Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Educación química
versión impresa ISSN 0187-893X
Educ. quím vol.22 no.3 Ciudad de México jul. 2011
Didáctica de la química
The knowledge of chemistry in secondary education: difficulties from the teachers' viewpoint
Ana Luiza de Quadros1, Dayse Carvalho Da-Silva1, Fernando César Silva1, Frank Pereira de Andrade2, Helga Gabriela Aleme2, Juliana Cristina Tristão2, Sheila Rodrigues Oliveira1, Leandro José Santos2, Gilson DeFreitas-Silva3
1 Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. <aquadros@qui.ufmg.br>; <daysecsm@yahoo.com.br>; <fcsquimico@yahoo.com.br>; <shetq@yahoo.com.br>.
2 Universidade Federal de Viçosa, Florestal, MG, Brasil. <frankimica@yahoo.com.br>; <hgaleme@yahoo.com.br>; <julitristao@yahoo.com.br>; <leandroj.santos@ufv.br>.
3 Universidade Federal da Bahia, Salvador, BA, Brasil. <gilsondfs@ufba.br>.
Fecha de recepción: 16 de abril de 2010.
Fecha de aceptación: 5 de agosto de 2010.
Abstract
The present work sought to investigate the chemistry content that high school Brazilian teachers find difficult to work with in the classroom, the strategies, and the pedagogical resources they use to teach such content, and how students view this content. The interviews with 79 chemistry teachers from Minas Gerais State of high schools revealed an overemphasis on calculations to the detriment of content comprehension.
Keywords: chemistry teaching, high school, teacher's difficulties, chemistry content.
Resumen
El presente trabajo investiga los contenidos de química que los profesores de bachillerato brasileños encuentran difíciles de trabajar dentro del aula, así como las estrategias y los recursos pedagógicos que emplean para enseñar tales contenidos y cómo los estudiantes los ven. Las entrevistas con 79 profesores de bachillerato de química del Estado de Minas Gerais revelan un sobreénfasis en cálculos en detrimento de la comprensión conceptual.
Introduction
Chemistry is considered a core science that can permeate several areas of knowledge, such as engineering, health, astronomy, biology, and geology, among others. According to the Brazilian secondary school curriculum parameters (PCNEM), chemistry is one of the curriculum components that can promote the intellectual development of the students through the search to understand nature and its transformations (Brazil, 1999). The chemistry disciplines in secondary education can afford unique opportunities to students understand the world in the "chemical" viewpoint and to help them learn important concepts.
Unfortunately, in Brazil and perhaps in the world, the word chemistry has acquired a negative connotation. It is common to hear about "chemical-free" products and treatments. It is almost automatically associated with the word "danger" because of the toxicity and inflammability of some products or because of some of their adverse effects on the environment or on human beings. Also is uncommon relate chemistry with the benefits that this science brings to society.
The school is a privileged place to broaden the view of what chemistry and chemical products are. The classroom is an environment for the construction of knowledge; teachers and students engage in the development of skills and competencies that are important for the education of students and citizens. Considering the socialhistorical psychology of Vigotski (1993), our view of learning is based on the development of mental structures that allow the student to use the "way of thinking" acquired in the classroom in another learning situation at school and/or in daily life. For such, the students must actively participate in classes. However, this is not what we observe!
The Brazilian Education System
According to Article 21 of the Law of Directives and Bases of National Education (Law n.° 9.394/96), the current Brazilian Education System is composed of: Basic Education and Higher Education (Figure 1). The aim of Basic Education is to allow the student to develop, guaranteeing the normal and necessary background to exercise his/her role as a citizen and providing the means to advance in work and further studies. It is subdivided into Preschool, Elementary and Middle School, and High School. Figure 1 provides an idea of the basic organization of the current Brazilian school system.
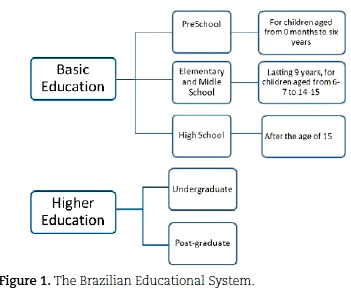
The first stage of basic education, that is, preschool, is for children ages zero to six years and is offered by day care centers and preschools. Although the State is obliged to provide this, the child's attendance is optional. Basic Education lasts nine years, is obligatory for all school-age children and offers a guaranteed place even for those who did not have access to schooling when they were at the appropriate age. High school is also obligatory for school age students, lasts at least three years and provides the student with a general education, with the option of technical/professional training if the time is extended beyond the three years.
Higher education in Brazil includes undergraduate programs in the different professional areas. It is open to candidates who have completed high school or the equivalent and have passed the college entrance exams. Graduate school is also part of higher education and includes masters' and doctoral programs, and specialization courses. It is at this educational level that Brazilian chemistry teachers are trained, in courses called "Teaching Degree Course in Chemistry".
Chemistry is taught from the sixth year of Basic Education, as part of the science curriculum, which includes biology, chemistry and physics. It is in high school, however, that chemistry is given as a separate subject. At most schools, the course is offered during all three years of high school, with a minimum of two classroom hours per week, with some variation from one school to another.
In these three years of high school, knowledge of properties, constitution and transformation of materials is developed. In traditional school curricula, the studies begin with the microscopic aspects and move on to the macroscopic aspects. The content, in these cases, covers atomic theory, chemical bonding, transformations, stoichiometric calculations, kinetics, thermochemistry, electrochemistry, and solutions, generally in the first two years, and organic chemistry is normally given in the third year of high school. Following the tendency in some parts of the world, Brazil now has high school chemistry textbooks with programs that begin study with the macroscopic aspects, normally with the observable phenomena, to only then go on to the theories and models. Teachers from schools that use these programs have reported satisfactory results in terms of the students' learning.
Most of the teachers invited to be part of this work develop the course content as per Table 1.
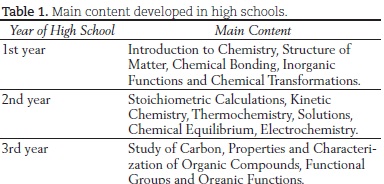
It is important to emphasize that this is not a standard program. In Brazil, what is considered basic and should therefore be present in the chemistry curriculum is the content on properties, constitution and transformation of materials. However, in the classroom, the curriculum ends up becoming standard, in a process which gives more consideration to tradition than to the students' educational needs.
Considerations for teaching Chemistry in Brazil
It is common in Brazilian classrooms to hear secondary students referring to chemistry as being difficult, abstract, unnecessary, and other similar adjectives. We teachers recognize the importance of chemistry for society, despite its complexity.
These and other issues have been topics of debate about science education and the education of citizens. This leads us to reflect on interdisciplinary practices as a means to improve science education (Lavaqui & Batista, 2007).
Maldaner and Piedade (1995) consider that the greatest problem in teaching chemistry is the way that concepts are introduced. The concepts should enable the student to truly learn chemistry, without rote learning of definitions or use of formulas and words devoid of meaning. Ideally, the words or concepts used by the students should gradually become a way of thinking.
Brazilian teachers often comment on the students' lack of interest, which raises other questions: would this lack of interest be a direct consequence of the methodology adopted to teach science? Is what students seek at school that which the teachers offer? What is certain is that this lack of interest on the students' part ends up discouraging the teachers from searching for innovative and more creative teaching and evaluation methodologies (Lima & Vasconcelos, 2006). Strack et al. (2009) state that:
Many teachers' practice is currently restricted to a rigid curriculum characterized by disconnected content, a lack of interdisciplinarity, and mainly a lack of connection with students' reality. This devalues the classroom, which should be a place of construction and change, both for the students and for the teachers themselves (Strack, et al., 2009, pp. 18).
According to Lopes (2005), school disciplines should not be the mere reproduction of scientific knowledge. They should have social purposes. Lopes (2005) points out that the best form of arguing in favor of the relevance of certain concepts and theories is to analyze the processes of organization and constitution of school knowledge, by curricular integration, and the different processes of mediation that constitute this knowledge. This includes transposition and didactic mediation, and the proper use of metaphors and analogies.
When we look at the school curriculum, we remember that its construction is based on the view of science of the person who creates the curriculum. This general view of science allows making connections between the several areas of content and judging them as more or less important in the curriculum. Thus, many areas of content that are considered important are organized and included in the curriculum, in school books, delivered in class, and sometimes without contributing significantly to the education of the student/citizen. This fact may contribute to the student's lack of interest and dedication, which creates a vicious circle of lack of understanding of concepts and a consequent teacher's lack of motivation.
Added to this is the extensive content included in the secondary education syllabus. In the case of chemistry, the syllabus initially proposed is so extensive that it cannot be thoroughly covered, or it is covered only superficially, which can give rise to the misconstruing of concepts and lack of correlation with previously taught content. Sometimes the curricula are grounded in a need to prepare for the university entrance examinations. The exam syllabus is always extensive and ends up defining the content to be dealt with in the classroom.
In general, the science teacher has historically been exposed to a series of challenges that include keeping up with scientific and technological discoveries, staying up to date on environmental problems, knowing at least the minimum about polemic issues in the media, and making this all information accessible and pleasant to the students, in other words, teaching involves making the unfamiliar familiar.
Besides the challenges science itself poses to the teacher are those posed by contemporaneous education trends. The teachers who seek innovation in the teaching of science come across some obstacles, as described by Leal and Mortimer (2008):
The school culture, supported by didactic books and a generally not very critical early education, and the lack of governmental directives and articulation by schools and teachers, is a great obstacle to the advance of innovative projects (Leal & Mortimer, 2008, pp. 228).
The teachers are also faced with a somewhat unwelcoming reality in Brazilian schools, such as crowded classrooms (more than forty students), professional devaluation, and outdated school facilities, especially in public schools. These factors also make it difficult to adapt to the proposal of the Brazilian curriculum parameters (Lima & Vasconcelos, 2006).
Carnoy, Gove & Marshall (2003), looking for to understand the different learning processes in Latin America, appointed by Latin American Laboratory for the Evaluation of Educational Quality (Laboratorio Latinoamericano de Evaluación de la Calidad de la Educación, 1998), had developed research in classrooms of Brazil, Chile and Cuba. The results showed significant differences between classrooms observed in that three countries, both in relation to the practice adopted by teachers in the management of classes, as regards the difficulty of content covered. The research in Brazilian's classroom identified that the level of cognitive demands of students is smaller than in other countries, less connection between knowledge, the use of "copy" via blackboard is larger and, consequently, there is more emphasis on memorization in detriment to the understanding of the concepts. The results of Cuban classrooms and Chilean private classrooms are positively compared with those observed in Brazilian schools and Chilean public schools.
It is known that Brazilian students see chemistry as a lesser school discipline and that they find it difficult, we looked at the teachers' work and at the syllabus-related difficulties perceived by the teachers. A large number of chemistry teachers in secondary schools also report difficulties and problems related to teaching this discipline. We are aware that it does not suffice to know the chemistry content in order to be able to teach it. Teachers must also have knowledge about how to teach, how to articulate content, and what the basic knowledge required from every student is, that is, what we want our students to learn. This study aimed to investigate syllabus-related difficulties faced by the teachers of chemistry in secondary schools.
The steps of this study
In 2009, the Chemistry Department of the Universidade Federal de Minas Gerais —UFMG— was in charge of organizing the Minas Gerais State's Chemistry Olympiad (Olimpíada Mineira de Química-OMQ), which is part of the national chemistry olympiad. The state-level contest brought together representatives of 170 schools of Minas Gerais State.
This event gathered about 120 basic education teachers from the state of Minas Gerais, Brazil, who were responsible for accompanying the students who would take part in the state chemisty contest, OMQ.
These teachers were invited to participate because they had demonstrated profession commitment and for participating in activities which motivate students to study chemistry. The 79 invited participants who filled out the questionnaire gave their informed consent of use of the collected data for research under the condition of non-disclosure of personal data.
In the data collection questionnaire distributed to the high school teachers, we asked them to describe their impressions about the teaching of chemistry, indicating the content that they considered difficult to work with in the high school classroom, the strategies and pedagogic resources used, and how the students related to the indicated content. Each participant was to write about the content with which they had the most difficulty in working with the students. Next, the teacher described the type of difficulty faced in developing that content and the ways in which he/she worked to overcome the difficulty. The questionnaire was previously validated with a group of volunteer teachers.
The data were submitted to qualitative textual analysis (Moraes, 2003; Navarro & Diaz, 1994), first by quantification and then by categorization. The unitary elements were correlated for data assessment (Cirino, et al., 2009; Galiazzi, 2000; Galiazzi & Moraes, 2006; Machado, 2002). Although the teachers' responses were rather terse in some cases, sometimes they allowed for interpreting the symbolic meaning of their conceptions of the chemistry content worked on in the classroom.
According to Moraes (2003), the textual analysis is based on suppositions about the relation between the reading of the material under analysis and its set of signifiers. In doing the analysis, the researcher attributes meanings that involve their own knowledge and the theories that they are dealing with. In this sense, we see that our understanding of the meaning of the difficulties reported by the teachers' results from meanings that we attribute to the text.
Description and analysis of the content problem
Some studies discuss the importance and difficulties related to a student and/or teacher oriented chemistry content. Finley et al. (1982) investigated the importance and the difficulties perceived by secondary school teachers of physics, biology, health science, and chemistry. This study reports and further classifies the content cited by the investigated teachers. Although the teachers considered the content difficult, there was not a specific concern about the type and cause of such difficulties. The present work investigated the content that the secondary school chemistry teachers considered difficult and the source of such difficulty.
The teachers were requested to choose the content that they teach to their students and that they considered the most difficult to teach. No restriction was made about class grades. As a result, they reported difficulties in the whole secondary school chemistry content, ranging from the atomic theory to organic chemistry, which are normally taught in the first and last grades of secondary school in Brazil, respectively. The cited content that emerged from the data was grouped according to similarity and is shown in Table 2.
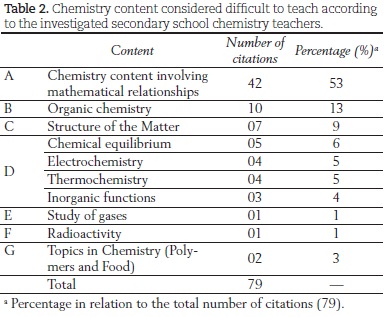
Before looking more closely at the content descriptions, we point out that very often the teachers attribute the difficulty to the students. Both in the case of mathematical knowledge and of specific chemistry content, the teachers describe the students as being unable to learn or as lacking basic requirements or something similar. Next, we describe each of the items given in Table 2 and attempt to analyze the teachers' comments.
A-Chemistry content involving mathematical relationships
The content in this category includes stoichiometry, chemical calculations, matter quantity, and others. In general, teaching this content is a great challenge because it requires that the students coherently articulate mathematical (measurement units and their conversion, second order polynomials, proportion, logarithm etc.) and chemistry knowledge (magnitude, symbols, representations etc.).
The fact that difficulties in the use of calculations accounted for over 50% concerned us. Most teachers commented that the main difficulty in teaching this content is associated with either the students' "poor math grounding" or "interpretation of question statements". Never did the teachers correlate the problems observed in the teaching of this content to their practice in terms of the required chemistry knowledge.
We have not observed a greater concern with understanding stoichiometry, matter quantity, or other content in this category in the teachers' comments. From experience, we know that proportion is necessary in stoichiometry and that it poses a challenge to the students. However, in looking for the source of difficulty, we considered the understanding of chemical transformations. Do the students fully grasp chemical transformations? Do the teachers give it due importance? We consider that to think in chemical terms about quantities in a chemical equation depends on understanding the transformations involved.
Rossi et al. (2008) investigated the teachers' knowledge of density and confirmed the direct relationship between this content and its mathematical expression. According to them:
Many students have difficulty in learning chemistry concepts when the discipline is restricted to fragmented content presented out of context, which results in generalized deficiency. [...] The questionnaire revealed that mathematical equations apparently are excessively emphasized in the study of density, which has grave consequences for higher education (Rossi et al., 2008, pp. 59).
For over a decade now, PCNem (Brasil, 1999) has recommended the creation of classroom situations that stimulate the students to develop the capacity to reason and use science as an element of interpretation and intervention. When they emphasize calculations, the teachers may be limiting the understanding of concepts and making their understanding and that of the subsequent content difficult.
As already pointed out by Maldaner and Piedade (1995) as to how concepts are initially presented, to truly learn chemistry, they can not be restricted to rote learning or the use of formulas and words void of meaning. The emphasis on the mathematics of the content will not contribute to the development of thinking in chemistry.
B-Organic chemistry content
Content requiring a greater knowledge of substances constituted of mainly carbon, classified as organic chemistry, was also mentioned. Carbon hybridization, atomic and molecular orbitals, organic reactions, and mechanisms of reaction are part of this category.
The separation of organic and inorganic chemistry in secondary school has been criticized for a long time. The teachers that mentioned organic chemistry probably still preserve the tradition of reserving this chemistry content for the third year of secondary school. However, more important than that is the depth intended in secondary level classes. We agree that as the students acquire basic content, the teacher gradually goes more into depth. One of our concerns is that schools with two hours of chemistry classes a week teach content like "reaction mechanisms". We wonder at the depth of such content. How much do secondary students learn about it? Probably, when citing such content, the teachers are aware of their level of abstraction. If the content is not understood from the chemical viewpoint, the students' option is to memorize. The atoms and molecules involved in these reactions have real structures that cannot be perceived by the senses, though. How can you study the imperceptible? The correlation between the behavior of these minute particles that constitute the microscopic and lend the properties to the macroscopic system was and continues to be the great challenge of chemistry, and consequently, of teaching chemistry. How to overcome it? (Roque & Silva, 2008.)
In an attempt to facilitate the teaching of such content, some teachers reported using analogies, contextualizations, multimedia resources (slides), and physical models (balloons or plastic models to represent molecules). The combination of these strategies/resources can make learning concepts more significant, increasing the interest of the students in organic chemistry. However, despite their efforts, the teachers still recognize that a large number of students do not learn and do not like this content.
C-Structure of Matter
The teachers considered the structure of matter the third most difficult content to teach. Some attributed these difficulties to its subjective and abstract nature, the difficulty to understand and visualize the models, and the lack of a physics grounding to understand the concepts of charge and electric and magnetic fields. In these cases, to facilitate teaching this content, the teachers resort to computer models, videos, and even drawings and analogies of the atomic models being studied. One of the teachers emphasized that it is necessary to believe in what has never been seen and to use "touchable" models, as he/she calls it, such as marbles and the solar system.
Another teacher mentioned the difficulty to teach electronic distribution and energy sublevels and that the students do not understand Bohr's model, despite the use of media to facilitate teaching. The team responsible for the ufmg's entrance examination (vestibular) has avoided formulating questions that involve energy sublevels because they consider this content as belonging to higher education, due to its depth.
From the comments of some Minas Gerais secondary school teachers, we could notice that they continue teaching this content and describe it as being difficult. The periodical properties were also mentioned by the teachers. They said that the students have difficulty to understand and correlate the properties in graphs and in the chemistry context. In relation to the students' interest in this content, one of the teachers said that they love the atomic theory subject and that in the case of those who do not, it is possibly because they do not understand the subject. Most teachers said that the students have little interest in the content.
Quadros et al. (2008) evaluated the exams of the state chemistry Olympiad and most of the students' errors involved the content structure of the matter. This content is abstract in nature and in most secondary schools, it is taught early in the chemistry course, and that probably the students have difficulty with abstractions. With regard to the number of students' errors in the state chemistry Olympiad and the level of abstraction required by this content, the number of citations of the content was small.
D-Chemical equilibrium, Electrochemistry, Thermochemistry, and Inorganic functions
Some teachers cited this content as being difficult to teach and learn, which is probably due to their abstract nature. Some studies have evidenced the students' difficulties in this content. Driel and Gräber (2002) discussed the students' difficulties with the concept of chemical equilibrium as being related to its level of abstraction and the fact that the concepts involved have different meanings from those of daily life, among others. Jong and Treagust (2002) investigated the learning of electrochemistry and found that the students' difficulty is in relating the chemical species involved in electrochemical phenomena with the actual processes. Several other studies (e.g., Garnett & Treagust, 1992a and 1992b; Posada, 1997; Sanger & Greenbowe, 1997; Lima, et al., 2005) have indicated the students' difficulties in electrochemistry.
Concerning thermochemistry, Mortimer and Amaral (1998) reported that the use of concepts whose meanings in science are different from those of daily language makes understanding difficult and that the students fail to clearly distinguish the limits and context of application of each one.
In relation to inorganic functions, Campos and Silva (1999) analyzed 12 secondary school chemistry books and considered the content a jumbled bunch of concepts that did not actually contribute to the understanding of the physical world before the eyes of teenagers. According to them, as presented in the books, the content is confusing and completely useless because of the presence of contradictory ideas, minimum coverage of the established principles, statements not contextualized in the students' daily life, an excessive number of unrelated concepts, the solvent at times being considered and at others ignored, and overemphasis of names, formulas, and classifications. If the investigated teachers follow the schoolbooks, this difficulty is expected.
E-Study of gases
The study of gases was mentioned only by one teacher, who associated the difficulty in this content with the short time available to explore it, since it is taught in the second year of secondary school. In some schoolbooks, the authors chose to limit this content to the standard conditions for temperature and pressure-STP. Naturally, this requires less time. However, contextualization, a current trend in education, is at the door of the classrooms; can we limit the content to conditions that do not exist in the students' life? Maybe the solution would be to redimension it, to teach concepts related to the students' daily life. If the teachers still have the traditional program of study of gases in mind, they will certainly not have time enough to deal with it properly.
F-Radioactivity
Some secondary school chemistry schoolbooks include the topic of radioactivity. It is logical to think that given the complexity of the content, the books bring more complementary information than chemistry content. One of the teachers cited it and its difficulty due to the students' lack of grounding; however, an interdisciplinary approach results in a satisfactory response of the students to this content. The only comment on this content reflects a tendency not to deal with it in secondary school.
G-Chemistry topics
The study of topics of interest in chemistry was evidenced after the publication of the guidelines for the national curriculum parameters (PCN+) (Brasil, 2002). Two teachers mentioned the chemistry topics Foods and Polymers. They remarked on the lack of didactic material, as these topics are not included in most course books and are not available as educational videos because of the lack of knowledge of practical activities and other reasons. The teachers see possibilities of contextualization and interdisciplinarity in these topics. However, they seem to have difficulty in preparing their classes, which makes clear the inadequacy of the didactic material available according to the current trends in education, especially the socalled more traditional materials. The current trends in education are more present in papers about teaching and learning chemistry published in specialized journals. However, to prepare classes, the teachers tend to resort more to books than to articles.
What we learn from the data
Found in our research in relation to teachers difficulties related to the chemistry content allowed us to draw a general profile of chemistry classes in secondary education. We noticed an emphasis on calculations and formulas and we propose that such an emphasis does not ensure the understanding of the chemistry content.
The current teaching trends, which are broadly discussed by chemistry educators in Brazil, highlight the importance of basic concepts to understanding the world. Chemistry is a science that studies materials and some emphases are made. According to the PCN+ (Brasil, 2002), chemistry must be presented based on three fundamentals: chemical transformations, materials and their properties, and explanatory models. Teaching based on these three fundamentals may provide the required ground knowledge of chemistry to the students, especially if accompanied by the proper pedagogical approach based on contextualization, which lends meaning to the content and makes establishing relationships with other fields of knowledge easy. The respect for the cognitive and emotional development of the students ensures close attention to the students' education and their interests, the development of skills suited to the topics and content being taught (Brasil, 2002). Despite all the efforts to help the Brazilian teachers, the pedagogical guidelines (PCNEM, PCN+, Basic Common Content of Minas Gerais, etc.) seem to be much more just a physical presence in the schools than they actually are appropriated and implemented.
Thus, the concern is about the way teachers understand the subjects in secondary education (chemical disciplines) and in how they supply citizens educational necessities. The content currently taught and emphasized probably does not increase the students' interest in science. The choice seems to be for a classical content based mostly on school book content rather than on what would be in accordance with their vision of the world, their ideas, their practices, their social representations and their symbols. The language of chemistry is a tool available to facilitate the understanding of the world. It is not an end in itself; that is, it only makes sense teaching it in context.
Several researchers have pointed out the use of words in chemistry with meanings different from their daily meanings as an obstacle to learning. However, chemistry takes microscopic entities and creates models to represent the world. The teacher is left with the hard task of drawing relationships between this world of entities and the macroscopic world. Roque and Silva (2008) remarked that the difficulty in learning the language of chemistry is associated with the distinction between common language and the almost hermetic specificity of chemistry, and most probably, with the difficulty of establishing the necessary relationships between the chemistry entities of the microscopic and macroscopic worlds.
As already stated by Carnoy, Gove and Marshal (2003), the emphasis is still on content storage and there is little relationship between these concepts and with the world of life. The mathematication of chemical content is an evidence that chemistry teachers are focusing on using formulas and, probably, meaningless knowledge for the student.
The data collected evidence the need to discuss and enrich the Brazilian teachers' conceptions of contextualization, interdisciplinarity, conceptual focus, and other subjects related to chemistry education, aiming to overcome a still prevailing simplistic view. We consider it essential that secondary school students learn more chemistry concepts than mathematical relations underlying such concepts.
Acknowledgment
Financial support from The Brazilian Research Council (CNPQ), Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) is gratefully acknowledged.
References
Brasil, Secretária da Educação Média e Tecnológica, Parámetros Curriculares Nacionais para o Ensino Médio (PCNEM)-Ciências da Natureza, Matemática e suas Tecnologias, Brasilia, Brazil: MEC, 1999. [ Links ]
Brasil, Secretária da Educação Média e Tecnológica, PCN+ Ensino Médio: Orientações Educacionais complementares aos Parâmetros Curriculares Nacionais-Ciências da Natureza, Matemática e suas Tecnologias, Brasilia, Brazil: MEC, 2002. [ Links ]
Campos, R. C. & Silva, R. C., Functions of the inorganic chemistry... do they work?, Química Nova na Escola, 5(9), 18-22, 1999. [ Links ]
Carnoy, M., Gove, A. K. & Marshall, J. H., The reasons of the academic performance differences in Latin America: qualitative data of Brazil, Chile and Cuba, Revista Brasileira de Estudos Pedagógicos, 84(206/207/208), 7-33, 2003. [ Links ]
Cirino, M. M., Souza, A. R., Filho, O. S. & Carneiro, M. C., The mediation of the notion of probability in the development of the chemical kinetics concepts in the High School, Ciência & Educação, 15(1), 189-219, 2009. [ Links ]
Driel, J. V. & Gräber, W., The teaching and learning chemical equilibrium. In: J. K. Gilbert, R. Justi, J. H. V. Driel, O. D. Jong & D. F. Treagust (eds.), Chemical Education: Towards Research-Based Practice (pp. 271-292). Dordrecht, Netherlands: Kluwer, 2002. [ Links ]
Finley, F. N., Stewart, J., & Mahmoud, N. A., Teachers' perceptions of important and difficult science content, Science Education, 66(4), 531-538, 1982. [ Links ]
Galiazzi, M. C. & Moraes, R., Discursive textual analysis: a multiple face recontructive process, Ciência & Educação, 12(1), 117-128, 2006. [ Links ]
Galiazzi, M. C., Educar pela pesquisa: espaço de transformação e avanço na formação do professor de Ciências, Tese de Doutorado, Faculdade de Educação, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, 2000. [ Links ]
Garnett, P. J. & Treagust, D. F., Conceptual difficulties experienced by senior high school students of electrochemistry: electric circuits and oxidation-reduction equations, Journal of Research in Science Teaching, 29, 121-142, 1992a. [ Links ]
Garnett, P. J. & Treagust, D. F., Conceptual difficulties experienced by senior high school students of electrochemistry (galvanic) and electrolytic cells, Journal of Research in Science Teaching, 29, 1079-1099, 1992b. [ Links ]
Jong, O. D. & Treagust, D. F., The teaching and learning chemical equilibrium. In: J. K. Gilbert, R. Justi, J. H. V. Driel, O. D. Jong & D. F. Treagust (eds.), Chemical Education: Towards Research-Based Practice (pp. 317-338). Dordrecht, Netherlands: Kluwer, 2002. [ Links ]
Laboratorio Latinoamericano de Evaluación de la Calidad de la Educación (LLECE). Primer Estudio Internacional Comparativo sobre Lenguaje, Matemática y Factores Asociados en Tercero y Cuarto Grado. Santiago: UNESCO, 1998. [ Links ]
Lavaqui, V. & Batista, I. L., Interdisciplinarity in Science and Mathematics education at High School level, Ciência & Educação, 13(3), 399-420, 2007. [ Links ]
Leal, M. C. & Mortimer, E. F., Appropriation of a Chemistry curricular innovation discourse for secondary school teachers: perspectives and tensions, Ciência & Educação, 14(2), 213-231, 2008. [ Links ]
Lima, A., Marcondes, V. R. & Eunice, M., Experimental activities in the chemistry teaching: reflections of a group of teachers starting from the theme electrochemistry, Enseñanza de Las Ciencias, Special issue, 1-4, 2005. [ Links ]
Lima, K. E. C. & Vasconcelos, S. D., Analysis of science teaching methodology used by teachers from public schools in Recife, Ensaio: Avaliação e Políticas Públicas em Educação, 14(52), 397-412, 2006. [ Links ]
Lopes, A. C., Curriculum discourses in school Chemistry, Ciência & Educação, 11 (2), 263-278, 2005. [ Links ]
Machado, D. M. Z., Compêtencias vivenciais tecidas a partir das práticas curriculares das alunas do curso de Fonoaudiologia-UFSM: sua articulação com a assessoria psicológica, Dissertação de Mestrado, Centro de Educação, Universidade Federal de Santa Maria, Santa Maria, 2002. [ Links ]
Maldaner, O. A. & Piedade, M. C. T., Rethinking the Chemistry: the formation of teams of teachers/researchers as effective form of change of the classroom in chemistry, Química Nova na Escola, 1, 15-19, 1995. [ Links ]
Moraes, R., A storm of light: comprehension made possible by discursive textual analysis, Ciência & Educação, 9(2), 191-211, 2003. [ Links ]
Mortimer, E. F. & Amaral, L. O. F., The more warm better: heat and temperature in the termochemistry teaching, Química Nova na Escola, 4(7), 30-34, 1998. [ Links ]
Navarro, P. & Diaz, C., Analisis de contenido. In: Delgado, J. M. & Gutierrez, J., Métodos y técnicas cualitativas de investigación en ciencias sociales, Madrid, Spain: Sintesis, 1994. [ Links ]
Posada, J. M., Conceptions of high school students concerning the internal structure of metals and their electric conduction: structure and evolution, Science Education, 81, 445-467, 1997. [ Links ]
Quadros, A. L., Vieira, F. T., Lopes, C. M., Correa, J. M. M., Pinto, P. L., Nogueira, R. K., Fátima, A., O desempenho dos estudantes de ensino médio na Olimpíada Mineira de Química. In: XXII Encontro Regional da SBQ/MG, 2008, Belo Horizonte. Anais do XXII Encontro Regional da SBQ/MG, 2008. [ Links ]
Roque, N. F. & Silva, J. L. P. B., Chemical language and organic chemistry teaching, Química Nova, 31 (4), 921-923, 2008. [ Links ]
Rossi, A. V., Massarotto, A. M., Garcia, F. B. T., Anselmo, G. R. T., Marco, I. L. G., Curralero, I. C. B., Terra, J. & Zanini, S. M. C., Reflections on what is teached and what is learned about density at school, Química Nova na Escola, 14(30), 55-59, 2008. [ Links ]
Sanger, M. J. & Greenbowe, T. J., Common student misconceptions in electrochemistry: galvanic, electrolytic and concentration cells, Journal of Research in Science Teaching, 34(4), 377-398, 1997. [ Links ]
Strack, R., Marques, M. & Del Pino, J. C., For another path in the construction of knowledge in Chemistry Education, Química Nova na Escola, 31 (1), 18-22, 2009. [ Links ]
Vigotski, L. S., Pensamento e Linguagem, São Paulo, Brazil: Martins Fortes, 1993. [ Links ]














