Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Educación química
versão impressa ISSN 0187-893X
Educ. quím vol.20 no.1 Ciudad de México Jan. 2009
De aniversario
Do Gifted Students View and Use Mental Models Differently from Others?
Richard K. Coll*
* University of Waikato, New Zealand. E-mail: r.coll@waikato.ac.nz
Abstract
There is now a substantial body of research into learners' mental models of abstract scientific concepts. This research mostly confirms the obvious; that many learners struggle to understand abstract mental models in the way scientists or science teachers do. There is, however, a paucity of research into how gifted and talented science students view and use mental models in science. This paper explores this issue by examining mental models of chemical bonding for three highly gifted science students; one a senior secondary school student, a second an undergraduate, and third a PhD candidate also near completion. As might be expected, these highly gifted students held sophisticated mental models for all types of chemical bonding. Additionally, it seems they understood the limitations, purpose, and use of mental models in a similar way to that of scientists. This work provides insights into how the gifted conceptualize and use mental models, and provides insights into how teachers might move less gifted students towards a more sophisticated view of mental models in chemistry.
Keywords: Mental models; chemistry; gifted and talented; chemical bonding.
Models and Mental Models in Chemistry
Models and mental models are used extensively in chemistry and the sciences. Indeed, Harrison and Treagust (1998) say to understand chemistry is to understand its models. Models are used in chemistry for a variety of purposes: to produce simpler forms of objects or concepts, to provide stimulation and support for the visualisation of some phenomenon and to provide explanations for scientific phenomena (Gilbert, 1980; Gilbert, & Rutherford, 1998a, 1998b; Suckling, Suckling & Suckling, 1978). Models are used to explain or understand a target concept or object, and the use of a model inherently involves simplification of the target, the extent to which the target and source share attributes thus varies. In other words, the model comprises an approximation or less accurate representation of the target (Maksic, 1990). Because the purpose of models and modelling varies, models themselves vary considerably from the simple to the extraordinarily complex (Gilbert & Osborne, 1980). As Zumdahl (1989) see it, models vary from simple models used to predict behaviour (e.g., equations, ball-and-stick models, etc.), to much more complicated models that are used to account very precisely for observed quantitative behaviour (e.g., quantum mechanics).
Because there are so many types of models, the literature contains a variety of attempts to classify models, with such typologies usually based on the nature of the model. Coll (2006) broadly classifies into physical models and conceptual-symbolic models. Physical models are such things as scale models of physical structures to be built (Harrison & Treagust 1998) or ball-and-stick models of molecular structures. Physical models are typically used to represent external physical characteristics of the target (Coll, France & Taylor, 2005). Conceptual/symbolic models consist of mental constructs that are used to explain the world, observations or results of experiments. These models are thus mental models, and include analogies (Justi & Gilbert, 2006), equations (Harrison & Treagust, 1998, 2000, 2006), maps (Coll, 2006; Harrison & Treagust, 1998), theoretical models (Harrison & Treagust 2006), and mathematical equations/models (Harrison & Treagust, 1998, 2006).
Experts' and Students' Mental Models for Science and Chemistry Concepts
Experts understand that mental models, like other models, are human constructs designed to serve a purpose; be that generation of new knowledge, explanation of complex or un-observable behaviour, and so on. A model seeks to simplify the target concept or object, and thereby is a 'facsimile' of the target. Thus, models by their very nature are approximations of reality — in other words, every model possesses some limitations. This is not a fault or flaw of models; the very simplification that arises from the model's inherent limitations is that which allows experts to focus on some particular aspect of the target.
Experts understand the nature of models, and understand that models including mental models possess inherent limitations, but use them in very pragmatic ways because they are useful in explaining or understanding chemistry concepts. For example, an expert may well use a simple model like Ligand Field Theory to explain the absorption spectra of organometallic substances, despite knowing this model fails to explain many other observations (such as the spectrochemical series). Likewise, a shorthand diagrammatic model of an aromatic compound may appear to indicate it possesses double bonds (Figure 1); the expert knows it does not, but finds such two-dimensional representations convenient in terms of electron bookkeeping, or to show electron movement when considering chemical reaction schemes.

The literature, as might be expected, suggests that students' mental models differ greatly from experts. A substantial body of research now indicates that students prefer realistic looking mental models, often confusing mental models with reality (see, e.g., Smit & Finegold, 1995; Stavy, 1991, 1995, Stavy & Tirosh, 1993). This is unsurprising in the case of younger students, especially when they are grappling with abstract or microscopic concepts. What is perhaps more surprising is that Coll and Treagust (2003a, 2003b) report that even advanced students (e.g., university undergraduates, and postgraduates) also prefer atomic/molecular level, mental that are like copies of reality (e.g., atoms are like small, hard spheres).
However, it remains unclear if gifted and talented students view and use mental models for abstract science concepts in ways that are different from their less able peers. First, we need to consider what it means to be gifted and/or talented, before looking at the present work, which looks at their views of and use of mental models for some chemistry concepts.
Gifted and Talented Students
Traditional views of gifted and talented students tended to see them as students who significantly out-perform their peers in tests or examinations (Moltzen, 2004). This is usually taken to be a relatively small proportion of any cohort. Gifted students are generally taken to be academically able students (Moltzen, Riley & McAlpine; Taber, 2007), whereas talented students are those who out-perform their peers in creative, sport or cultural pursuits (Taber, 2007). Gifted and talented students are those with attributes of both the gifted and talented. Taber (2007) notes that in the UK the Department for Education and Skills (DfES) requires schools to identify the "top 5-10% of pupils in each relevant year group" (Taber, 2007, p. 2). He further notes that the UK DfES further classifies the top 1% of pupils in a given year group as 'exceptionally able'.
It remains unclear how any school or teacher is supposed to actually identify the gifted, talented, gifted and talented, and exceptionally able. Moltzen, Riley and McAlpine (2001) along with Riley (2003) comment that in New Zealand at least, gifted students are identified using multiple means. The three most common approaches are a combination of standardised measures of achievement, observations by teachers and parents, and standardised evaluations of portfolios or performances (Frasier, 1997). However, the literature suggests that there remains heavy reliance on a few techniques used singly; namely, teacher identification and standardised testing. In particular, cultural, spiritual, and emotional giftedness are of-ten overlooked. Such identification practice, it seems likely, excludes many individuals that are gifted in ways not measured by such traditional means. For example, some indigenous peoples see gifted as encompassing not just exceptional ability in terms of academic performance, but also in terms of performance in the arts, sport, leadership and service to the community as well as spiritual and emotional qualities and pride in self— or cultural—identity (Bevan-Brown, 1996; Keen, 2001). Such disparity may be as a result of differing perceptions of gifted and likely leads to students from some minority groups being underrepresented in those labelled gifted.
Strenberg (1993) proposes five criteria we might use to judge gifted students: excellence, that is, they are extremely good at something relative to their peers; rarity, that is, a high level of an attribute that is rare amongst their peers; productivity, that is, excellence must potentially lead to productivity; demonstrability, that is, they are able to demonstrate their ability via one or more valid tests; and value, that is, the excellence demonstrated must be considered of value in the society that judges them as gifted.
Research Aims
The research reported in this work emerged from a large study of students' preferred mental models for chemical bonding. During the main study, several participants stood out in terms of academic ability (based on teacher identification, and examination of academic transcripts). To illustrate the level of academic achievement, the secondary school student (at the time) was one of few New Zealanders awarded a tertiary education 'scholarship' in external summative examinations (usually about 2-3% of the population see Benson-Pope, 2005), the undergraduate students and masters student achieved grades of A+ (above 85%) or A++ (above 90%) for all courses and A++ for all their chemistry courses, as did the PhD candidate. The researcher did not seek to identify if these participants were talented or gifted in other ways (e.g., sport, cultural, etc.). Hence, they represent classically-defined gifted students based on the summary definition of gifted provided by Taber (2007); that is, those who are able to achieve exceptionally in academic terms, and those who are able to undertake some science-related task at a level of demand. The former facet is based on their prior performance in exams as described above, and the latter as we shall see based on data presented in the research findings.
Methodology
Sample
The sample comprised three individuals identified during the main study as described above. Claire, a mature 17 year-old European Year 13 student, was confident and outspoken with a strong academic record in chemistry and the sciences. She had carried out some school-level peer-tutoring, although not in the sciences. Her future plans included a career in engineering, applied science, or a technology-oriented field such as biotechnology. Steve, a second-year undergraduate, by way of contrast, was a much quieter and a rather reserved individual. An outstanding academic achiever, he was very measured and thoughtful in his responses. He stated that he enjoyed chemistry and said he had done no tutoring and intended pursuing postgraduate studies to the doctoral-level (an ambition he subsequently realized). Jason, a 26 year-old European, was a student of extremely high academic ability who graduated with BSc and first class MSc (Hons). At the time of interview, he had just secured a post-doctoral research fellowship at one of the world's most prestigious universities (which subsequently led to a faculty appointment). His research area was concerned with organometallic syntheses and the spectrometric investigation of organometallic complexes. He was a relaxed and confident individual, with considerable chemistry tutoring experience, particularly at the first-year level.
Research Methods: Theoretical Basis and Overview
The main study sought to ascertain the preferred mental models participants held about chemical bonding in a variety of substances. The researcher first developed a conceptual theme for the inquiry and this is detailed in Figure 2. Substances were classified into three types; metallic, ionic and covalent, in terms of their chemical bonding. Such classification is a little arbitrary since modern theories of chemical bonding make no such distinctions. Interestingly, inspection of common chemistry textbooks and curriculum material encountered by learners, including those involved in this inquiry, reveals that there is considerable discrepancy in treatment of the models used to explain theories of chemical bonding. For example, Gillespie, Humphreys, Baird, and Robinson (1986) offer one view of metallic bonding "because of their small core charges, metals have little tendency to accept electrons to form negative ions. Covalent bonding is also not possible in solid metals, because a metal atom does not have enough valence electrons to form covalent bonds to all its 12 or 8 neighbouring atoms" (p. 491). However, elsewhere metallic bonding is described as covalent in nature: "Metals are just covalently bonded solids with partially filled energy bands, and do not require any special bonding mechanism for their understanding" ("Metallic Bonding", 1994, p. 1965). How is it possible to reconcile such disparate views and have confidence that a description of mental models for chemical bonding represents the scientists' views? In this work, the dilemma has been addressed from a social constructivist view of learning.
It is an illusion that there is knowledge in textbooks or documents. They contain language, which is a string of words, deposited in them by authors. The words have meaning for the authors and the readers and interpreters, each one of whom has built up [his or] her subjective meanings according to [his or] her individual experience. Though these individual meanings are constructs that have been through a certain amount of social adaptation (because their users have socially interacted with others), they remain subjective and to some extent idiosyncratic. (Von Glasersfeld, 1993, p. 30)
This inquiry is based on a social constructivist standpoint; it is the view of this researcher that the scientists' view thus represents a social construction and the way this was addressed is described below beginning with the theoretical basis to the work.
The theoretical basis for this inquiry is Norman's (1983) typology of mental models; that is, a target system, the conceptual model of the target system, the user's mental model of the target system and the scientists' conceptualisation of the target system. Based on Norman's typology, chemical bonding has been classified into a series of three target systems, namely, metallic, ionic and covalent bonding. Examination of curriculum material and interviews with instructors resulted in the identification of a series of target models for each of these target systems (Figure 2). These models were then socially-negotiated with chemistry educators, a process that formed the first phase of the data collection (see below).
Research Methods: Socially-negotiated Mental Models and Interview Protocol
Data collection comprised two phases. The first phase involved a detailed examination of curriculum material combined with informal interviews with the instructors involved in the inquiry; lesson plans, lecture notes, textbooks, and workbooks used by learners. The researcher then synthesized these data sources to produce a description of each of the target models in Figure 2. A panel of eight experts with no contractual interest in the study validated these descriptions and negotiated with the researcher a series of criterial attributes (Gilbert,Watts & Osbourne,1985);facts considered the minimum necessary knowledge which participants needed to possess before they could be said to understand the target models.
The second phase of data collection involved face-to-face interviews, including an IAE approach (Gilbert et al., 1985; White & Gunstone, 1992). The data corpus thus consisted of participant-validated verbatim transcripts, together with drawings produced by the learners during interviews. For each target system in turn, there were three phases to the interview. The interview began with the researcher showing the participant a familiar sample or object; in the case of metallic bonding, aluminium foil and steel wool, sodium chloride and lithium chloride in the case of ionic bonding, and molecular iodine (I2) and chloroform (CHCl3) in the case of covalent bonding. The participant was then asked to explain the bonding in the substance in terms of his or her understanding of chemical bonding. Participants were encouraged to draw their models, and at the beginning of the interview, most learners indicated a preference for a given model, for example, the sea of electrons model or the octet rule. Once learners had indicated such a preference, the researcher probed his or her familiarity with the model using the criterial attributes deemed by the independent experts to comprise the essential facts about the model (e.g., for the sea of electrons model, the lattice like structure of the positive cores, the delocalization of electrons, etc.). If a learner did not identify a preferred model, the researcher simply probed his or her understanding of chemical bonding for that target system during the interviews.
Research Findings
The mental models preferred by these gifted students differed markedly from those of their peers. We use the term preferred mental model here deliberately. What has happened in effect is that each participant has been asked to describe the mental model they first thought of when probed — what we consider to be their preferred mental model. Less gifted peers preferred simple realistic-appearing mental models, and this was reflected in their discourse and drawings. Their models also frequently contained alternative conceptions. Each target system is now considered in turn.
Metallic Bonding
David. The way I think of it is as metallic bonding 'cos we learnt it in chemistry, and I think the image I have got of that is just the metal with a sea of electrons, it's just how I think of it.
Interviewer. I am just trying to get an idea of what that
actually means to you when you say sea of electrons. What picture do you have of that?
David. Oh just like, the sort of mental image I would have is like, sort of sandwich type thing.
Interviewer. Do you want to draw that, just so I am clear on what you mean?
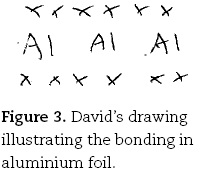
David. Yeah sure [draws Figure 3]. I don't think I know if there is any stuff between the atoms.
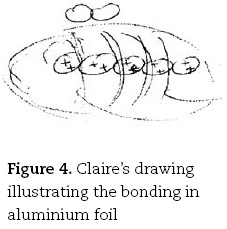
This contrasts with the mental models preferred by more gifted students. Claire, for example, like her less able peers, identified the sea of electrons as her preferred mental model for the bonding in metals, but quickly identified the salient features in her description and drawing (Figure 4):
Claire: The electrons are all free, but the, nuclei are together.
Interviewer. Right.
Claire. I don't know, in layers or not, and the electrons are free between [drawing row of circles with + signs inside them, and surrounded by an ellipsoid] those sort of move around [draws curved lines from top to bottom of ellipse, Figure 4] which is why they conduct because the electrons are free to move and that's the current.
Interviewer. Right.
Claire. Because you can see that the electrons are all moving around, and they are not attached, and you have got the nuclei there.
Hence, whilst gifted students and less gifted identified the same preferred mental model (the sea of electrons here), the gifted provided more detailed, complete descriptions and drawings that made more sense. The mental models described by less gifted undergraduates and graduates were very similar to those of their school level counterparts. However, gifted undergraduates and graduates used more scientific terminology, but essentially provided a description the same as Claire's. Steve, for example, said metallic bonding consisted of "a row of monovalent cations, cations in a delocalised sea of electrons around it, and that's metal bonding ... the electrostatic forces of attraction between cations, the aluminium cations and the electrons. So they form a sort of network of attraction ...". Jason likewise provided a fairly detailed description (although his drawing is fairly simple) peppered with jargon, and tried to illustrate his understanding of aspects of the lattice (Figure 5):

Jason. A whole lot of metal atoms basically all stacked as closely as possible together. There's a bunch of different metal structures like cubic and hexagonal close-packing and then the body-centred cubic, so basically all the metal atoms are as close together as they can possibly be.
Jason. I know that for example that you can have structures such as hexagonal close-packed and cubic close-packed which I am not sure I can describe. It's not until you get an actual model yourself and you can show that they are different and you can actually see they are different, but metals will hold to one structure or another.
The gifted participants also were able to use their preferred mental models for the bonding in metals to explain the nature of alloys, and the properties of metals such as conductivity and malleability. Less gifted participants particularly struggled with these concepts — Brian's simplistic observation that "the bonding in an alloy would be identical" to that in a pure metal, contrasts with Jason's more detailed description:
Jason. How I would see the bonding, the bonding would be fairly similar to the previous sample [i.e., aluminium foil]. The only difference is that this is actually steel, and the only difference is that it has got some interstitial carbon in it.
Interviewer. OK, could you just tell me what you mean by interstitial carbon?
Jason. Well when you pack the iron spheres together there's still space you can't, they're not cubes, so they can't pack completely, so there's no space at all.
Interviewer. So they are not cubes?
Jason. The atoms, no. I am sort of regarding them as spheres. So there's space and if you do the sort of geometric modelling, there's about twenty-four percent or so I think.
Interviewer. Twenty-four percent?
Jason. Space, and so the rest of this can be filled up by other smaller atoms and steel is, um, carbon and iron all mixed together in a certain percentage. Those carbon atoms are incorporated in the lattice, so the carbon atoms almost take on a, they help to lock the structure in place and that's why that why sort of steel is harder than iron.
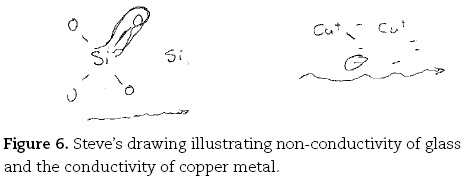
Likewise Anne's comment that "copper's got ions that can allow a flow of electrons to go through it", contrasts sharply with Steve's complex explanation fo conductivity of metals in comparison with non-conductors like a glass rod (the probe used here):
Steve. OK with the glass rod you have got, it is basically a silica structure inside the glass, and that's silicon covalently bonded in a sort of silica structure with oxygen's [drawing SiO4 unit, LHS Figure 6], sort of units like so. Whereas in the copper wire, you have got the metal bonding that we talked of before, with a delocalised sea of electrons [draws two Cu+ with negative signs around them, RHS Figure 6] and so when you apply a potential to the copper wire, it enables the electrons to flow freely in the delocalised sea from one side to another [draws arrow].
Interviewer. Oh yeah, I see.
Steve. Setting up a current and that's why you get your light glowing. But because you have got covalent bonding, and electron sharing rather than the electrostatic sort of bonding that you have got with the metal bonding, it's not possible for the electrons to move through the system freely [draws arrow under SiO4 unit].
Interviewer. Why is that not possible in that case?
Steve. Because the electrons are tied into this covalent bond here [draws line enclosing one Si-O], where they are shared between the silicon and the oxygen rather than being in a sea of delocalised electrons.
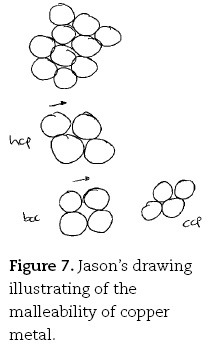
The more complex the problem, the more sophisticated the explanation provided by the gifted students. Jason, for example, went into considerable detail when explaining the reason for the malleability of copper metal:
Jason. I am drawing spheres together, but because the forces, because they are not particularly directional, each one has sort of 12 nearest neighbours, they can be pushed past one another with reasonable ease.
Interviewer. So what happens when they are pushed past each other?
Jason. Well I guess I basically see this [draws four more circles close together; middle part of Figure 7] go through a process where they start in this kind of configuration here, which is hexagonal-close packed [writes hcp next to middle circles].
Interviewer. hcp, yep.
Jason. OK, and then it experiences a stress in one direction or another. It slips [draws arrow above middle circles] into this kind of configuration [drawing four spheres in cubic arrangement; lower LHS of Figure 7, writes bcc] in which it has less nearest neighbours. I've just drawn it in two dimensions here to make it simple.
Interviewer. Yep, OK.
Jason. Like this one would have six nearest neighbours [indicating the hcp structure] and this one would just have four [indicating bcc]. So because the directionality isn't too strong, it can endure going from twelve, ah, six nearest neighbours, to four, and so that's bcc. Then as it experiences more stress, it sort of flops back over into the other one here [draws four circles in diamond shape; lower RHS of Figure 7, writes ccp].
Interviewer. So it's going from sitting in the gap to sitting on top and then back into the gap.
Jason. Exactly.
Ionic Bonding
As for metallic bonding, gifted students' preferred mental models for ionic bonding were described in considerably more detail than their less gifted counterparts. So, for example, David drew the structure in Figure 8, and said: "the Na is the positive one and the Cl the negative one ... because there's the attraction between the positive and negative charge they are bonded together".

Contrast this with Claire's description, in which she went further explaining via the octet rule why the sodium and chloride ion from in the first place:
Sodium is over here somewhere [drawing Periodic Table outline; draws square on LHS], and the chlorine is up here somewhere [draws square on RHS]. The electronegativity goes that way [indicating from left to right on the Periodic Table]. Chlorine, chloride, has a really strong attraction for electrons [drawing Cl— with arrows pointing toward it] and, um, the sodium ion is holding that one electron in the outer shell really loosely [writes Na+] so it is easily attracted to the chloride [draws arrow towards Cl]. The sodium loses and the chloride gains, spends most of its time with the chloride, so it is sort of ionic, yeah you can almost say it has been transferred.
Some of the other less gifted school students also related the formation of ionic compounds to the octet rule, but here, Claire has gone further, and related this to periodic trends.
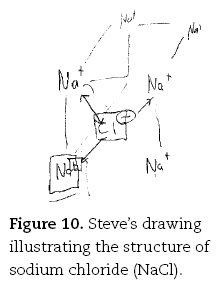
Descriptions of participants' preferred mental models for ionic bonding of the gifted undergraduates were likewise more sophisticated than their less gifted counterparts, and also more sophisticated than the school students. So Bob was able to note numerous features of the structure and bonding in sodium chloride: "You have got sodium cations and chloride anions ... the anions tend to be bigger than the cations ... the anions pack together in similar ways to the metal atoms in like cubic close-packed or hexagonal close-packed ... you are actually going to have a gap in here where as sodium can fit in". But Steve was more specific and detailed in his description of the bonding in sodium chloride, mentioning, for example, the regular array and the repeating nature of the arrangement of ions:
Well once again you have got a regular repeating, a regular sort of repeating structure in an infinitely extended network of sodium plus [draws Na+, Figure 10] in cubic arrangement ions, ah, sodium plus cations and chloride minus anions [draws Cl— in centre]. So you have, I can't quite remember what the exact structure is — I should remember that. But you have got a regular array of say Na+ and then they have a Cl—, and they have repeating thing in three dimensional units. I can't quite remember, it's either face-centred cubic or body-centred cubic.
Again Jason's description of his preferred mental model for the bonding in sodium chloride was substantially more sophisticated that that of his less gifted peers. Other less gifted graduates provided descriptions similar to those of the less gifted undergraduates, but Jason's model contains some sophisticated features; for example, the notion of repulsion by like ions at a distance, and again he presents a considerable amount of scientific terminology:
Jason. OK, sodium chloride is a sort of a classic cubic structure where you have each sodium and each chlorine surrounded by a six ... [drawing Cl in a circle with Na in circles surrounding it, Figure 11]. So you get something that looks like this. So you get a chlorine which is surrounded by six sodiums and ...
Interviewer. OK so you are just drawing a central chlorine and then there's six sodium, and you're drawing lines between them.
Jason. This is not really indicating the bonding in any way.
Interviewer. So what are those lines indicating to you what do you mean by those lines?
Jason. They're just showing the three dimensional nature of the diagram, I have drawn them more than to indicate bonding. In this case, I see the chlorine as being not a chlorine atom, but a chlorine ion. So a chlorine which has gained an electron from the sodium atom, so that the chlorine atom has a negative charge. The sodium has a positive charge. It's like this structure is essentially held together by electrostatic interactions.
Interviewer. OK I see what you mean.
Jason. Now there's also repulsions as well, because in this area here you also have another chlorine atom which is reasonably close to that chlorine atom so there's repulsions between the two.
Interviewer. Between those two chlorines.
Jason. Yep. Well it obviously this is extended into three dimensions.
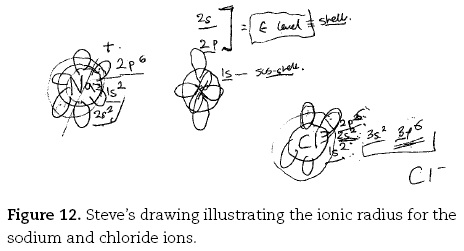
As seen in the case of metallic bonding, the gifted students were able to use their mental models to explain some of the unusual characteristics of ionic bonding (such as the ionic covalent continuum), and of the properties of ionic substances more easily than their less gifted counterparts. So whilst most undergraduates said the bonding in lithium chloride was similar to that in sodium chloride — Bob, for example, saying "lithium one plus would be a lot smaller than sodium", Steve provided a complex explanation of ionic size. Without the use of a Periodic Table, Steve, provided a comprehensive explanation, relating the size of the ions to the electronic sub-shells or orbitals in the respective atoms.
The Na+ ion has simply the 1s2,the 2s2 and the 2p6, whereas the chlorine's filled the 1s2, the 2s2,the 2p6,the 3s2 and the 3p6 [Figure 12]. Because it's filled more orbitals, and as the orbital energy, um... I am just trying to think about this. As you get more and more orbitals, they go out further and further and further away form the centre of the nucleus itself. So the radius, r, increases. So that as you go to a new energy level, so that's filled up to the end of the two. But that's filled up to the end of the three, and so that's going to be a larger radius by quite a bit.
Likewise, in the case of the graduates, Jason introduced the notion of polarisability of electron clouds to explain some differences in bonding between sodium and lithium chloride:
Jason. I see them [the chloride ion and sodium ion], because neither of them are particularly polarisable, they are fairly spherical.
Interviewer. OK, you mentioned polarisable. What do you mean by that term?
Jason. I kind of regard polarisability as sort of the floppiness of the anion [respondent laughs]. It's sort of big and easily deformed. I guess I sort of associate it with the softness of the anions as well. Now I have to describe what floppiness and softness is?
Interviewer. OK that's alright, you can keep going. So you are saying floppy or soft...
Jason. Yeah, and if you have a polarising anion, ah, cation, the cation would be small and highly charged and so it would have a tendency to distort the electrons that are near by, of the nearby anion. So, for example, if you had your sodium and your iodide or something, you'd have a very strongly polarising cation and a very polarisable anion. So you're probably not going to have strict ionic bonding like you have here quite a different thing altogether.
Interviewer. So what would be different? What would be the different type of bonding other than ionic?
Jason. That's when you would get more into covalent with the sharing of the electron.
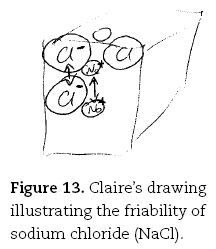
Likewise, school students typically said the reason sodium chloride was friable (i.e., easily crushed) and silica was not, was as Anne put it "the bonding in sodium chloride is weak" — seemingly unaware of the fact that the high melting point indicates otherwise. In contrast, Claire offered a rich explanation:
The sodium chloride, they are held in place by really strong electromagnetic forces between them, between the atoms because of the big electronegativity difference [drawing cube with Na and Cl inside circles, Figure 13]. So when the force pushes them closer together these two strongly negative ions will repel each other, same with the positive ions [draws double-headed arrows between ions]. Because they are very strongly charged so that causes the crystal to break up into smaller bits.
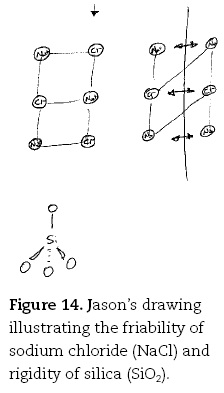
Not surprisingly, whilst his peers also said friability was due to weak bonds in sodium chloride, Jason once again provided a highly detailed explanation:
Jason. The sodium and sodium chloride [drawing rows of Na+ and Cl—, top LHS of Figure 14], you start with a situation where opposing charges are lining up and that's providing the attractive force. OK so you have this sort of system, and then when you apply a force, so say a shear force which pushes [drawing an arrow at top of one row of ions].
Interviewer. Along one line yep OK.
Jason. There's sodium, chlorine, sodium and then that force has all of a sudden pushed the chlorine next to the chlorine [drawing second row of Na+ and Cl— alongside each other]. So there is now no sort of bonding forces in this direction now [draws diagonal lines linking Na+ and Or, top RHS of Figure 14].
Interviewer. So those are the illustrating the friability of ones on the angle, and they sodium chloride (NaCl) and are the bonding forces?
Jason. Yep, and now you have a repulsive force which is right next to each other [drawing double-headed arrows between ions] pushing each other apart, and so therefore you get a shear down there [draws a line between rows of ions].
Interviewer. Right down the middle OK.
Jason. So it's broken apart. Silica on the other hand is a lot stronger. So that's why we don't end up with grains of sand on our beaches. Silica has got covalent bonding where each silicon is surrounded tetrahedrally by a by four oxygen atoms [drawing SiO4 unit in tetrahedral configuration] and these are covalent bonds which are extremely strong, and they don't break particularly easily at all.
Covalent Bonding
It was in the case of covalent bonding that the biggest differences were evident between the preferred mental models of gifted and less gifted students. Less gifted students explained covalent bonding almost exclusively in terms of the rather simple octet rule, despite in the case of undergraduates and graduates being exposed to a large variety of fairly sophisticated models (i.e., those in Figure 2) in their studies. When shown substances such as molecular iodine (I2) and chloroform (CHCl3) they talked about "sharing of electrons" which was due to the fact that for instance carbon "has got room for four more [electrons] and it can get one, like share one with Cl and H". In other words, the octet rule dominated explanations for covalent bonding. Claire also drew upon the octet rule, but introduced notions of electronegativity and unequal sharing:
Claire. That one is covalent, because they are identical [drawing two 'I's inside overlapping circles, Figure 15] and there's no electronegativity difference and...
Interviewer. Between what?
Claire. Between the two atoms that are bonded together. So it's not polar the electrons spend an equal amount of time between each atom. The shared one's that is. They share two electrons because each of them needs to gain one [draws two crosses between 'I's].
Interviewer. Each 'I' is it?
Claire. Yeah, that's it yeah.
Interviewer. Why is gaining that one, why does it need to gain that one?
Claire. Um, to get a full shell [draws two 'I's surrounded by circles with seven dots on one circle, and seven crosses on second circle].
Interviewer. OK, I see OK.
Claire. And you join those two [circles one cross and one dot] that one can count that one.
Less gifted undergraduates provided descriptions not unlike Claire's (a Year 13 school student), as can be seen in Reneé's description of the bonding in molecular iodine:
Reneé. OK in this case they are sharing electrons equally, like neither of them has a higher affinity if you like, and they are forming an electron pair which makes it into a molecule.
Interviewer. OK can you just tell me what that sharing looks like to you? How does that come about if you like?
Reneé. Lewis dot diagrams [respondent laughs].
Interviewer. OK that's fine. Whatever way you see it. That's what I am interested in.
Reneé. OK [draws two 'I's, one with small crosses, and the other with dots around it, Figure 16].
Interviewer. OK you have drawn a couple of 'I's there, and you have drawn some crosses around one [respondent laughs]. OK that's fine. So the crosses and dots, what are they indicating?
Reneé. They are the electrons for each respective atom, and, um, just to complete the octet the, the single electron, one from each iodine, forms a covalent bond.
Interviewer. Right OK. So the cross and dots they are...?
Reneé. Oh they are just sort of representative of the electrons, like seven electrons for one, and seven electrons for the other.
This might look like a reasonable description of the bonding in a covalent substance, but it contrasts dramatically with Steve's highly sophisticated molecular orbital description, which is presented below. It is interesting to note that Steve at first presented a description of the bonding in molecular iodine very much like that of Reneé. However, Steve spontaneously introduced the molecular orbital theory, and went on to produce a description that was enormously rich and detailed; all the more remarkable given the spontaneous nature of his response. He began by considering the atomic orbitals involved in the formation of a bond.
Steve. Of course I suppose you could look at that in terms of a molecular orbital diagram and draw the molecular orbital which covers both [draws larger perimeter encircling I-I group, Figure 17] which covers both atoms as well.
Interviewer. OK how do you see that?
Steve. Well if you were to take the iodine, if I remember it, you are going to have your 1s2, and your 2s2, 2p6, your 3s2, and then it's going to be 3p6 as well isn't it, 4s2? Oh hang on a sec, 4s2, 3 d10, 4p that would be 5, I guess, if I can remember it right [draws 1s2, 2s2, 2p6, 3 s2, 3p6, 4s2, 3d10 and 4p5 in a column, RHS top of Figure 17]. It's a bit of a struggle sometimes. So you would have atomic orbitals there [draws two sets of three closely spaced lines, writes AO next to them], there, and there, and then they come together and form the same number of molecular orbitals. I could draw a diagram I suppose. You don't need to worry about these ones because they are completely filled [draws line through 1s2, 2s2, 2p6, 3s2 and 3p6], so it's just the overlap of the, of these.
Interviewer. OK the outer ones.
He then constructed an energy level diagram from the atomic orbitals that he described previously.
Forming the bonding and antibonding combinations [draws series of lines between two previous sets of three lines and links up]. OK yeah [writes bonding and antibonding at top and bottom of lines respectively] and of course you would have to have the same number of molecular orbitals as atomic orbitals.
This was followed by a detailed description of the role of bonding and antibonding molecular orbitals.
Interviewer. OK. The bonding and antibonding you talked about there. Can you just tell me little bit more about those?
Steve. OK well if you take the case of iodine [writes I2] you have got in this case two atomic orbitals, one for each iodine—two atomic orbitals. Now when the covalent bond is formed the atomic orbitals overlap and they form the same number of molecular orbitals.
Interviewer. Right.
Steve. That's the conservation of orbital rule.
Interviewer. Right.
Steve. Four and six is actually going to give you three isn't it? Interviewer. Three, OK.
Steve. So you have got three different p orbitals.
Interviewer. Yep, OK.
Steve. So you form these two overall combinations depending on the phase of the lobes when they overlap [draws two sets of dumbbells linked by line at centre].
Steve then related the boundary surface depictions to molecular orbital energy levels which he used to relate to bond formation.
Interviewer. That's those sort of dumbbells you have drawn is it?
Steve. Yeah dumbbell drawings, and so you get the two molecular orbital states; bonding, which is lower in energy than the individual atomic orbitals that you started with, and the antibonding, which is higher in energy that the two atomic orbitals that we started with.
Interviewer. Could you just sort of relate those lobes you drew to the bonding and antibonding, just so I am clear on that?
Steve. I need to be sure of my signs in this case, I believe, I am just trying to think about it now...
Interviewer. What is it that those signs are indicating, that you have drawn there?
Steve. OK I am trying to think of how to describe that. Well it's basically the sign of the wavefunction [writes Ψ under each lobe and + and - beside them]. So you have got the wavefunction describing each individual atomic orbital, and each one has overall a positive sign or a negative sign, and so when the two are, I think when the two are positive may be the bonding combination, the positive and negative the antibonding I can't actually quite remember.
Finally he used the energy level diagram to deduce bond order for molecular iodine (I2).
Interviewer. OK that's fine. OK so in this case how would you see the bonding form in relation to say that energy level diagram you have drawn there?
Steve. Well take each one of these orbitals here, they would be occupied by, in this case there is five, so you would have, one, two three, four five [draws half arrows in atomic orbital levels].
Interviewer. Right, that's in your atomic orbitals, yep.
Steve. Yep and I am just trying to think how the degeneracies, and what have you work out, but I think some of these would be degenerate [indicates closely spaced lines].
Interviewer. That's your molecular orbitals you have drawn there right?
Steve. Yeah, and I think there maybe a non-bonding level. I can't quite remember [draws two lines in centre of molecular orbital diagram]. But they would fill from the lowest energy to the highest energy, so you get X number in the bonding, and maybe something similar in the non-bonding, and some in the anti-bonding, and the number in the antibonding cancels out some in the bonding.
Interviewer. Cancels out, right.
Steve. That determines the overall strength of the bond. So it's essentially the net number of electrons that is in the bonding orbital [draws rectangle around lower levels in molecular orbital diagram].
Interviewer. OK so it'd be that rectangle you have drawn. So the net number in there?
Steve. Yeah, after you have taken into account the number in the antibonding it's an indicator of the bond strength.
Steve also provided a molecular orbital-based description for the bonding in chloroform (CHCl3) although it was not as sophisticated as his description for iodine, presumably because of the complexity of the energy level diagrams (and likely that he was never taught this).
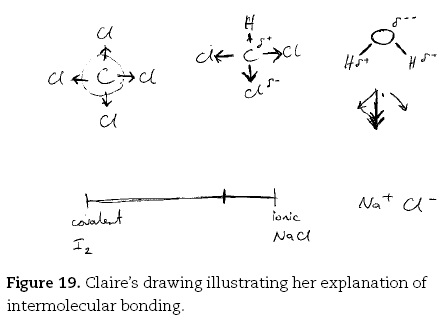
Again the gifted students' descriptions of the minutiae of their preferred mental models were impressive. Claire returned to her theme of the importance of electronegativity when describing the polar nature of the chemical bonding in chloroform (CHCl3):
Claire. They are all kind of covalent bonds. The carbonchloride bond is polar. It will be polar because of that electronegativity difference, the same as the sodium. It's not enough of a difference to become an ionic bond. So it's just polar covalent with the chloride.
Interviewer. Can you tell me what you mean by polar covalent so I am clear on that?
Claire. The electrons that they are sharing, they are sharing two electrons. Because the chloride, the chlorine, wants to gain one, so it just borrows one of the carbon ones. But these two shared electrons aren't, they are not equally shared. Because the chloride's got a strong, a more strong attraction. So that's going to go [draws arrow from C to Cl, Figure 18], and the hydrogen is still a polar bond because it can't be exactly non-polar because the atoms aren't identical like the iodine ones. But it's not much of a difference so it's a little bit polar.
Similarly, Claire was able to use her preferred mental model for the bonding in covalent substances to explain one IAE card showing the deviation of streams of liquid from a burette as they flowed past a charged rod (water, chloroform and carbon tetrachloride):
They sort of all equally pull on this carbon, and they all cancel each other out. So the molecule is non-polar. So there's no effect, whether you put a positive or negative rod, because the molecule itself is not charged. Chloroform on the other hand [draws planar CHCl3 molecule, top middle part of Figure 19] has got those three, which are highly polar bonds [draws arrows from C to the Cls]. That one is not as polar, so you have got those and just a little one [draws small arrow from C to H]. I don't know which way it goes, but those two cancel each other out [indicating 'Cl's opposite each other]. So you are left with the effect of those two [indicating the H and remaining Cl] which makes that one delta minus and that delta plus [writes δ+ near H and δ- near Cl] so they line up. The delta minus end is attracted towards the positively charged rod. Water is quite highly charged because that's [indicating oxygen] the second most electronegative atom [drawing bent H2O molecule] apart from chloride and fluoride. So you have got two polar bonds here, which can be represented by those two vectors [draws arrows under H2O molecule] which add to give that [draws heavy arrow between arrows].
Claire went on to explain the origin of the polar covalent bond which she related to the concept of the ionic-covalent continuum and to samples she had encountered previously during her interview, that is, NaCl and I2.
Interviewer. Can you just tell me a little bit about those delta minus and delta plus signs. What are they indicating to you?
Claire. Because it's a polar bond. There's the continuum between the covalent like iodine [draws line, writes covalent and I2 at one end, and ionic and NaCl at other, lower part of Figure 19], and the ionic which is usually taken to be sodium chloride, and like all the other bonds fall into the sort of continuum. I don't know where the carbon chlorine would be, but it'd be, it's quite polar.
Steve was likewise able to provide an explanation for variations in boiling point trends with periodic group number (Figure 20 which was used as a probe in the interviews about the use of preferred mental models of covalent bonding):
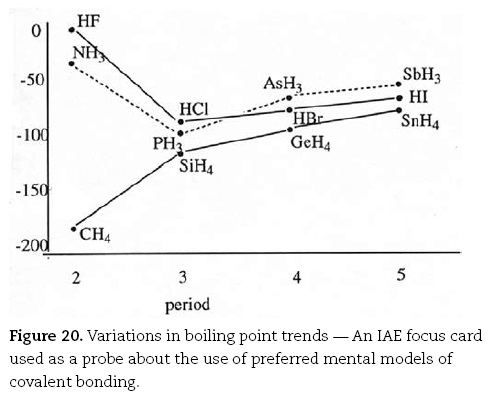
Steve. Well you have got a lone pair of electrons on the nitrogen. I suppose it means that part of the molecule is slightly electronegative, and the other part is slightly electropositive. So naturally there is going to be some sort of attraction between the two, which is van der Waals. In this case here [indicates PH3] you have got the same thing.
Interviewer. That's the PH3 is it?
Steve. Yes. But in this case now with the PH3, the electronegativity of the phosphorus with respect to the nitrogen is once again significantly reduced. So that's not so electronegative so therefore the interaction is not as strong.
Characteristically, Jason provided the most sophisticated explanation of the trends in boiling points for covalent substances:
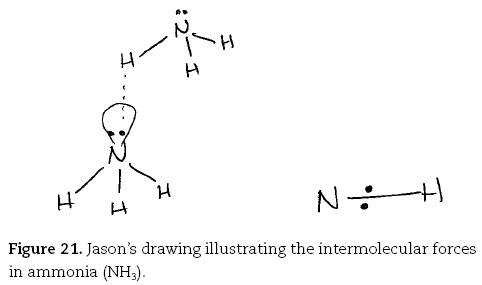
Looking at the general trend in this line in this diagram [i.e., Figure 20] you can see that as you increase the molecular weight of whatever hydride you are dealing with. For example, the carbon, silicon, germanium, tin series is a good one. As the hydride gets heavier the boiling point increases. However, that trend is broken by both the group three and the group five and the seven one by ammonia and HF. In this case the reason the boiling points are different is because of something called hydrogen bonding. This is where you have a bond between ammonia, for example, where you have [drawing ammonia molecule, Figure 21] ... and hydrogen bonding is the bonding between the hydrogen of an adjoining ammonia [drawing second ammonia]. In this case say this hydrogen, and a lone pair on another nitrogen [draws dotted line from H to lone pair]. I think the reason for this bonding is because the within each nitrogen to hydrogen bond you have a covalent bond which is sharing of the electrons. But because nitrogen is quite electronegative, that means it likes to hold the electrons as close to it as possible. It holds the shared electrons very close to it, rather than the hydrogen. So the hydrogen is effectively electron deficient, and so you can get a bond with the lone pair on the nitrogen, because a lone pair of course contains spare electrons if you like. And they're attracted to the electron deficient hydrogen's. Now you only get that in cases where you have a very electronegative main atom. So that's why you see it mainly for HF and ammonia.
Summary and Conclusions
The researcher has deliberately presented a considerable amount of raw data here. There are two reasons for this. First, such an act represents part of what Guba and Lincoln (1989, 1994) refer to as an audit trail, a way to show readers the process used to make interpretations and draw conclusions in educational research. Second, the amount of data presented shows clearly the rich, detailed nature of the expressed mental models for the target systems of chemical bonding that form the focus of this work.
Examination of the data presented here shows how the mental models expressed by these three gifted science student differ markedly from their less gifted peers. That is not to say the models are perfect, or that they represent the consensual scientific view totally. However, the models are far more in accord with the scientific view, and unlike their less gifted counterparts, these gifted students' mental models contain few alternative conceptions (see Coll & Taylor, 2001 for a detailed description of student alternative conceptions held by the less gifted in the main study). So, for example, Steve was confused about the exact number of electrons involved in the I2 molecule (allocating 8 electrons instead of 10 into the molecular orbitals), but nonetheless he evidenced a sound understanding of the molecular orbital theory as it applies to molecular iodine (I2). In addition, he was able to use the salient points of molecular orbital theory to describe the bonding in chloroform (CHCl3), and this suggests his understanding of molecular orbital theory is not confined to simple molecules such as homonuclear diatomics. Likewise, Jason claimed that "we don't end up with grains of sand on our beaches", whereas sand in fact arises from the weathering of silicate rocks (see, e.g., Schaetzl & Anderson, 2005). Claire did tend to focus on the octet rule, which Taber and Coll (2002) note is a feature of learning at the school level, and which points to a view of bonding involving only atoms and molecules; that is, bonding consisting of the filling shells or pairing of electrons rather than being an electrostatic interaction. Her lack of exposure to more sophisticated models with greater explanatory power is probably why she focussed on the octet rule. However, her very detailed descriptions of the target models are evident of far more advanced understanding of models that her less gifted peers (Taber, 2007).
In each case then, these gifted students showed detailed understanding of mental models they had been taught for chemical bonding. But how do they view such mental models? Do they understand the limitations of mental models in the same way scientists do, and do they use such models in the same pragmatic fashion? To answer these questions we need to consider the situation when these gifted students were confronted with choice. When confronted with a need to explain something, scientists choose to use the models and explanations that are the most economical (Coll, Taylor & Lay, 2008, in press). That is to say, they use the simplest model that explains the data or facts in they are presented with. They resort to more sophisticated models when their simpler models break down. The Rutherford-Bohr model for the atom is a case in point. It explained some aspects of atomic structure (the Stern-Gerlach experiment, atomic spectra, etc.), but was inconsistent with other data or physics theories, and so was eventually abandoned in favour of a quantum mechanical, wave model for atomic structure. So is there any evidence here that these gifted science students used models in these sorts of ways? This is now considered by examination of several common themes that emerged from the data.
Each of these gifted students was able to introduce new terminology and concepts as the demand probe increased. For example, Jason introduced the notion of interstitial substitution to explain the bonding and structure in the alloy steel, and used this to explain the greater strength of steel. He introduced terms such as directionality when describing the bonding in metals, and polarization to describe the bonding in polar covalent substances. In a similar way, Claire drew upon the Periodic Table and identified periodic trends to describe differences in the bonding in ionic substances such as sodium and lithium chloride. She further introduced the non-intuitive notion of the ionic-covalent continuum suggesting she was capable of moving away from her atomistic notions of bonding.
These gifted students also were able to utilize two-dimensional visual clues in the way a scientist might; with, for example, Jason noting that the lines he drew (in Figure 11) were not intended to indicate chemical bonds, but lattice orientation and replication of units. This contrasts markedly with literature reports, which suggest that in depictions of ionic lattices students frequently confuse geometric lines with chemical bonds (Butts & Smith, 1987; Coll & Taylor, 2001; Taber, 1994). Similarly, Steve did not confuse the boundary surface of molecular orbitals with spheres or orbits; a subtle and sophisticated difference, that again the literature suggests students frequently confuse (Coll & Taylor, 2001; Taber & Coll, 2002). The way Steve systematically increases the level of sophistication of his molecular orbital description of the bonding in molecular iodine (I2) is highly impressive. He begins with reasonably simple ideas about electron configuration, and subsequently introduces terms such as atomic orbital, molecular orbital, bonding and antibonding molecular orbitals, phases of lobes, wavefunctions, degeneracy, and bond order. This suggests he was able to recognise the need for increasing sophistication in modelling in order to explain various facts (such as bond order, spatial orientation, etc.).
In our previous work about the less gifted students involved in the main study that the present work derived from, we noted that in order to move students on in terms of models and modelling we needed to provide them with reasons to use more sophisticated and complex models. This is supported here. These gifted students identified limitations of simple models more readily than their peers, perhaps as a result of their greater capacity to remember fine details of all models they had encountered. Hence, one recommendation to arise from the present work is to explicitly identify the limitations of simple models for chemical bonding (like the octet rule), and to point out explanatory limitations. For example, the power of the molecular orbital theory to explain the unusual properties of molecular dioxygen (O2) such as its paramagnetism highlights the limitations of the Lewis dot structures based on the octet rule. Second, we need to explicitly teach students about the nature of models (Harrison & Treagust, 2000). There are some textbooks that already do this rather well (e.g., Zumdahl, 1989), but we suggest here teachers need to place greater emphasis on the nature of models before teaching chemistry models. This might include reference to the purpose of models, with the literature indicating, for example, that students seldom understand the generative nature of models (Harrison, 2008; Gentner, 1983, 1989; Gentner & Stevens, 1983), or the fact that for some science concepts the model is the scientific explanation (Harrison, 2008; Harrison & Treagust, 2000).
References
Allinger, N.L., Cava, M.P., De Jongh, D.C., Johnson, C.R., Lebel, N.A., & Stevens, C.L. (1971). Organic chemistry. New York: Worth. [ Links ]
Benson-Pope, D. (2005). Government responds to scholarship review. Retrieved 30 July 2008 from http://www.beehive.govt.nz/node/22568 [ Links ]
Bevan-Brown, J. (2004). Gifted and talented Maori learners. In D. McAlpine & R. Moltzen (Eds.). Gifted and talented: New Zealand perspectives (2nd ed., pp. 171-197). Palmerston North, New Zealand: ERDC Press, Massey University. [ Links ]
Coll, R.K. (2006). The role of models, mental models and analogies in chemistry teaching. In: P.J. Abusson, A.G. Harrison & S.M. Ritchie (Eds.), Metaphor and analogy in science education (pp. 65-77). Dordrechdt, The Netherlands: Kluwer. [ Links ]
Coll, R.K. (2007). Opportunities for gifted science provision in the context of a learner-centred national curriculum. In K.S.Taber (Ed.), Science education for gifted learners (pp. 59-70). Milton Park, UK: Routledge. [ Links ]
Coll, R.K. France, B., & Taylor, I. (2005). The role of models and analogies in science education: Implications from research, International Journal of Science Education, 27(2), 183-198. [ Links ]
Coll, R.K., & Taylor, N. (2001). Alternative conceptions of chemical bonding held by upper secondary and tertiary students, Research in Science and Technological Education, 19(2), 171-191. [ Links ]
Coll, R.K., Taylor, N., & Lay, M.C. (2008, in press). Scientists' habits of mind as evidenced by the interaction between their scientific training and religious beliefs, International Journal of Science Education. [ Links ]
Coll, R.K., & Treagust, D.F. (2003a). Investigation of secondary school, undergraduate and graduate learners' mental models of ionic bonding, Journal of Research in Science Teaching, 40(5), 464-886. [ Links ]
Coll, R.K., & Treagust, D.F. (2003b). Learners' mental models of metallic bonding, Science Education, 87, 685-707. [ Links ]
Frasier, M. (1997). Multiple criteria: The mandate and the challenge, Roper Review, 20(2), A4-A7. [ Links ]
Geertz, C. (1973). The interpretation of cultures. New York: Basic Books. [ Links ]
Gentner, D. (1983). Structure-mapping: A theoretical framework for analogy, Cognitive Science, 7, 155-170. [ Links ]
Gentner, D. (1989). The mechanisms of analogical learning. In S. Vosniadou & A. Ortony (Eds.), Similarity and analogical reasoning (pp. 199-241). Cambridge, UK: Cambridge University Press. [ Links ]
Gentner, D., & Stevens, A.L. (Eds.). (1983). Mental models. Hillsdale, NJ: Lawrence Erlbaum. [ Links ]
Gilbert, J.K. (1980). The use of models in science and science teaching. European Journal of Science Education, 2(1), 3-13. [ Links ]
Gilbert, J.K. (Ed.). (1993). Models and modelling in science education. Hatfield Herts, UK: Association for Science Education. [ Links ]
Gilbert, J.K., & Osborne, R.J. (1980). The use of models in science and science teaching, European Journal of Science Teaching, 2(1), 3-13. [ Links ]
Gilbert, J.K., & Rutherford, M. (1998a). Models in explanations Part 1: Horses for courses?, International Journal of Science Education, 20(1), 83-97. [ Links ]
Gilbert, J.K., & Rutherford, M. (1998b). Models in explanations Part 2: Whose voice? Whose ears?, International Journal of Science Education, 20(2), 187-203. [ Links ]
Gilbert, J.K., Watts, D.M., & Osborne, R.J. (1985). Eliciting student views using an interview-about-instances technique. In L.H.T. West & A.L. Pines (Eds.), Cognitive structure and conceptual change (pp. 11-27). Orlando, FL: Academic Press. [ Links ]
Gillespie, R.J., Humphreys, D.A., Baird, N.C., & Robinson, E.A. (1986). Chemistry (2nd ed.). Boston: Allyn & Bacon. [ Links ]
Guba, E.G., & Lincoln, Y.S. (1989). Fourth generation evaluation. Newbury Park, CA: Sage. [ Links ]
Guba, E.G., & Lincoln, Y.S. (1994). Competing paradigms in qualitative research. In N.K. Denzin & Y.S. Lincoln (Eds.), Handbook of qualitative research (pp.105-117). Thousand Oaks, CA: Sage. [ Links ]
Harrison, A.G. (2008). Teaching with analogies: Friend or foe? In A.G. Harrison & R.K. Coll (Eds.), Using analogies in middle and secondary science classrooms (pp. 6-21). Thousand Oaks, CA: Corwin. [ Links ]
Harrison, A.G., & Treagust, D.F. (1996). Secondary students' mental models of atoms and molecules: Implications for teaching chemistry, Science Education, 80(5), 509-534. [ Links ]
Harrison, A.G., & Treagust, D.F. (1998). Modelling in science lessons:Are there better ways to learn with models?, School Science and Mathematics, 98(8), 420-429. [ Links ]
Harrison A., & Treagust, D.F. (2000). Learning about atoms, molecules and chemical bonds: A case study of multiple-model use in grade 11 chemistry, Science Education, 84, 352-381. [ Links ]
Harrison, A.G., & Treagust, D.F. (2006). Teaching and learning with analogies: Friend or foe? In: P.J. Abusson, A.G. Harrison & S.M. Ritchie (Eds.), Metaphor and analogy in science education (pp. 11-24). Dordrechdt, The Netherlands: Kluwer. [ Links ]
Justi, R., & Gilbert, J.K. (2006). The role of analog models in the understanding of the nature of models in chemistry. In P.J. Abusson, A.G. Harrison & S.M. Ritchie (Eds.), Metaphor and analogy in science education (pp. 119-130). Dordrechdt, The Netherlands: Kluwer. [ Links ]
Keen, D. (2001, December). Talent in the new millennium. Paper presented at the AARE Conference. Freemantle, Australia. [ Links ]
Maksic, Z.B. (1990). Theoretical models of chemical bonding Part 1: Atomic hypothesis and the concept of molecular structure. New York: Springer-Verlag. [ Links ]
Merriam, S.B. (1988). Case study research in education. San Francisco: Jossey-Bass. [ Links ]
Metallic bonding. (1994). In R.B. King (Ed.), Encyclopaedia of inorganic chemistry (pp. 1965-1983). New York: Wiley. [ Links ]
Moltzen, R. (2004). Historical perspectives. In D. McAlpine & R. Moltzen (Eds.), Gifted and talented: New Zealand perspectives (pp. 1-21). Palmerston North, New Zealand: ERDC Press, Massey University. [ Links ]
New Zealand Qualifications Authority. (2004). Report to New Zealand Qualifications Authority Scholarship Review Committee regarding scholarship 2004 issues. Wellington, New Zealand: Minter-Ellison-Rudd-Watts-NZQA. [ Links ]
Norman, D.N. (1983). Some observations on mental models. In D. Gentner & A.L. Stevens (Eds.), Mental models (pp. 7-14). Hillsdale, NJ: Lawrence Erlbaum. [ Links ]
Riley, T. (2004). Curriculum models: The framework for gifted and talented education. In D. McAlpine & R. Moltzen (Eds.), Gifted and talented: New Zealand perspectives (2nd ed., pp. 309-343). Palmerston North, New Zealand: ERDC Press, Massey University. [ Links ]
Schaetzl, R., Anderson, S. (2005). Soils, genesis and geomorphology. Cambridge, UK: Cambridge University Press. [ Links ]
Smit, J.J.A., & Finegold, M. (1995). Models in physics: Perceptions held by final-year prospective physical science teachers studying at South African universities, International Journal of Science Education, 17(5), 621-634. [ Links ]
Stavy, R. (1991). Using analogy to overcome misconceptions about conservation of matter, Journal of Research in Science Teaching, 28(4), 305-313. [ Links ]
Stavy, R. (1995). Conceptual development of basic ideas in chemistry. In S.M Glynn & R. Duit (Eds.), Learning science in the schools: Research reforming practice (pp. 131-154). Manwah, NJ: Lawrence Erlbaum. [ Links ]
Stavy, R., & Tirosh, D. (1993). When analogy is perceived as such. Journal of Research in Science Teaching, 30(10), 1229-1239. [ Links ]
Sternberg, R.J. (1993). The concept of giftedness: A pentagonal implicit theory. In G.R. Bock & K. Ackrill (Eds.), The origins and development of high ability, CIBA Foundation Symposia Series (pp. 5-21). Chichester, UK: Wiley. [ Links ]
Suckling, C.J., Suckling, K.E., & Suckling, C.W. (1978). Chemistry through models: Concepts and applications of modelling in chemical science, technology and industry. Cambridge, UK: Cambridge University Press. [ Links ]
Taber, K.S. (2007). Science education for gifted learners? In K.S. Taber (Ed.), Science education for gifted learners (pp. 1-14). Milton Park, UK: Routledge. [ Links ]
Taber, K.S., & Coll, R.K. (2002). Bonding. In J.K., Gilbert, O. De Jong, R. Justi, D.F. Treagust, & J.H. Van Driel (Eds.), Chemical education: Towards research-based practice (pp. 213-234). Dordrecht, The Netherlands: Kluwer. [ Links ]
Von Glasersfeld, E. (1993). Questions and answers about radical constructivism. In: K. Tobin (Ed.), The practice of constructivism in science education (pp. 23-38). Hillsdale, NJ: Lawrence Erlbaum. [ Links ]
White, R., & Gunstone, R. (1992). Probing understanding. London: Falmer. [ Links ]
Zumdahl, S.S., & Zumdahl, S.S (2000). Chemistry (5th ed.). Lexington, DC: Heath. [ Links ]