Introduction
Recent advances in chemistry and specifically the emergence of green chemistry require that students be trained in catalytic organic reactions (Lenardão, Freitag, Dabdoub, Batista, & Silveira, 2003). Microwave heat, a global trend in eco-friendly synthesis, that can be applied in chemistry classes is very efficient and produces excellent results with a short reaction time (Dalmás et al., 2013; Konrath, Piedade, & Eifler-Lima, 2012; Teixeira et al., 2010). Thus, teaching mini-projects that involve more complex organic syntheses (e.g., CC cross-coupling reactions) that are prepared using microwave heat presents a good method for a thorough training in organic chemistry (Aktoudianakis et al., 2008; Soares, Fernandes, Chavarria, & Borges, 2015). Therefore, the use of microwave heat technology in organic synthesis must be included in the training of future professionals.
To provide students with this experience, a three-week experiment (lab period = 3 h/week) is described based on the Suzuki-Miyaura cross-coupling reaction of phenylboronic acid (1) with 4-iodoanisole (2), which is catalyzed by Pd/C, to synthesize 4-methoxybiphenyl (3) via refluxing under air in an adapted domestic microwave oven (Scheme 1; Dalmás et al., 2013).
The primary goal of this experiment is to introduce students to a research environment by familiarizing them with the scientific literature and detailed laboratory procedures. At the conclusion of the three-week mini-project, students should be more comfortable with synthesizing organic compounds via CC cross-coupling reactions, and they should also be able to identify reaction products using current chromatographic techniques (TLC and GC-MS). They also learn to report their results in a journal-ready format.
Experiment
The students work in groups of three. Prior to the initial laboratory period, students read journal articles related to the experiment (Aktoudianakis et al., 2008; Oliveira et al., 2015; Soares et al., 2015). During week one, a quick introduction to the microwave synthesis technique is provided. Students synthesize compound 3 via the Suzuki-Miyaura cross-coupling reaction that is catalyzed by Pd/C. Phenylboronic acid (1.5 mmol), 4-iodoanisole (1.0 mmol), Pd/C 10 wt.% loading (15 mg, 1.4 mol% of Pd), K2CO3 (2.0 mmol), and 8 mL of dimethylformamide (DMF) are refluxed under air in an adapted domestic microwave oven for an assigned time (30, 45, 50, 60, or 90 min). After the reaction time is complete, the mixture is stored under air at room temperature until the next class.
During week two, the retention factors (Rf) of compounds 1, 2 and 3 (provided by professor) and the reaction mixture are determined using thin layer chromatography (TLC). To determine if the cross-coupling product forms, the students perform the experiment to isolate the 4-methoxybiphenyl from initial reaction mixture.
Week three focuses on the characterization of compound 3. The students collect 4-methoxybiphenyl as white crystals and determine its melting range and mass chromatogram using GC-MS. The detailed procedures are in the Supporting Information.
Results and discussion
The mini-project was implemented with 18 students in 2014 in an upper-division undergraduate course, Physical Chemistry II, Course Agroindustrial Engineering with an emphasis on agrochemistry. The course covered the basic techniques of catalysis that are applied in organic synthesis of bioactive compounds. Additionally, the mini-project was tested with 15 students in 2014 of Technical Course in Chemistry, discipline of Organic Chemistry III, in the Dom Feliciano School (Gravataí - RS, Brazil).
The students found it interesting to use an adapted domestic microwave oven to heat the Suzuki-Miyaura reaction mixture. This apparatus has already been used successfully in NC cross-coupling reactions by our group (Dalmás et al., 2013). The yield ranged from 41% to 92% (Table 1), according to the heating time used. The low yields of compound 3 were associated with variables related to purification and the short reaction time. However, even with unsatisfactory results from the reaction, a fruitful discussion concerning the technical errors can occur. The reported melting range of the pure 4-methoxybiphenyl is 81-83.5 °C (Rosa, Rosa, Rominger, Dupont, & Monteiro, 2006). Each student group reported melting points that were within this range (Table 1). Moreover, the students analyzed the mass chromatogram obtained for compound 3 using the GC-MS technique. Representative data from a student group is provided in the Instructor's Notes in Supporting Information.
Table 1 The results obtained for each group during the mini-project.
| Group | Affiliation | Time (min) | Melting range (°C) | Yield (%) |
|---|---|---|---|---|
| 1 | Dom Feliciano | 30 | 74-76 | 61 |
| 2 | FURG | 30 | 81-82 | 41 |
| 3 | Dom Feliciano | 45 | 84-86 | 60 |
| 4 | FURG | 45 | 81-83 | 53 |
| 5 | Dom Feliciano | 50 | 82-85 | 52 |
| 6 | FURG | 50 | 86-88 | 57 |
| 7 | Dom Feliciano | 60 | 81-84 | 74 |
| 8 | FURG | 60 | 86-89 | 79 |
| 9 | FURG | 60 | 81-82 | 83 |
| 10 | Dom Feliciano | 90 | 84-85 | 78 |
| 11 | FURG | 90 | 82-85 | 92 |
a Suzuki-Miyaura cross-coupling conditions: 4-iodoanisole (1.0 mmol), phenylboronic acid (1.5 mmol), Pd/C (15 mg, 1.4 mol% of Pd), K2CO3 (2.0 mmol), DMF (8 mL), refluxed under air in the adapted domestic microwave oven to produce the isolated yields.
The students found it interesting to use an adapted domestic microwave oven to heat the Suzuki-Miyaura reaction mixture. This apparatus has already been used successfully in NC cross-coupling reactions by our group (Dalmás et al., 2013). The yield ranged from 41% to 92% (Table 1), according to the heating time used. The low yields of compound 3 were associated with variables related to purification and the short reaction time. However, even with unsatisfactory results from the reaction, a fruitful discussion concerning the technical errors can occur. The reported melting range of the pure 4-methoxybiphenyl is 81-83.5 °C (Rosa, Rosa, Rominger, Dupont, & Monteiro, 2006). Each student group reported melting points that were within this range (Table 1). Moreover, the students analyzed the mass chromatogram obtained for compound 3 using the GC-MS technique. Representative data from a student group is provided in the Instructor's Notes in Supporting Information.
The longer reaction times resulted in higher yields. Approximately 90 min was necessary to fully convert the reagents to the cross-coupling product (previously tested). Group 1 obtained a higher than expected yield after 30 min. For this time, the conversion is low (∼45%), and we believe the product contains traces of compound 2 (mp 50-53 °C) with 3. There were no major differences in the yield obtained by the students in the technical course (Dom Feliciano) as opposed to those in the undergraduate (FURG) course for the reaction times of 45-60 min. However, the data from the undergraduates were more consistent (see Fig. 1). This shows the flexibility of the mini-project, which can be applied to both levels of education. For 90 min, group 10 likely lost the mass of the isolated product during the procedure.
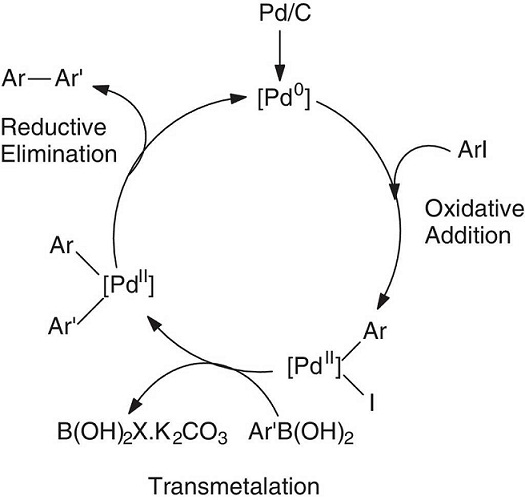
These results were explained using the classic catalytic cycle for the Suzuki-Miyaura cross-coupling reaction (only the FURG students, Fig. 2). The adopted catalytic system is eco-friendly because of the use of microwave heat and phosphine-free conditions, which are global trends in modern organic synthesis. Other Pd sources (commercial salts) can also be tested as catalysts in this CC cross-coupling reaction (Zim, Monteiro, & Dupont, 2000).
Conclusion
This three-week mini-project introduced students to a modern organic synthesis process. This study was designed as a low-cost, practical class that promotes the use of materials and reagents available in laboratories with limited resources. Starting with basic information, students prepared compound 3 via the Suzuki-Miyaura cross-coupling reaction, purified it via crystallization, determined its melting range and obtained a mass chromatogram to confirm its purity. Additionally, the students used a common procedure in modern organic chemistry laboratories. To conclude this mini-project, the students provided an explanation for the phenomenon in a laboratory report (in manuscript format).
The pedagogical objectives proposed for this mini-project were fully achieved. The sequence of the related activities motivated and effectively engaged all students, creating a cooperative spirit between the workgroups. The students participating in similar activities exhibited outstanding performance in the research groups.











 nueva página del texto (beta)
nueva página del texto (beta)