Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Educación química
versión impresa ISSN 0187-893X
Educ. quím vol.26 no.1 Ciudad de México ene. 2015
Para quitarle el polvo
Paul Schützenberger
Jaime Wisniak*
* Department of Chemical Engineering, Ben-Gurion University of the Negev, Beer-Sheva, Israel 84105. Correo electrónico: wisniak@exchange.bgu.ac.il
Fecha de recepción: 19 de julio de 2014.
Fecha de aceptación: 25 de septiembre de 2014.
Abstract
Paul Schützenberger (1829-1897), a French physician and chemist, carried on important research in the areas of inorganic, organic, and physiological chemistry, He established the composition of a large number of alkaloids and dyes of vegetable origin, studied the hydrolysis of proteins and the nature of cellulose, discovering cellulose acetate. Prepared a large number of new compounds by replacing the metal of a salt by an halogen or an electronegative radical; discovered hyposulfurous acid, synthesized many bisulfites, and showed their used for the dosage of dissolved oxygen; discovered the carbonyls of chloroplatinic and phosphoplatinic acids, and established the existence of silicon carbide (carborundum).
Keywords: albuminoids, alkaloids, cellulose, cellulose acetate, chloroplatinic acid, hyposulfurous acid, hyposulfites, phosphoplatinic acid, platinum, proteins, vegetable dyes.
Resumen
Paul Schützenberger (1829-1897), médico y químico francés que llevó a cabo importantes investigaciones en química inorgánica, orgánica, y fisiológica. Estableció la naturaleza y composición de un gran número de alcaloides y colorantes de origen vegetal, estudió la hidrólisis de proteínas y la naturaleza de la celulosa, descubriendo así el acetato de celulosa. Preparó muchísimos nuevos compuestos reemplazando el metal de una sal por un halógeno o un radical electronegativo, descubrió el ácido hiposulfuroso, sintetizó muchos bisulfitos y demostró su uso para dosificar oxígeno disuelto, descubrió los carbonilos de los ácidos cloroplatínico y fosfoplatínico, y estableció la existencia del carburo de silicio (carborundum).
Palabras clave: albuminoides, alcaloides, celulosa, acetato de celulosa, ácido cloroplatínico, ácido hiposulfuroso, hiposulfitos, ácido fosfoplatínico, platino, proteínas, tintes vegetales.
Life and career (Friedel, 1898; Willm, 1889; Urbain, 1928; Battegay, 1929; Davis, 1929)
Paul Schützenberger was born on December 23, 1829, in Strasburg, the son of Georges Frédéric Schützenberger (1779–1859), a former major of the city, who occupied a chair in the Faculty of law. He was part of an old family of the city who distinguished themselves in public, industrial, and artistic activities. One of his uncles, Charles Schützenberger (1809–1881), was professor of chemical medicine of the University of Strasburg, and another, Louis, was proprietor of a large brewery. Paul received his basic education at the Lycée de Strasburg (Collège Royal), and received his diploma of baccalauréat ès lettres (bachelor of letters) in 1847; in 1849 he also passed the examination for the baccalauréat ès science. Influenced by his uncle he begun medical studies at the Faculté de Médecine de Strasburg University of Strasburg and graduated in 1855 after defending a thesis about the bone system under normal and pathological conditions, from the viewpoint of structure and composition (Schützenberger, 1855). During his medical studies he worked for Louis-Victor Amédée Cailliot (1805-1884) as aide préparateur (1850) and then as préparateur en chef (1850).
Schützenberger's academic career was fast, intense, and diversified. In 1853, Jean-François Persoz (1805–1868), professor of chemistry at the Conservatoire des Arts et Métiers in Paris, invited him to be his préparateur for the course in dyeing and printing. A year later he returned to Alsace as chargé de cours in the École Professionnelle de Mulhouse and in 1855 he was appointed professor of chemistry at the École Supérieure des Sciences Appliquées of Mulhouse. While there he found the time to continue his studies; he obtained a licence-ès-sciences physiques (1859) and the agrégation en chimie médicale of the Faculty of Medicine of Strasbourg (1860); all this, while preparing a doctoral thesis for the Faculté des Sciences of Paris. In 1863 he was awarded his doctorat-ès-sciences physiques degree after successfully defending his thesis about the substitution of metals salts by electronegative elements (Schützenberger, 1863a). Immediately thereafter, Schützenberger moved to Paris to work as préparateur for Antoine-Jérôme Balard (1802-1876), professor of mineral chemistry at the Collège de France.
In 1868, Victor Duruy (1811-1894), the French Minister of Public Instruction, created at the Sorbonne the École Pratique des Hautes Études and appointed Henry Sainte-Claire Deville (1818-1881) as its nominal director. Deville promptly chose Schützenberger as assistant director. In 1874 Schützenberger replaced Würtz in the Académie de Médicine, and in 1876, after Balard's death, Schützenberger replaced him at the chair of mineral chemistry in the Collège de France. In 1882 he became directing professor at the municipal École de Physique et de Chimie, which he had organized with the help of Alsatian friends after the model of the school at Mulhouse. He was the first director of the École Supérieure de Physique et de Chimie Industrielles de la ville de Paris, founded in 1882. In 1888 Schützenberger was elected to the chemistry section of the Académie des Sciences, replacing Henri Debray (1827-1888), who had just passed away.
Paul Schützenberger passed away on June 26, 1897, at Mézy, Seine et Oise, after a serious illness.
Schützenberger received many awards and honors for his contributions to science and industry. He was awarded the Grand Médaille d'Or of the Société Industrielle de Lille, the gold medal of the Exposition Universelle of 1867, the Jecker Prize of the Académie des Sciences (1872), the gold medal of the Société Industrielle du Nord de France (1881), and the Grand Médaille d'Honneur of the Société Industrielle de Mulhouse. He was elected member of the Société Chimique de France (1860) and served three times as its president (1871, 1872, 1874); he was member of the Conseil d'Hygiene et de Salubrité de Seine (1877) and of the Académie de Médicine. He was appointed chevalier de la Legion d'Honneur (1868) and promoted to officier in 1885.
The research activities of Schützenberger covered a wide variety of subjects. He worked on pigments and dyes of vegetable origin, the composition of alkaloids, the hydrolysis of proteins, prepared a large number of combinations in which the halogens chlorine, bromine, and fluorine or an electronegative radical, substituted the metal of a salt, prepared many new platinum compounds (carbonyl chloroplatinates and phosphoplatinates), established the existence of silicon carbide (carborundum) and the chemical nature of cellulosic materials, prepared cellulose acetate by the reaction of cellulose with acetic anhydride at 160°C, discovered hydrosulfurous acid and the hydrosulfides and their usage for the dosage of free oxygen, etc.
Scientific contribution
Schützenberger was a very prolific writer, he wrote over 150 papers in a wide range of subjects: alkaloids, albuminoids, cellulose and its derivatives, dyes, electronegative radicals, ferments, hyposulfurous acid and hydrosulfites, medicine, petroleum, phosgene, platinum derivatives, rare earths, etc. etc. He also published several books summarizing his findings in different areas (Schützenberger, 1863b, 1867, 1875ab, 1880-1894, 1882; Schützenberger and Bouduard, 1898). As was customary for candidates to the Académie des Sciences, he published a summary of his experimental projects and results (Schützenberger, 1868, 1888). Here we describe a few of his researches.
Vegetable dyes
The industry of dyeing clothes was a central industrial activity in Mulhouse and this subject was a natural starting activity for Schützenberger's researches. In 1856, he measured the water solubility of the coloring matter of madder (Rubia tinctorum) in the range 100° to 250°C (Plessy and Schützenberger, 1856). Mathieu Plessy and Schützenberger realized madder and its flowers contained the coloring principle mixed with too many impurities to allow a single treatment with water in a closed vessel to give a satisfactory measurement of the solubility. For this reason they decided to use a concentrated extract prepared according to the method described by Jean Gerber and Edmund Dollfus, in which madder flowers were first macerated with boiling wood spirit (methanol), and then filtered. Addition of distilled water to the filtrate produced an abundant yellow precipitate, which could be collected in a filter, washed with distilled water, and then dried, yielding a shinning powder of a bright yellow brown color, having the same dyeing power as 40 times its weight of crude madder.
Plessy and Schützenberger used the dry extract to separate the coloring matter in a crystalline state: 10 grams of the extract were mixed with 100 g of distilled water in a copper tube closed by a screw stopper. The apparatus was placed in an oil-bath and heated for fifteen minutes to 250°C. On cooling, the liquid was entirely filled with crystalline needles of a fine pale red. These crystals were easily separated by decantation from the undissolved excess of extract, The procedure was repeated for 10 times until the coloring matter was exhausted and the water no longer acquired color. The residue was a brown resin, the alcoholic solution of which no longer acquired a violet color with ammonia. The coloring matter was in a state of great purity and was crystallized a second time in water of 250°C, to get rid of the little resin which it might still retain. Its physical properties and analysis showed it to be identical with the sublimed alizarin of Pierre Jean Robiquet (1780-1840) and Jean Jacques Colin (Robiquet and Colin, 1826). Its dyeing power was also identical, and much greater than that of madder (40 times) or its flowers (80 times). The solubility of alizarin in water was found to increase from 0.031-g/100 g of water at 100°C, to 3.16-g/100 g of water at 250°C (Plessy and Schützenberger, 1856).
In another work, Schützenberger and H. Shiffert proved commercial purpurin was actually a mixture of four different coloring substances, each different from alizarin; two red ones which they named purpurin and pseudo purpurin, another colored dark orange red, and a fourth one colored light yellow (xanthopurpurin). The procedure for separating them was based on their relative solubility in distilled benzene and alcohol. Digestion of commercial purpurin with benzene at 50° or 60°C separated xanthopurpurin, an isomer of alizarin. The fraction insoluble in benzene was dried and then extracted at 50°C with alcohol. On cooling, the alcohol extract deposited crystalline lumps of alizarin hydrate. Purpurin and pseudo purpurin were separated using the fact the latter was insoluble in boiling alcohol (Schützenberger and Shiffert, 1864). According to Schützenberger and H. Shiffert, purpurin was little soluble in cold benzene or alcohol, but very soluble in the boiling solvents. It was soluble in ammonia, yielding a beautiful purple red solution. It could be employed to dye when using the same mordants as those for alizarin. Pseudo purpurin was little soluble in cold benzene or alcohol, and a little more soluble in the hot solvent. Its behavior with ammonia and mordants was similar to that of purpurin. The orange red principle was very soluble in alcohol and sparingly soluble in benzene. It dissolved in ammonia yielding a dark red to orange solution. It tinted red orange alumina mordants. The yellow principle was very soluble in benzene and alcohol and yielded a red orange solution with ammonia. It gave a strong and live yellow color to alumina mordants.
In a following publication Schützenberger (1865b) reported the percent composition of the four principles, as follows:
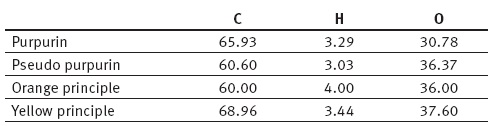
as well as the preparation of ethylpurpurin and purpuramide.
In another memoir, Schützenberger described the results of his experiments about the composition of the coloring matter of cochineal, Coccus cacti (Schützenberger, 1858a). Previous publications had reported this colorant contained a red dye, named carmine, acid in nature, which did not contain nitrogen. Others had isolated carminic acid, an amorphous compound, which treated with nitric acid produced nitrococcusic acid, a nitrated and well-defined yellow product (De la Rue, 1845-48). It was also known that cochineal in contact for some time with aqueous ammonia, changed completely its properties: the red colorant transformed into a violet colored one, which could not be considered an ammonia salt of carminic acid, because the color did not return to red under the influence of acids. In other words, it behaved like a new coloring principle.
Schützenberger decided to carry on additional experiments in order to determine the precise composition of carminic acid. In the first stage he prepared carminic acid by treating an aqueous brew of cochineal with calcium chloride, to separate the colorant material as a calcium lacquer. The dry lacquer was decomposed by an alcoholic solution of oxalic acid and the resulting red solution was filtrated and concentrated by evaporation. This syrup precipitated the carminic acid as clear red crystalline lumps, containing a high percentage of nitrogen, which Schützenberger assumed was a combination of carminic acid with a particular nitrogen derivative, possible tyrosine. The red solution accompanying these lumps was separated by filtration, diluted with water, and precipitated with lead acetate. The precipitate was separated, treated with hot water, and decomposed with sulfuric acid. The final product was an amorphous inodorous red acid tasting solid, which did not contain nitrogen, having all the properties of the carminic acid reported in the literature (De la Rue, 1845-48), and elemental composition 52.5% carbon, 4.16% hydrogen, and 43.34% oxygen (not very different from the actual values, 53.66% carbon, 4.09% hydrogen, and 42.24% oxygen). The acid reacted with aqueous ammonia yielding carminamide (Schützenberger, 1858ab).
Schützenberger reported he had carefully repeated his experiments with other species of cochineal and obtained carminic acid of different composition, particularly the percentage of oxygen. This led him to assume cochineal contained at least two coloring matters, differing in their oxygen content, and estimated their empirical formulas as C18H8O10 (carminic acid) for the less oxidized species, and C18H8O14, for the more oxidized one (oxycarminic acid). Each of these acids reacted with ammonia producing the respective amide (Schützenberger, 1858ab).
Additional work in the area of vegetable dyes included luteolin, the coloring matter present in weld (Reseda luteola) (Schützenberger and Paraf, 1861); catechin, from yellow wood (Schützenberger and Rack, 1865); isatine, by oxidation of indigo (Schützenberger, 1865a); reduction of isatine by hydriodic acid (Schützenberger, 1865c); reduction of indigo by powdered zinc and barium hydroxide (Schützenberger, 1877); the coloring matter from Persian berries, Nerpruns tinctoria (glucosides that are split by sulfuric acid rhamnegins α and β) (Schützenberger, 1868de); etc.
Alkaloids
Between 1858 and 1865 Schützenberger published a series of short memoirs about alkaloids. He reacted quinine sulfate with nascent hydrogen produced by the reaction between zinc and sulfuric acid, treated the solution with ammonia, and separated from it a quinine tetrahydrate. Heating the latter to 140° and 150°C led to elimination of water, with formation of quinine trihydrate and dihydrate, respectively. The dihydrate was a non-crystallizable resinous solid, melting at 100°C, soluble in ether and alcohol; its salts were less soluble than the pertinent ones of quinine. Under similar conditions, cinchonine yielded a resinous non-crystallizable tetrahydrate, very soluble in cold alcohol ad ether. This hydrate also lost one equivalent of water at 140° and at 150°C. Schützenberger remarked he could not explain why nascent hydrogen led to water fixation in alkaloids (Schützenberger, 1858d).
He also showed benzoyl chloride acted on dry cinchonine and dry quinine with formation of monohydrochloride of monobenzoylquinine and monohydrochlorhydride of monobenzoylcinchonine respectively. Both reactions were exothermic. Both benzoyl derivatives were completely soluble in alcohol and ether, insoluble in water, tasteless, and non-crystallizable; acetyl chloride generated semi fluid resinous bases of apparently analogous composition. Schützenberger reported that under the same conditions strychnine gave origin to benzoylstrychnine, a colorless slightly bitter solid, sparingly soluble in water, very soluble in alcohol and ether, and insoluble in bases (Schützenberger, 1858h).
In 1852, Charles-Julien Desnoix published his degree thesis in which he claimed the seeds of Nux vomica contained, in addition to strychnine and brucine, a third alkaloid, which he named igasurin. Igasurin was a white bitter solid, crystallizing as prisms arranged in a feather form; its properties were very similar to those of brucine, except it was five times more soluble in boiling water (Blondeau, 1853). This report led to many researches trying to provide more information about this new alkaloid. In 1858 Schützenberger published a note in which he reported he had separated igasurin into nine different varieties, differing between them only in their solubility of hot water. The nine bodies had a strong and persistent bitter taste, all crystallizing as transparent or pearly needles, having a poisonous effect similar as strychnine, and being colored red by cold nitric acid, the same as brucine. Schützenberger believed all these varieties could be considered as the product of transformation of one into the other, under the influence of oxygen and "vital forces". Their elemental composition was that of brucine minus carbon and more oxygen or water (Schütenberger, 1858e). Eventually William Ashwell Shenstone (1850-1908) proved that igasurin was simply a mixture of strychnine and brucine (Shenstone. 1880).
Additional work included the reaction of strychnine, quinine, and cinchonine with sulfuric and benzoic acids (Schützenberger, 1858fghi), and the products of the oxidation of morphine (Schützenberger, 1865d).
Substitution of metals by electronegative bodies
One important work of Schützenberger was related to the possible replacement of the metal present in an oxygenated salt by an electronegative body such as chlorine, bromine, iodine, cyanogen, sulfur, etc. In the resulting compounds these electronegative bodies appeared replacing the basic hydrogen, and hence, being a kind of particular "salts". The series of papers published on the subject led to his doctoral thesis presented to the Faculté des Sciences of Paris (Schützenberger, 1861abc, 1862abc, 1863a; Schützenberger and Sengenwald, 1862). The basic principle of the project was based on Jean-Baptiste André Dumas's (1800-1884) statement that "chlorine had the singular property of separating the hydrogen present in certain bodies and replacing it atom by atom" (Dumas, 1834), and after discovering trichloroacetic acid. Dumas had given to the substitution law its real significance, by showing the modified substance kept its chemical type, and the substituend element played the same role as the hydrogen eliminated.
According to Schützenberger, the different genres of salts were a striking example of chemical types; in a nitrate, acetate, etc. we found all the essential characteristics of a type: the same number of atoms and their same grouping. The basic purpose of the thesis was to investigate the possibility of replacing the metal of a salt by a simple electronegative body such a chlorine, bromine, and iodine, and thus prepare what Schützenberger named temporarily, salts of chlorine, bromine, and iodine, having a constitution and arrangement similar to that of the corresponding metallic salts. Schützenberger understood there were three main ways for preparing a salt: (1) a double decomposition, that is, reacting one salt with another one (e.g. reacting an acetate with iodine monochloride or bromine chloride), (2) the direct combination between and acid and a base, and (3) the displacement of one metal by another (e.g. reacting a chlorine salt with bromine and iodine, having a stronger affinity for oxygen than chlorine. That is, displacing the chlorine in the same way that a metal displaces another, and forming salts of bromine and iodine) (Schützenberger, 1863a).
In a salt, it was possible to displace the metal by another, equivalent per equivalent, as long as the second metal was more oxidizable than the first one. Schützenberger was bent in proving an analogous phenomenon occurred with negative salts. Thus the chlorine in chlorine acetate could be displaced and replaced by bromine, iodine, and the metals.
Schützenberger prepared and determined the physical and chemical properties of a large number of these salts, among them, chlorine, bromine, iodine, and cyanogene acetates, bromine and iodine butyrates, iodine benzoate, etc. etc. Some details are a follows: (a) Chlorine acetate: The only method applicable to the preparation of this compound, and of chlorine salts of chlorine in general, was direct synthesis: the combination of anhydrous acetic acid with chlorine oxide. The mixture of the two substances in equivalent proportions had initially a blood color tint and showed no signs of immediate action. Kept in a refrigerant mixture it rapidly lost its color without releasing gas or losing weight. From this color disappearance, it was reasonable to infer that combination, or some kind of chemical action, had taken place. In order to confirm that the acetic acid had been saturated with hypochlorous acid, Schützenberger added to the mixture an excess of hypochlorous acid, which communicated to it a persistent red tint. On subsequent heating in a water-bath to 30°C, the excess of hypochlorous acid was driven off, and the liquid became colorless as before. The same result was obtained bubbling dry hypochlorous acid rapidly through anhydrous acetic acid surrounded with cold water, until the liquid acquired a decidedly yellow color. The gas was readily absorbed and combined immediately; afterwards, heating to 30°C expelled the excess of hypochlorous acid. The resulting chlorine acetate was a very pale bright yellow liquid, with having a powerful and irritating smell, reminding that of its two constituents. It exploded violently when heated to about 100°C, sometimes with production of light, if the quantity of substance operated upon was sufficient. The compound was stable in the dark but in direct or diffused daylight and room temperatures it gradually decomposed, releasing oxygen and chlorine, and leaving acetic acid. It dissolved instantly and in all proportions in water, giving a mixture of hydrated acetic and hypochlorous acids, which decolorized a solution of sulfate of indigo sulfate, disengaged oxygen when heated, and in fact presented all the characteristic reactions of the two acids. This result showed the compound had been produced by a simple reaction (Schützenberger, 1863a).
In order to determine the composition of this acetate, Schützenberger studied its reaction with metals, manganese and lead dioxides, sulfur, phosphorus, and arsenic, carbon, organic matter (sugar, alcohol, etc.), etc. Some of his results were (a) there was no reaction with platinum, iridium, gold, silver, and palladium, unless finely divided. Platinum black or sponge decomposed chlorine acetate, even at ordinary temperatures, into chlorine, oxygen, and anhydrous acetic acid; (b) metals such sodium, potassium, and magnesium reacted strongly while, aluminum, iron, copper, cadmium, mercury, etc., reacted with varying degrees of energy, (c) chlorine acetate was strongly attacked by sulfur releasing SO₂ and chlorine; phosphorus also reacted violently releasing chorine and forming phosphoric acid, and (d) organic substances such as sugar and alcohol were oxidized and released chlorine. An elemental analysis of chlorine acetate indicated it contained 38.77% chlorine by weight and its empirical formula could be represented by (C₂H₃O,Cl)O (Schützenberger, 1863a).
Schützenberger wrote that although he did not prepare pure bromine acetate, its properties were very similar to those of chlorine acetate. Iodine acetate was prepared by bubbling dry hypochlorous acid through a mixture of equivalent amounts of anhydrous acetic and iodine, maintained in a coldwater bath. The iodine disappeared by degrees, and then an abundant crop of yellowish-white acicular crystals was formed. If the bubbling of hypochlorous acid was continued, the crystals gradually dissolved, and at the same time there was be an abundant evolution of chlorine, until the liquid next became colorless. After the bubbling of hypochlorous was stopped, small crystalline grains of iodine acetate, sensible to light, begun to deposit. These crystals deliquesced rapidly when in contact with moist air, and iodine was set free. They were also decomposed instantly by water or absolute alcohol, giving iodine, iodic acid, and hydrated acetic acid. The crystals heated to temperatures below 100°C they slowly decomposed releasing iodine; at above 100°C they decomposed rapidly, sometimes with a slight explosion, liberating free much iodine. Elemental analysis indicated the empirical formula of iodine acetate was C₆H₉IO₆. These results indicated that when the chlorine in chlorine acetate was replaced by iodine, the latter behaved like a triatomic radical instead of a monatomic one (Schützenberger, 1863a).
Schützenberger also studied the reactions of benzoyl chloride with silver cyanide, the preparation of the acetates of arsenious and boric acids, the combinations of sulfuric acid, tartaric acid, phosphoric acid with acetic anhydride, the combination of sulfuric acid with hypochlorous anhydride, etc. (Schützenberger, 1863a).
In another memoir, Schützenberger and Edouard Lippmann (a visiting chemist) described the results of the reaction between chlorine acetate and ethylene. They bubbled dry ethylene through a cold mixture of chlorine acetate and acetic anhydride and noticed that the gas was completely absorbed when all the chlorine acetate had disappeared. Adding water easily separated the product of the reaction; the excess acetic anhydride was hydrolyzed, and a heavy layer of colorless liquid separated under the water. This liquid was separated, washed several times with water, and then distilled. According to Schützenberger and Lippmann, the fraction that passed over at 148°C was glycol acetochloride, which was easily hydrolyzed by KOH yielding ethylene oxide. Heated in the presence of water it released HCl and acetic acid (Schützenberger and Lippmann, 1865).
Platinum derivatives
Schützenberger began his discoveries of new platinum compounds by accident. He had tried to synthesize carbonyl dichloride (phosgene) without the intervention of light by passing a mixture of dry mixture of CO and chlorine over platinum sponge heated to about 400°C (Schützenberger, 1868ab). To his surprise, he found considerable amounts of phosgene were formed but also the platinum was not acting only by its presence (as a catalyst). Entrained in the gaseous stream was a new solid and volatile compound of platinum, which could be recovered as a light yellow flocculent powder in the cool part of the tube. This compound melted at about 150°C yielding a dark yellow transparent liquid that on cooling, coagulated into a yellow crystalline mass. In contact with water, it decomposed releasing CO2 and leaving a finely divided black powder of platinum. The synthesis procedure and the action of heat and water indicated it was a compound of platinum chlorine and oxygen; chemical analysis indicated its composition corresponded to the formula (CO)2PtCl2.
According to Schützenberger, the variability of the melting point of the new compound indicated it was possible to prepare many substances of variable composition (Schützenberger, 1868c).
Schützenberger and C. Fontaine studied the action of phosphorus chlorides on these new carbonyl compounds (Schützenberger and Fontaine, 1869). Their results indicated PCl5 reacted with platinum to form a definite compound, crystallizing as yellow red needles, having the formulas PCl5·Pt or rather PCl₃PtCl₂ (chloroplatinous acid). The latter combined with a one molecule of PCl₃ to form a lemon yellow crystallizable substance having the formula P2Cl6PtCl2 (chloroplatinic acid). These two new acids were the source of a large number of chemical reactions. For example, they were rapidly decomposed by water forming HCl and polybasic acids derived from the substitution of the chlorine by OH, for example P(OH)₃PtCl₂ and P2(OH)6PtCl2 (phosphoplatinous and phosphoplatinic acid respectively). They reacted with ethanol homologues to form the corresponding ether, for example, P(RO)₃PtCl₂ and P2(RO)3PtCl2, where R represents the alkyl radical of the alcohol. The ethers of the phosphoplatinous chloride reacted with PCl₃, CO, ethylene, and toluidine to form compounds such as P(RO)₃C₂H₄·PtCl₂. Ammonia combined directly with the phosphoplatinic ethers to form the dichlorhydrate of a diamine, P2(RO)6Pt-(NH2·HCl)2, etc.
Schützenberger and Fontaine also provided a detailed description of the preparation and properties of phosphoplatinous chloride, PCl₃·PtCl₂, phosphoplatinic chloride, P2Cl6·PtCl2, and phosphoplatinous acid, P(OH)₃·PtCl₂ (Schützenberger and Fontaine, 1869; Schützenberger, 1870d); the methyl and ethyl phosphoplatinous ether and their ammonia derivatives (Schützenberger and Fontaine, 1872a), as well as the action of zinc on the above ethers and the reaction of chloroplatinous chloride with glycerin, allyl alcohol, benzyl alcohol, ammonia, and ammonia compounds (Schützenberger and Fontaine, 1872b).
In another publication (Schützenberger, 1870b) Schützenberger wrote that the original compound was actually a mixture of two, a yellow one melting at 130°C and corresponding to the formula (CO)₃·2PtCl₂, and the other a colorless substance sublimating as white needles and having a formula (CO)₂·PtCl₂ or (CO)₄·2PtCl₂. The latter substance could be obtained by contacting the original one with pure CO at 155°C. Similarly, (CO)₂·PtCl2 heated at 210°C in the presence of CO2 or air, lost CO and became (CO)₃·2PtCl₂. In summary, by changing the temperature, it was possible to obtain three different combinations of PtCl₂ with CO: (1) CO·PtCl₂, carbonyl chloroplatinate, a yellow or orange yellow solid melting at 194°C and sublimating at 240°C, in a stream of CO2, as long golden yellow needles; (2) (CO)₂·PtCl₂, dicarbonyl chloroplatinate, a light yellow solid melting at 142°C and sublimating slowly at 150°C, in a stream of CO2, as white needles, and (3) (CO)₃·PtCl2, representing a combination of one mole each of the previous two derivatives; a orange yellow solid melting at 130°C, and decomposing at 250°C into the monocarbonyl derivative. These three bodies reacted instantly with water forming finally divided platinum, HCl and CO2, or HCl and a mixture of CO2 and CO:
COPtCl₂ + H₂O = Pt +2HCl + CO
(CO)₂PtCl₂ + H₂O = Pt + HCl + CO₂ + CO
They were also decomposable by alcohol, releasing platinum and forming a chloroxycarbonic ether (Schützenberger, 1870ef).
The mono and dicarbonyl reacted with ammonia with different consequences, depending on the temperature level. At their fusion temperature they decomposed releasing platinum and forming ammonium chloride. At room temperature, saturating with ammonia a solution of the carbonyl chloroplatinate in carbon tetrachloride precipitated a large amount of light yellow flakes according to
CO·PtCl₂ + 2NH₃ = CO·Pt·2NH₂·2HCI,
(CO)₂·PtCl₂ + 2NH3 = (CO)₂·Pt·2NH₂·2HCl
On heating, these derivatives melted and decomposed into platinum, ammonium chloride, nitrogen, hydrogen, and a volatile liquid (probably formyl chloride) (Schützenberger, 1870bc).
Hyposulfurous acid
In 1854 Christian Friedrich Schönbein (1799-1868) reported that an aqueous solution of SO₂ in contact with zinc, turned rapidly yellow and acquired the property of strongly decolorating a solution of indigo and litmus. After a short time the solution deposited sulfur and lost its activity. Schönbein believed the combined oxygen in contact with zinc and SO₂ converted into ozone, which destroyed the color (Schönbein, 1854). Schützenberger believed the process was actually to a reduction because the solution recovered its color in contact with air. The decoloration process also took place when the liquid was separated from the metal; hence instead of assuming the latter took part in the process, it was reasonable to presume the formation of a particular compound having strong reducing power. Since none of the known compounds of sulfur with hydrogen or sulfur had this property, it would be of interest to study this reaction in more detail (Schützenberger, 1869).
Schützenberger's many experiments to try to isolate the active compound were unsuccessful because the rapidity with which the decolorizing power was lost (as tested with potassium permanganate). It acquired a maximum value after a few minutes and then decreased rapidly, at the same time the solution lost its yellow color and became milky with the deposited sulfur. The maximum reducing value was about 50% higher that of the starting solution. He also found that adding cupric sulfate to the reducing liquid produced a red-strong precipitate of copper hydride, which converted rapidly into copper sulfide. Silver, mercury, or gold salts were similarly reduced to metallic silver, mercury, or gold. No hydrogen release was observed during the solution of zinc in the sulfurous acid and the same phenomena were obtained when replacing the zinc with iron, manganese, or magnesium (Schützenberger, 1869).
Better results were obtained if the sulfurous acid solution was replaced by a concentrated solution of sodium bisulfite. In this case the reducing power was stronger and lasted for substantially more time, if the solution was kept out of contact with air. In these circumstances the zinc dissolved partially, without release of hydrogen and the reaction ended after about 30 minutes. On cooling the liquid deposited crystals of a double sulfite of zinc and sodium. According to Schützenberger, the mother solution could advantageously be used as a eudiometric liquid for absorbing the oxygen: agitated with air it eliminated the oxygen in a few seconds, faster than an alkaline solution of pyrogallate (Schützenberger, 1869).
Schützenberger described the preparation of the active component as follows: A wide-neck flask of about 0.5 L capacity, containing zinc shavings and a concentrated solution of sodium bisulfite, was closed and submerged in cold water. After 30 minutes the solution was transferred to a larger flask containing concentrated alcohol. The precipitate of impure double sulfite of zinc and sodium was separated and the clear filtrate cooled. In a short period of time, a new crystalline precipitate of fine colorless needles was formed. After separation and drying under vacuum, the crystals turned into a white powder, very soluble in water, soluble in diluted alcohol, and insoluble in concentrated alcohol. This powder represented the active principle because its aqueous solution strongly bleached indigo sulfate and litmus, and precipitated copper hydride from copper sulfate, and silver from silver nitrate (Schützenberger, 1869).
Schützenberger analyzed the active principle and found it was the salt of a particular acid, analogue to hypophosphoric acid, which he decided to name hydrosulfurous acid. The same procedure could be used to prepare the hydrosulfites of iron, manganese, and magnesium. The formation of a hyposulfite was simply a secondary phenomenon due to the slow and spontaneous destruction of zinc (or other metal) hydrosulfite. Schützenberger demonstrated the last argument using a voltaic pile. In his arrangement the bisulfite was put in a porous vase, immersed in water acidified with sulfuric acid, and the negative pole aqueous was bisulfite. During the electrolysis, a constant release of oxygen was observed at the positive pole, while no gas was generated at the negative one. The bisulfite became rapidly bleached and active, while becoming charged with hydrosulfite. It was enough to substitute in the Bunsen element the nitric acid by sodium bisulfite to corroborate this result. A couple was then formed were the positive instead of being oxidant, was reducer, although it functioned in the same way as an oxidant by absorbing the hydrogen that tended to deposit on the carbon, and avoiding polarization (Schützenberger, 1869).
Schützenberger and C. Risler showed hyposulfite could be used to dosage oxygen in gaseous mixtures and of oxygen and oxygenated substances dissolved in water, as well as the oxidation power of blood (Schültzenberger and Risler, 1873abc).
Cellulose
In 1855 Marcelin Berthelot (1827-1907) showed that the different sugars of vegetables origin, and their congeners, had a strong analogy with glycerin; they combined with acids forming neutral compounds, having the properties and reactions of neutral fatty materials, and could be prepared and purified in the same manner (Berthelot, 1855). Berthelot prepared and described the properties of the products obtained reacting mannite, quercetin, erythritol, orcinol, and common sugar, with acetic, butyric, stearic, oleic, palmitic, and benzoic acids. The results indicated that sugars and their congeners functioned as polyatomic alcohols of a high order, and were susceptible, as glycerin, of forming truly composed esters. These esters were prepared by treating the sugars and congeners with a hydrated acid, at temperatures that varied with the stability of the material. Although the procedure was very general, it had the disadvantage that it required a very long time (40-50 hours) and yielded only small quantities of the esters (Berthelot, 1855).
In a paper published in 1865, Schützenberger proved that the acetates of sugar and cellulose could be prepared faster and in higher yield by replacing the crystallizable acetic acid by its anhydride; the esterification was complete in a few minutes and did not require a temperature higher than the boiling point of acetic anhydride. The organic substance begun to decompose between 138° and 140°C, and once the reaction had started, it continued of itself, accompanied by lively ebullition, without further application of external heat. The only products were hydrated acetic acid and an acetic derivative, soluble in the hydrated acetic acid, soluble or insoluble in water, according to the nature of the substance employed. In the last case, it was enough to pour the thick final syrup into water and wash the precipitate with water. If the product was soluble in water, the final solution was diluted with more water, bleached with animal charcoal, and finally evaporated under vacuum over lime (Schützenberger, 1865e).
Schützenberger described the results of the reaction of acetic anhydride with starch, cellulose, sugar cane, glucose, mannite, galactitol, tannic acid, salicin, amygdaline, hematin, etc. Starch formed two products, one insoluble in water and soluble in alcohol and acetic acid, the second, a bitter derivative soluble in water and in alcohol. Cellulose reacted with the anhydride only at temperatures above 160°C; cellulose acetate was a white amorphous solid, insoluble in water and alcohol, and soluble in hydrated acetic acid; boiling KOH decomposed it regenerating the cellulose (Schützenberger, 1865e).
In a following work, Schützenberger and C. Naudin remarked that the reaction of acetic anhydride allowed determining the atomicity of the alcohol, if the immediate principle played or not the role of alcohol, and the preparation of acetic derivatives hard to prepare by other methods. The reaction with the anhydride was more advantageous than using acetyl chloride because it did not form HCl, which could affect the results of the reaction. They went one to describe in more detail the reaction of cellulose, starch, dextrin, glycogen, Arabic gum, inulin, glucose, lactose, etc. with acetic anhydride, and the properties of the acetates formed. For example, cellulose triacetate was insoluble in water, alcohol, and ether, and soluble in concentrated acetic acid. With starch, the products differed with the reaction temperature. At 140°C, the product was starch triacetate, a while amorphous solid, insoluble in water, alcohol, ether, and acetic acid; it did not blue iodine and was hydrolyzed by the alkalis regenerating starch. At 160°C, the product was dextrin triacetate, soluble in glacial; the alkalis decomposed it liberating dextrin (Schützenberger and Naudin, 1869a).
The results indicated cellulose and its isomers having the formula C5H10O3, formed triacetates, easily distinguished form other products by the nature of their solubility and the nature of the products regenerated by hydrolysis (Schützenberger and Naudin, 1869a).
Schützenberger and Naudin also described in much detail the reaction of glucose and cane sugar with acetic anhydride (Schützenberger and Naudin, 1869b).
References
Battegay, M., Schutzenberger, Savant Industriel, Bull. Soc. Industr. Mulhouse, 95, 680-688, 1929. [ Links ]
Berthelot, M., Sur les Combinaisons Neutres des Matières Sucrées avec les Acides, Compt. Rendus, 41, 452-456, 1855; [ Links ] Ann, Chim. Phys. [3], 47, 297-354, 1856.
Blondeau, P., Rapport sur une Thèse de M. Denoix ayant par Sujet: Étude de la Famille des Loganiacées et de la Igasurine, J. Pharm. Chim. [3], 202-205, 1853. [ Links ]
Davis, T. L., Paul Schutzenberger, J. Chem. Educ., 6, 1403-1414, 1929. [ Links ]
De la Rue, W., On Cochineal (Coccus cacti), Mem. Chem. Soc., 3, 454-479, 1845-48. [ Links ]
Dumas, J.-P., Suite de Recherches de Chimie Organique sur le Prétendu Chlorure de Carbone de M. Liebig et de l'Hydroiodure de Carbone de M. Sérullas, Proc. Verb. Acad. Sci., January 13, 1834. [ Links ]
Friedel, C., Notice sur la Vie et les Travaux de Paul Schutzenberger, Bull. Soc. Chim., 29, i-xxxiv, 1898. [ Links ]
Plessy, E, M., Schützenberger, P., De la Solubilité de la Matière Colorante de la Garance dans l'Eau entre 100 et 250°, Compt. Rendus, 43, 167-168, 1856. [ Links ]
Robiquet, P. J., Colin, Sur un Nouveau Principe Immédiat des Végétaux (l'Alizarin) Obtenu de la Garance, J. Pharm., 12, 407-412, 1826. [ Links ]
Schönbein, C. F., Über Farbenveränderungen. J. Prak. Chem., 61, 193-224, 1854. [ Links ]
Schützenberger, P., Considérations sur le Système Osseux Normal et Pathologique au point de Vue de sa Structure et de sa Composition, Thèse Médecine, Université de Strasbourg, Strasbourg, 1855. [ Links ]
Schützenberger, P., Mémoire sur la Composition de l'Acide Carminique et de quelques-uns de ses Dérivés, Ann. Chim. Phys. [3], 54, 52-64, 1858a. [ Links ]
Schützenberger, P., Recherches sur la Cochenille, Compt. Rendus, 46, 47-48, 1858b. [ Links ]
Schützenberger, P., Note sur Deux Nouveaux Dérivés de la Quinine et de la Cinchonine, Compt. Rendus, 46, 1065-1068, 1858d. [ Links ]
Schützenberger, P., Recherches sur les Alcaloïdes de la Noix Vomique, Ann. Chim. Phys. [3], 54, 65-73, 1858e. [ Links ]
Schützenberger, P., Recherches sur la Strychnine, Compt. Rendus, 47, 79-81, 1858f. [ Links ]
Schützenberger, P., Recherches sur la Quinine, Compt. Rendus, 47, 81-82, 1858g. [ Links ]
Schützenberger, P., Note sur les Dérivés Benzoïques de la Quinine, de la Cinchonine, et de la Strychnine, Compt. Rendus, 47, 233-235, 1858h. [ Links ]
Schützenberger, P., Note sur les Dérivés Sulfuriques des Alcaloïdes Végétaux, Compt. Rendus, 48, 235-237, 1858i. [ Links ]
Schützenberger, P., Substitution des Corps Électro-négatifs (Chlore, Brome, Iode, Cyanogène, Soufre, etc.) aux Métaux dans les Sels Oxygénés: Production d'une Nouvelle Classe de Sels dans lesquels les Corps Électro-négatifs Remplacent l'Hydrogène Basique, Compt. Rendus, 52, 135-139, 1861a. [ Links ]
Schützenberger, P., Mémoire sur les Produits de Décomposition du Benzoate d'Iode sous l'Influence de la Chaleur, Compt. Rendus, 52, 963-965, 1861b. [ Links ]
Schützenberger, P., Sur les Combinaisons des Acides Entre Eux, Compt. Rendus, 53, 538-541, 1861c. [ Links ]
Schützenberger, P., Mémoire sur l'Acétate de Cyanogène, Compt. Rendus, 54, 154-156, 1862a. [ Links ]
Schützenberger, P., Action du Protochlorure d'Iode sur Quelques Substances Organiques, Compt. Rendus, 54, 197-199, 1862b. [ Links ]
Schützenberger, P., Nouvelles Recherches sur l'Acétate d'Iode, Compt. Rendus, 54, 1026-1029, 1862c. [ Links ]
Schützenberger, P., Essai sur les Substitutions des Éléments Électronégatifs aux Métaux dans les Sels, et sur les Combinaisons des Acides Anhydres entre Eux, Silbermann, Strasbourg, 1re Thèse; Propositions de Physique Donnés par la Faculté, 2e Thèse; Thèses de Doctorat Présentées a la Faculté des Sciences, Paris, 1863a. [ Links ]
Schützenberger, P., Traité de Chimie Appliquée à la Physiologie et à la Pathologie, Animale, Masson, Paris, 1863b. [ Links ]
Schützenberger, P., Sur quelques Nouveaux Dérivés de l'Indigotine, Compt. Rendus, 61, 284-286, 1865a. [ Links ]
Schützenberger, P., Recherches sur les Matières Colorantes de la Garance, Bull. Soc. Chim., 4, 12-17, 1865b. [ Links ]
Schützenberger, P., Sur les Produits de Réduction de l'Isatine, Bull. Soc. Chim., 4, 170-176, 1865c. [ Links ]
Schützenberger, P., Sur les Produits d'Oxydation de la Morphine, Bull. Soc. Chim., 4, 176-181, 1865d. [ Links ]
Schützenberger, P., Action de l'Acide Acétique Anhydre sur la Cellulose, l'Amidon, les Sucres, la Mannite, et ses Congénères, les Glucosides et Certaines Matières Colorantes Végétales, Compt. Rendus, 61, 485-486, 1865e. [ Links ]
Schützenberger, P., Traité des Matières Colorants, Masson, Paris, 1867. [ Links ]
Schützenberger, P., Sur un Nouveau Composé de Platine, Compt. Rendus, 66, 666-668, 1868a. [ Links ]
Schützenberger, P., Sur Quelques Réactions donnant lieu à la Formation de l'Oxychlorure de Carbone, Compt. Rendus, 66, 747-748, 1868b. [ Links ]
Schützenberger, P., Sur Quelques Réactions donnant lieu à la Formation de l'Oxychlorure de Carbone et sur un Nouveau Composé Volatil de Platine, Ann. Chim. Phys. [4], 15, 100-106, 1868c; [ Links ] Bull. Soc. Chim., 10, 188-192, 1868c.
Schützenberger, P., Sur les Matières Colorantes de la Graine de Perse, Compt. Rendus, 67, 176-178, 1868d. [ Links ]
Schützenberger, P., Mémoire sur les Matières Colorantes des Graines des Nerpruns Tinctoriaux, Ann. Chim. Phys. [4], 15, 118-130, 1868e. [ Links ]
Schützenberger, P., Sur un Nouvel Acide de Soufre, Compt. Rendus, 69, 196-201, 1869; [ Links ] Ann. Chim. Phys. [4], 20, 351-361, 1870a.
Schützenberger, P., Sur les Combinaisons du Protochlorure de Platine avec l'Oxyde de Carbone, Compt. Rendus, 70, 1134-1136, 1870b. [ Links ]
Schützenberger, P., Recherches sur le Platine, Compt. Rendus, 70, 1287-1290, 1870c. [ Links ]
Schützenberger, P., Note sur les Composés Phospho-platiniques, Compt. Rendus, 70, 1414-1415, 1870d. [ Links ]
Schützenberger, P., Sur les Composés Phosphoplatiniques, Compt. Rendus, 71, 69, 1870e. [ Links ]
Schützenberger, P., Sur une Nouvelle Classe de Composés Platiniques, Ann. Chim. Phys .[4], 21, 350-363, 1870f. [ Links ]
Schützenberger, P., Recherches sur l'Albumine et les Matières Albuminoïdes, Masson, Paris, 1875a. [ Links ]
Schützenberger, P., Les Fermentations, Baillière, Paris, 1875b. [ Links ]
Schützenberger, P., Note sur un Nouveaux Dérivé de l'Indigotine, Compt. Rendus, 85, 147-149, 1877. [ Links ]
Schützenberger, P., Traité de Chimie Générale Comprenant les Principales Applications de la Chimie aux Sciences Biologiques et aux Arts Industriels, Hachette, Paris, 1880-1894; 7 volumes. [ Links ]
Schützenberger, P., Rapport sur les Procédés Chimiques de Blanchiment, de Teinture, d'Impression, d'Apprêts, Imprimerie Nationale, 1882. [ Links ]
Schützenberger, P., Exposé des Titres et des Travaux Scientifiques de M. Paul Schützenberger, Gauthier-Villars, Paris, 1868, 1888. [ Links ]
Schützenberger, P., Boudouard, O., Leçons de Chimie Générale: Professées au Collège de France, Pendant l'Année 1895-96, Doin, Paris, 1898. [ Links ]
Schützenberger, P., Fontaine, C., Sur une Nouvelle Classe de Composés Platiniques (1re Partie), Bull. Soc. Chim., 12, 482-496, 1869. [ Links ]
Schützenberger, P., Fontaine, C., Mémoire su les Composés Phosphoplatiniques (2e Partie), Bull. Soc. Chim., 18, 101-113, 1872a. [ Links ]
Schützenberger, P., Fontaine, C., Mémoire su les Composés Phosphoplatiniques (3e Partie), Bull. Soc. Chim., 18, 148-159, 1872b. [ Links ]
Schützenberger, P., Lippmann, E., Note sur l'Action de l'Acétate de Chlore sur l'Éthylène, Bull. Soc. Chim., 4, 438-440, 1865. [ Links ]
Schützenberger, P., Naudin, C., Première Mémoire Sur les Dérivés Acétiques des Substances Hydrocarbonées, Compt. Rendus, 68, 814-818, 1869a. [ Links ]
Schützenberger, P., Naudin, C., Deuxième Mémoire sur les Dérivés Acétiques des Sucres, Bull. Soc. Chim., 12, 204-209, 1869b. [ Links ]
Schützenberger, P., Paraf, A., Mémoire sur la Matière Colorante de la Gaude, Compt. Rendus, 52, 92-94, 1861. [ Links ]
Schützenberger, P., Rack, A., Note sur la Catéchine, Matière Colorante du Cachou, Bull. Soc. Chim., 4, 5-10, 1865. [ Links ]
Schützenberger. P., Risler, C., Recherches sur le Pouvoir Oxydant du Sang, Compt. Rendus, 76, 440-442, 1873a. [ Links ]
Schützenberger. P., Risler, C., Dosage de l'Oxygène Libre ou Dissous au Moyen d'une Solution Titrée de Hydrosulfite, Bull. Soc. Chim., 19, 152-156, 1873b. [ Links ]
Schützenberger. P., Risler, C., Mémoire sur l'Emploi de l'Hydrosulfite de Soude comme Moyen de Titrage de l'Oxygène et des Composés Oxygénés et sur quelques Faits Nouveaux Concernant l'Oxygène, Bull. Soc. Chim., 20, 145-159, 1873c. [ Links ]
Schützenberger. P., Sengenwald, R., Action du Protochlorure d'Iode sur Quelques Substances Organiques, Compt. Rendus, 54, 197-199, 1862. [ Links ]
Schützenberger, P., Shiffert, H., Sur les Matières Colorantes Contenues dans la Garance de l'Alsace, Bull. Soc. Industr. Mulhouse, 34, 70, 1864. [ Links ]
Shenstone, W. A., Note on Igasurine, J. Chem. Soc., 37, 325-326, 1880. [ Links ]
Urbain, G., Centenaire de P. Schutzenberger Bull. Soc. Chim., 43, 913-922, 1928. [ Links ]
Willm, E., Paul Schützenberger, in Biographies Alsaciennes et Portraits en Photographie par Ant. Meyer, ed. A. Ingold, 5ème série, Ant. Meyer, Colmar, 1889. [ Links ]














