCN?
Calculate the number of valence electrons, the number of non-binding electrons, and the oxidation state of [Au(CN)2]- complex.
Post-lab Questionnaire











 text new page (beta)
text new page (beta)