Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Educación química
versión impresa ISSN 0187-893X
Educ. quím vol.19 no.4 Ciudad de México oct. 2008
Para quitarle el polvo
William Ramsay
William Ramsay
Jaime Wisniak*
*Department of Chemical Engineering, Ben-Gurion University of the Negev. Beer-Sheva, Israel 84105. E-Mail: wisniak@bgumail.bgu.ac.il
Abstract
William Ramsay (1852-1916) was awarded the 1904 Nobel Prize for Chemistry "in recognition of his services in the discovery of the inert gaseous elements in air, and his determination of their place in the periodic system." In addition, he made significant contributions in the fields of organic chemistry, thermodynamics, and radioactive processes, identifying the gases released during the transmutation of elements such as thorium and radium.
Key words: boiling point, PVT behavior, surface tension, inert gases, argon, radioactivity.
Resumen
William Ramsay (1852-1916) recibió el Premio Nobel en Química (1904) por "su descubrimiento de los gases inertes en el aire y la determinación de su lugar en la Tabla Periódica." Hizo además, importantes contribuciones en las áreas de química organica, termodinámica, y procesos radioactivos, identificando los gases desprendidos durante la transmutación de elementos como el torio y el radio.
Life and career
William Ramsay was born in Glasgow, Scotland, October 2, 1852, and died in Hazlemere, Buckinghamshire, on 22 July 1916). He was the only child of Catherine Robertson and William Ramsay, a civil engineer and businessman (Moore, 1918; Collie, 1917).
From his fourth to his tenth year William Ramsay went to an elementary school and then to the Glasgow Academy. Afterwards (1866) he matriculated at the University of Glasgow where he also attended the chemistry lectures of John Ferguson (1837-1916) and the physics lectures of William Thomson, (Lord Kelvin, 1824-1907). In May 1869 he entered the laboratory of Robert Rattray Tatlock (1855-1950), the Glasgow city analyst. During the winter he attended lectures on chemistry in the university and for six months he worked in Lord Kelvin's laboratory (Collie, 1917; Travers, 1956).
After the Franco-Prussian work he worked for a short time under Robert Wilhelm Bunsen (1811-1899) in Heidelberg and thereafter he transferred to the University of Tübingen to study and work under Rudolf Fittig (1835-1910). There he studied platinum-ammonium compounds and the properties and reactions of the toluic acids (Bottinger and Ramsay, 1874ab).
In 1873 he obtained his Ph.D. and returned to Glasgow to work first as assistant to Georg Bischof (1834-1906) and then to John Ferguson (1837-1916) at the University of Glasgow. Afterwards he took over the chemistry chair in University College, Bristol, and in 1881, he was appointed principal of the College. In 1887 Ramsay succeeded Alexander Williamson (1824-1904) in the chair of chemistry and he remained there during the rest of his active academic life (Moore, 1918).
In August 1881 Ramsay married Margaret Buchanan. They had two children.
In 1900 Ramsay was appointed adviser to the Indian Government on the question of university and technical education in connection with the bequest of Jamsetji Nusserwanji Tata (1839-1904) who had left nearly half-a-million sterling to build and endow a university. This institution was started in Bangalore and eventually became the Indian Institute of Science (Collie, 1917).
Ramsay's researches on the rare gases brought him international fame and many honors were conferred on him by universities and learned societies throughout the world. The most important recognition was the 1904 Nobel Prize for chemistry "in recognition of his services in the discovery of the inert gaseous elements in air, and his determination of their place in the periodic system."
Scientific contribution
Ramsay was a prolific writer; he published more than 300 papers and 15 books. Among the latter we can mention Argon a New Constituent of the Atmosphere, written with Lord Rayleigh (Rayleigh and Ramsay, 1895), A System of Inorganic Chemistry (Ramsay, 1891b), Theoretical Modern Chemistry (Ramsay, 1900a), Introduction to the Study of Physical Chemistry (Ramsay, 1900b), and Experiments in Radio-Activity and the Production of Helium from Radium (Ramsay and Soddy, 1904b), and Stoichiometry, (Ramsay, 1908b).
The research activities of Ramsay may be classified into three general categories: (1) Pure organic and physico-organic chemistry, (2) Critical states of liquids and vapors and discovery of the inert gases and study of their properties, and (3) Researches in radioactivity and allied physico-chemical problems.
Chemistry
Ramsay's first academic papers were on a new antimony phosphide with a formula SbP (Ramsay, 1874a), on the properties and methods of preparation of hydrogen persulfide (Ramsay, 1874b), on the preparation of sodium ethylthiosul-fate and proof of the constitutional formula for sodium dithionate and the reaction that takes place when this compound is heated with phosphorus pentachloride (Ramsay, 1875), on the analysis of a bismuth mineral carrying cobalt and nickel (Ramsay, 1876a), and on the dehydration of hydrates (Ramsay, 1877).
His early chemical work was mainly concerned with organic chemistry, and was initially based on a large collection of pyridine bases left at the University of Glasgow by Thomas Anderson (1819-1874; discoverer of pyridine). Ramsay used these chemicals to synthesize by oxidation a variety of pyridinic acids as well as pyridine itself from hydrocyanic acid and acetylene (Ramsay, 1876b). He published several papers on the carboxylic acids resulting from oxidation of the pyridines, demonstrating their relationship to the benzene carboxylic acids. Together with John Gray McKendrick (18411926), the professor of physiology, he investigated the physiological action of various anesthetics (Coats et al., 1879), and with James Johnston Dobbie, (1852-1924), quinine and its decomposition products, and then the decomposition of allied alkaloids, quinidine (conquinine), cinchonine and cinchonidine by oxidation with permanganate (Moore, 1918; Ramsay and Dobbie, 1878; Dobbie and Ramsay 1879).
Ramsay then begun to study physico-chemical problems related to organic chemistry, for example, the heat of formation of aniline, picoline, toluidine, glycerin and other compounds (Ramsay, 1879b), the molecular volumes of the benzene, naphthalene, anthracite, and phenanthrene series (Ramsay, 1881a), the volumes of sodium and phosphorus at their boiling points (Ramsay, 1879a; Ramsay and Mason, 1881), and the atomic volume of nitrogen (Ramsay 1881b; Moore, 1918).
In 1884 he published a paper on the halogen compounds of selenium in which he compared the stability of the halogen compound of selenium with those of sulfur by measuring the degree of disassociation caused by increased temperature (Evans and Ramsay, 1884). With Sidney Young (1857-1937) he studied the decomposition of ammonia by heat, paying special attention to the temperature at which decomposition took place and to the influence of the material of the vessel or tube containing the gas (Ramsay and Young, 1884d). Ramsay was one of the first scientists to offer a plausible explanation for Brownian (pedectic) movement (Ramsay, 1892).
Thermodynamics
Many different formulas and representations have been developed for the dependence of the vapor pressure with temperature for pure liquids and their mixtures.
Between 1885 and 1886 Ramsay and Young (Ramsay and Young, 1885abc) developed an important expression relating the boiling points of two pure substances, which may be derived from the integrated Clausius-Clapeyron equation:
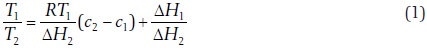
Assuming that both compounds have also the same vapor at the temperatures T'1 and T'2, then it must be that

where c is a constant given by

Ramsay and Young reported the values of the constant c for 30 different pairs of substances (Ramsay and Young, 1885b), including such systems as water + ethanol, aniline + water, water + oxygen, and ammonium chloride + water, and indicated that it has a very small value (<2x10− 4) and for chemically similar substances (like water and ethanol) it is almost zero. The last case corresponds to

In other words, the ratio of the absolute temperatures at which two substances boil under the same pressure is constant.
Curiously, in all their publications related to the boiling temperature of pure compounds, Ramsay and Young made no reference to a very important paper on the subject published by Karl Eugen Dühring (1833-1921), almost ten years before (Dühring, 1878), in which he showed that plotting the boiling point of a substance against the boiling point of water at the same pressure yielded a straight line, that is

where T'1 and T'2, are the boiling points of one substance at two different pressures, T'1 and T'2, are the corresponding boiling points of a second substance (water in this case), and q is a constant that is the slope of the line (Dühring's rule).
In 1873, James Thomson discussed his findings on the behavior of a pure substance in the P-T domain (Thomson, 1873), where the three phase-change curves, solid-gas, solid-liquid, and liquid-gas, yielded three continuous curves, which seemed to cross each other at one common point, which Thomson named triple point. The gas-liquid curve, named by Thomson the boiling curve, separated the regions of the plane corresponding to the ordinary liquid and those corresponding to the ordinary gaseous state. Andrews (Andrews, 1869, 1876) had already demonstrated that this separating boundary came to an end at a point, which he called the critical point, and that the transition from any liquid state to any gaseous state could be gradually effected by an infinite variety of courses passing round the extreme end of the boiling line. Thomson then proceeded to calculate the value of the slope of each the three phase-change curves and to demonstrate that its value was
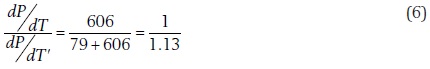
a result showing that for any small descent in temperature from the triple point the equilibrium pressure of steam with ice falls off 1.13 times as much as does the equilibrium pressure of steam with liquid water (In today values it is 2375.3 J/kg / 2709 J/kg = 1/1.14) (Thomson, 1873). The mathematical significance of these numbers is clear: the triple point corresponds to a discontinuity in the curves of phase change and the sublimation curve reaches the triple point with a slope larger than that of the vaporization curve.
In 1881 Thomas Carnelley (1852-1890) demonstrated that ice, mercuric chloride, and camphor did not melt below certain pressures peculiar to each substance but that above these pressures they melted when heated (Carnelley, 18801881). This discovery led to the expression hot ice. In 1884, Ramsay and Young provided the experimental verification of Carnelley's findings (Ramsay and Young, 1884abcd).
The findings of Carnelley and Thomson may be considered the catalyst that propelled Ramsay and Young into a vast research of the characteristics of the P-T-V domain of a pure substance, and were the subject of many of their joint publications.
In their first paper Ramsay and Young (Ramsay and Young, 1884b) tried to find out whether solids have definite volatilizing (sublimation) points under different pressures, as liquids have definite boiling points, and whether these pressures are identical with their vapor pressures at those temperatures. They reported that during freezing of ice at low pressures supersaturation was nearly always observed. The temperature fell occasionally as low as -11°C, while the water remained liquid. An abrupt formation of ice then occurred and the temperature rose to 0°C. Additional experiments performed with acetic acid, benzene, naphthalene, and camphor clearly showed that the pressures corresponding to the temperatures of volatilization coincided with the vapor pressure of the solid at the same temperature, that solids also have definite temperatures of volatilization, which depend on the pressure to which they are subjected, and these are sensibly coincident with those of their vapor pressures (Ramsay and Young, 1884b).
In their second paper (Ramsay and Young, 1884c) Ramsay and Young provided an experimental proof of the theory advanced by Thomson (Thomson, 1873) that the pressure exerted by the vapor of a solid substance at a given temperature is less than that of the vapor of the substance in the liquid state at the same temperature. To confirm this theory Thomson used the empirical formulae developed by Regnault to represent the dependence of the vapor pressure with temperature for the equilibrium of water vapor with ice and liquid water, and proved that Regnault's results pointed to a discontinuity in the curve, occurring at a temperature nearly coincident with 0°C, the melting point of ice under normal pressure (Ramsay and Young, 1884c) (the triple point of water is located at 273.16 K and 4.579 mmHg).
For dissociating substances the explanation was more complicated. The abnormal vapor density of many compounds had been ascribed to their dissociating to a greater or less degree while in the gaseous state. Since dissociation was accompanied by an increase of volume, the vapor density of the mixture of gaseous molecules decreased with an increase in temperature. This phenomenon was not confined to dissociating compounds alone. It was recognized that many, if not all, liquids acquired an abnormal vapor density in the proximity of their point of saturation. The question then arose, for associating liquids, how much abnormality should be attributed to the one cause and how much to the other? In order to answer it Ramsay and Young decided to study and compare the behavior of substances belonging to the four types (a) liquids, the vapor of which are not known to dissociate, (b) liquids, the vapors of which probably dissociate into like molecules, (c) bodies which dissociate gradually in the gaseous state into unlike molecules, (d) bodies which dissociate completely on passage into the gaseous state (Ramsay and Young, 1885cefg).
Simultaneously with the efforts of Ramsay and Young, Philippe-Auguste Guye (1862-1922) developed a method for calculating the molecular weight at the critical point, which was particularly applicable to compounds like water and methanol that deviated more pronouncedly from relations that had been derived previously. This procedure led Guye to conclude that methanol at its critical point seemed to exist as a dimer (Guye, 1894). Ramsay and John Shields (1818-1879) (Ramsay and Shields, 1893) studied the molecular complexity of 55 different liquids and found that most of them had the same degree of polymerization in the liquid and vapor phases, but for compounds such as methanol, fatty acids, acetone, propionitrile, nitroethane, and water, the phenomenon of polymerization was particularly strong and the degree of polymerization decreased with increased temperature. Analysis of the available information indicated that although water and the lower alcohols were strongly polymerized in the liquid phase, this was not true for the vapor phase; at temperatures approaching that of boiling the density of the vapor was slightly larger than that predicted by the ideal gas law. At higher temperatures both densities became essentially identical (Ramsay and Young, 1886abc).
Experiments were also conducted with substances dissociating in different manners, such as chloral hydrate, butyl chlorate hydrate, chloral methyl alcoholate, choral ethyl alcoholate, ammonium carbamate, ammonium chloride, phthalic acid, succinic acid, aldehyde ammonia, paraldehyde and metaldehyde, nitrogen peroxide, and acetic acid (Ramsay and Young, 1884de, 1885h, 1886abc, 1887abc; Aston and Ramsay, 1894; Ramsay and Aston, 1894ab, 1902).
Other publications reported the determination of the thermal properties of several liquids and their mixtures (ethyl ether, methyl, ethyl, and propyl alcohols, acetic acid, and a mixture of ether and ethyl alcohol) (Ramsay and Young, 1885h, 1886bcf; 1887abcd).
Another contribution was related to the phenomenon of surface tension. If we consider a liquid mass of cubic shape and containing one mol of a substance, then the square faces of the cube are equimolar and have a surface (M/d)2/3= s, where d is the density of the substance. The product of this surface multiplied by the surface tension y (measured by the ascension of a liquid inside a capillary tube) is called the molecular surface energy and represents the work that must be used to overcome the tension over the surface. In 1886 Lorand Eötvös (1848-1919) assumed that bodies that are in corresponding states (according to van Waals), should possess similar mechanical energies. On this assumption he imagined that there is a proportionality between yv2/3, where v = M/d, the surface energy, and the product Pv, the volume energy (Eötvös, 1886). Two substances in corresponding states will remain so when the change on condition with temperature at any corresponding temperatures is proportional to the temperatures. Mathematically, this situation means that
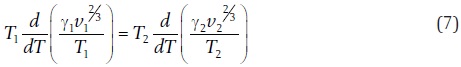
that is, the rate of change with temperature of the molecular surface energy of any two liquids is the same. Eötvös integrated the equation to get

where Tc is the critical temperature and T the temperature at which y and (Mv)2/3 are observed.
The Eötvös's equation was further investigated by Ramsay and Shields (Ramsay and Shields, 1893) and proved to be wrong because the value of ys not only should be zero at the critical temperature, but it should first decrease slowly with temperature until it reached a nearly steady rate of increase. Hence the slope of the curve described by eq (8) will not be constant from the origin but only after a certain temperature is reached. In other words, the correct functionality should be

The data needed to calculate the molecular surface energy of a liquid are thus the height to which the liquid will rise in a capillary tube of known diameter, the density at the temperature of observation, and the molecular mass.
Ramsay and Shields applied their equation to a large number of liquids such as ethyl ether, methanol, ethanol, carbon tetrachloride, benzene, benzene, and acetic acid, and concluded that many hydroxyl compounds, including water and alcohols, are characterized by greater complexity of their molecules, or molecular association. In other words, their chemical representation when in the liquid state will be different from that when in the gas state, for example, (H2O)n and (C2H5OH)n etc. Thus, alcohol appears as a triple and associated molecule and sulfuric acid, in the range 10° to 130°C, is constituted by the grouping of 32 simple molecules, which become separated as the temperature is increased.
Several papers published on the critical state of gases and the critical point (Ramsay 1882, Ramsay and Young, 1887bd) led Ramsay to make some very definite statements concerning gases and liquids, and to define the critical point as that point at which the liquid owing to expansion and the gas, owing to compression, acquire the same specific gravity and refractive index, and consequently are capable of mixing with one another.
Inertgases
The discovery of argon has been described in detail a previous publication and only the main points will be given here (Wisniak, 2007).
In 1784 and 1785 Henry Cavendish (1731-1810) published two extensive memoirs reporting his experiments on air (Cavendish, 1784; 1785), which may be considered the earliest clear evidence that air contains more gases than oxygen and nitrogen. Nevertheless, these findings were not given proper importance and up to the 1890's the generally accepted composition of air was described by Victor Regnault (1810-1878) as follows (Regnault, 1850): "Air is essentially a mixture of oxygen and nitrogen in the same proportion in every point of the earth...It contains very small amounts of CO2, water vapor, and other gases or vapors released by the decomposition of organic matter."
In letter published a letter in Nature (Lord Rayleigh, 1892b) Rayleigh reported that his experimental results definitely showed that nitrogen prepared from chemical means was lighter by about 1/1000 part than that derived from atmospheric air. Since a difference of this magnitude could not be accounted for on the basis of experimental error, Rayleigh invited criticism from "chemical readers" who might be interested in such questions.
Ramsay asked from Rayleigh and received his permission, to undertake some experiments designed to explain the possible anomalous behavior of atmospheric nitrogen. Ramsay was interested in the subject because years before he had tried to combine hydrogen and nitrogen directly by passing them over heated metals. For this reason, he believed that the way to solve the problem was to absorb carefully purified nitrogen in order to discover whether any portion of it was different from the rest. Passing nitrogen over soda lime, phosphorus pentoxide, magnesium at red heat, copper oxide, and soda lime, Ramsay found that prolonged treatment left only 1/80 of the original volume of the gas, with a density of 19.086 (Tilden, 1918; Hiebert, 1963). Examination of the spectrum of the gas revealed "the bands of nitrogen.some-what hazy bands, red, orange, yellow, and yellow green in color. and showed certain groups of red and green lines which did not appear to belong to the spectrum of any known gas" (Lord Rayleigh and Ramsay, 1895).
The possibility of a new substance was a tantalizing question. Rayleigh thought that "regarding it as established that one of other of the gases must be a mixture, containing as the case might be, an ingredient much heavier or much lighter than ordinary nitrogen, we had to consider the relative probabilities of the various possible interpretations... The simplest explanation in many respects was to admit the existence of a second ingredient in air from which oxygen, moisture and CO2 had already been removed. The proportional amount required was not great. If the density of the supposed gas were double that of nitrogen, 0.5 per cent only by volume would be needed, or if the density were half as much again as that of nitrogen, then 1 per cent would suffice. But in accepting this explanation, even provisionally, we had to face the improbability that a gas surrounding us on all sides, and present in enormous quantities, could have remained so long unsuspected" (Lord Rayleigh and Ramsay, 1895).
On May 24, 1894, Ramsay wrote to Rayleigh (Travers, 1956): "Has it occurred to you that there is room for gaseous elements at the end of the first column of the periodic table? Thus: Li, Be, C, C, N, O, F, X, X, X... etc. Such elements should have a density 20 or whereabouts, and 0.8 per cent (1/120th about) of the nitrogen of the air could raise so the density of nitrogen that it would stand to pure nitrogen in the ratio 230:231" (Tilden, 1918).
On August 13, 1894, at the Oxford meeting of the British Association, Rayleigh made a brief announcement that he and Ramsay had found atmospheric nitrogen, carefully purified from every other known constituent of air, to be contaminated to the extent of 1 per cent with another gas even more inert than nitrogen (Lord Rayleigh, 1892; Anonymous, 1894). This discovery was also communicated to the French Académie des Sciences through Marcelin Berthelot (18271907) (Berthelot, 1895ab).
In the search for argon compounds Ramsay also looked on the possibility of their existence in natural form. Henry Alexander Miers (1858-1942), a famous mineralogist, told Ramsay of some curious results published by William Francis Hillebrand (1853-1925) (Hillebrand, 1892). Hillebrand analyzed different minerals containing uranium and discovered "a hitherto unsuspected element in uraninite, existing in a form of combination not before observed in the mineral world.nitrogen, which is given off in a gaseous form on heating the mineral with a non-oxidizing acid, or.by fusing it with an alkaline carbonate.The gas was colorless, odorless, a non-supporter of combustion, unchanged by mixture with air...not absorbed by alkafis...the gas afforded the fluted spectrum of pure nitrogen." Ramsay repeated Hillebrand's experiments using clévite, a similar uranium mineral, and found that the gas released was not argon and its spectrum was different from that of nitrogen. He sent a sample to William Crookes (1832-1919) who reported that the new line was coincident in length with the line of helium, which had been previously discovered by Joseph Norman Lockyer (1836-1920) and Edward Frankland (1825-1899) in 1869 (Crookes, 1894-1895; Ramsay, 1896). On 27 March 1895 Ramsay announced the existence of terrestrial helium; a result that was independently confirmed by Per Theodor Cléve's (1840-1905) laboratory in Uppsala (Cléve, 1895). In August of the same year, Heinrich Kayser (1853-1940) announced the presence of atmospheric helium in natural gas emanating from the Black Forest.
Further work by Ramsay and Morris Williams Travers (1872-1961) confirmed the inert nature of helium and identified its physical characteristics. With the confirmation in 1898 by Edward Baly of the presence of atmospheric helium in unexpectedly large quantities with respect to the less volatile gases, the existence of helium in thorium and uranium minerals was considered an explanation for the replenishing of atmospheric helium.
It was now probable that other gases besides helium and argon existed in the atmosphere, as suggested by Karol Olszewski's (1846-1915) work on the liquefaction of argon (Olszewski, 1885). Thus Ramsay and Travers began to search for a third inert gas. After examining a large number of minerals and the gases from mineral waters and seawater (Kellas and Ramsay, 1895; Ramsay, 1895abc; Ramsay and Travers, 1897; Ramsay 1912; Ramsay and Masson, 1912), Ramsay and Travers decided to fractionate argon on a large scale. The last fraction of this process was collected separately and purified further from oxygen and nitrogen; the spectrum of the residual gas showed a bright green line and a bright yellow line not belonging to the argon spectrum. The density of the sample was also greater than that of argon. The new element was called krypton (Moore, 1918). Further fractionation of the argon produced a light fraction containing the gases with the lowest boiling points, which was also separated and examined. Its density was about 15, and the spectrum showed a large number of brilliant red and yellow lines. This new gas was called neon. After confirming the presence of atmospheric krypton and neon, Ramsay and Travers continued to develop techniques to obtain quantities of these gases sufficient for the research on their chemical and physical properties. The krypton residue collected from preliminary attempts to obtain such quantities was found to contain about twenty percent of a new element, which was named xenon.
Radioactivity
In 1900 Rutherford showed "that the compounds of thorium, besides being radioactive in the same sense as uranium compounds, also continuously emit into the surrounding atmosphere, under ordinary conditions, something which... behaves in all respects like a radioactive gas. This emanation.is the source of rays, which.will darken a photographic plate, and will render a gas capable of conducting an electric current.The chemical nature of the emanation itself showed that it possessed the property of inertness which characterizes the gases of the argon family.The position is reached that radioactivity is at once an atomic phenomenon and the accompaniment of a chemical change in which new kinds of matter are produced. These consideration force us to the conclusion that radioactivity is a manifestation of subatomic chemical change" (Rutherford, 1900). Further work (Rutherford and Soddy, 1902ab) indicated that a chemical change was proceeding in thorium whereby a nonthorium material (ThX) is produced; the latter undergoes a further transformation, generating a gaseous product, which in the radioactive state constitutes the emanation. Both the rate of production of the new material and the rate of decay of its activity appeared to be independent of the physical and chemical condition of the system, while the intensity of the radiation seemed to depend only on the quantity of active element present. These results led Rutherford and Soddy to believe that radioactivity was a manifestation of sub- atomic chemical change and that the well-known excess of atmospheric and terrestrial helium might be connected with radioactivity (Rutherford and Soddy, 1902b).
In 1903 Ramsay invited Soddy to join him to initiate research on radioactivity. Ramsay was particularly interested in determining whether radioactivity was a general characteristic of inert gases since Rutherford had asserted that the alpha particle was a helium atom. Ramsay and Soddy decided to test this assumption by determining the spectrum of the radium emanation; they collected the emanation from a solution of radium bromide in a spectrum tube and observed the spectrum of helium, thus giving a strong experimental confirmation of the disintegration theory proposed by Rutherford and Soddy. Ramsay and Soddy's result was also the first actual visual proof of the transmutation of one element into another and gave birth to his later ideas regarding the transmutation of elements by disintegration from a metal of higher atomic weight to one of lower atomic weight by a process of inorganic devolution (Ramsay and Soddy, 1904ab; Moore, 1918; Chaudhuri, 1918).
Between 1908 and 1910 Ramsay with Alexander Thomas Cameron (1882-1947) and Whytlaw-Gray experimentally confirmed the spectrum of radium emanation and determined the density of the gas (assuming it was monatomic) and that its atomic weight is 223; that is, about four units lighter than radium. This determination was an additional proof that the transition from radium to radon involves the expulsion of helium (Cameron and Ramsay, 1907ab; 1908ab). In 1904 Ramsay suggested the names "ex radio" (Ramsay, 1904), "exthorio," and "exactino" for these emanations; and later, the term "niton" for the emanation from radium. These names did not catch; in 1918 Gerhardt Carl Nathaniel Schmidt (1865-1949) gave the name radon to the emanation from radium to distinguish it from the emanations from thorium (thoron) and actinium (actinon) (Whytlaw and Ramsay, 1911; Collie, 1917). Between 1909 and 1913, Ramsay and Whytlaw-Gray determined the atomic weight of radium, and the density, vapor pressures, liquid volumes, and critical constants of radon (Ramsay and Whytlaw, 1909ab; Whytlaw and Ramsay, 1913).
On the basis of his and others results Ramsay adopted the view that the atom consisted of a vast number of electrons, some of them more constitutive than others. His theory was that when an alpha particle struck a non-radioactive atom a glancing blow near the surface the atom was ionized; if it struck the atom squarely in the centre, the latter was broken up with the formation of new elements. Ramsay argued that if sufficient radium emanation were brought into actual contact with atoms, the energy set free from the decomposing emanation might be powerful enough to shatter some of the atoms. In a first work he electrolyzed water in the presence of radium emanation and found that hydrogen was produced in excess (Ramsay, 1905, 1907). This suggested the idea that if this type of electrolysis were applied to a cupric sulfate solution, the process would give a deposit of metallic copper equivalent to the hydrogen. The results were not as predicted (Cameron and Ramsay, 1907ab): No copper was deposited and on analyzing the products a trace of lithium and a considerable amount of sodium were now present in the solution. Similar results were found when the nitrate salt replaced the sulfate. In addition, the inert gas produced from the electrolysis of cupric sulfate showed a brilliant spectrum of helium with possibly a trace of neon; while the one produced from cupric nitrate was mainly helium. In the case of pure water, the gas phase contained neon and a trace of helium (the latter probably coming from the non dissolved and gaseous portion of the emanation). The contention of Ramsay and Cameron was that under certain conditions not only helium but some one of the other rare gases of the atmosphere might constitute one of the disintegration products of the radium emanation. Marie Curie (1867-1934) repeated the work in which lithium was obtained from copper, and reported a negative result (Moore, 1918).
References
Andrews, T., On the Continuity of the Gaseous and Liquid States of Matter, Phil. Trans., 159, 575-590 (1869). [ Links ]
Andrews, T., On the Gaseous State of Matter, Phil. Trans., 166, 421-449, 1876. [ Links ]
Anonymous, Nature, 50, 1894. [ Links ]
Aston, E. A., Ramsay, W., The Molecular Formulae of Some Liquids, as Determined by their Surface Energy, J. Chem. Soc., Trans., 65, 167-173, 1894. [ Links ]
Berthelot, M., Sur l'Argon, Nouveau Constituant de l'Atmosphére, Decouvert par MM. Rayleigh et Ramsay, Compt. Rendus, 120, 1895; 235-239, 1895a. [ Links ]
Berthelot, M., Sur l'Argon, Compt. Rendus, 120, 521-522, 1895b. [ Links ]
Böttinger, C., Ramsay, W., New Method of Forming o-Toluic acid, Ann. Chem. Pharm., 168, 202, 1874a. [ Links ]
Böttinger, C., Ramsay, W., On m-Toluic acid, Ann. Chem. Pharm., 168, 253, 1874b. [ Links ]
Cameron, A. T., Ramsay, W., Some Properties of Radium-Emanation, J. Chem. Soc., Trans., 1266-1282, 1907a. [ Links ]
Cameron, A. T., Ramsay, W., The Chemical Action of Radium-Emanation. Part II. On Solutions Containing Copper and Lead, and on Water, J. Chem. Soc., Trans., 91, 1593-1606, 1907b. [ Links ]
Cameron, A. T., Ramsay, W., The Chemical Action of Radium-Emanation. Part III. On Water and Certain Gases, J. Chem. Soc. Trans., 93, 966-992, 1908a. [ Links ]
Cameron, A. T., Ramsay, W., The Chemical Action of Radium-Emanation. Part IV. On Water, J. Chem. Soc., Trans., 93, 992-997, 1908b. [ Links ]
Carnelley, T., Preliminary Note on the Existence of Ice and other Bodies in the Solid State at Temperatures far above their Ordinary Melting Points, Proc. Roy. Soc., 31, 284-291, 1880-1881. [ Links ]
Cavendish, H., Experiments on Air, Phil. Trans., 74, 119-153, 1784. [ Links ]
Cavendish, H., Experiments on Air, Phil. Trans., 75, 372-384, 1785. [ Links ]
Chaudhuri, T. C., Sir William Ramsay as Scientist and Man, Butterworth (Calcutta), 1918. [ Links ]
Clève, P. F., Sur la Presence de I'Helium dans la Clévéite, Compt. Rendus, 120, 834, 1895. [ Links ]
Coats, J., Ramsay, W., McKendrick, J. G. The Effects of Chloroform, Ethidene, and Ether on the Blood-Pressure: Being the Third Provisional Report of the Committee on Anaesthetics to the Scientific Grants Committee of the British Medical Association, J. Anatomy Physiol., 13, (Pt 3), 387-582, 1879. [ Links ]
Collie, J. N., Sir William Ramsay, 1815-1916, Proc. Roy. Soc., 93A, xlii-liv, 1917. [ Links ]
Crookes, W., On the Spectra of Argon, Proc. Roy. Soc., 57, 287-289, 1894-1895. [ Links ]
Dobbie, J. J., Ramsay, W., Decomposition Products of Quinine and Allied Alkaloids; Part II, Oxidation with Permanganate, J. Chem. Soc., 189-196, 1879. [ Links ]
Dühring, K. E., Neue Grundgesetze zur Rationelle Physik und Chemie, Fues Verlag Leipzig, 1878. [ Links ]
Eötvös, L., Über den Zusammenhang der Oberfiáchenspan-nung der Flüssigkeitenmit ihrem Molekularvolumen, Wied. Ann. Phys. Chem., 27, 448-459, 1886. [ Links ]
Evans, F. P., Ramsay, W., The Halogen Compounds of Selenium, J. Chem. Soc. Trans., 45, 62, 1884. [ Links ]
Guye, P. A., Sur la Polymérisation Moléculaire des Liquides, Arch. Sci. Phys. Nat., [3], 31, 38-48, 164-175, 1894. [ Links ]
Hiebert E N, Historical Remarks on the Discovery of Argon, the First Noble Gas, in H. H. Hyman, Noble Gas Compounds (University of Chicago, Chicago), 1963; pp 3-20. [ Links ]
Hillebrand W F, On the Occurrence of Nitrogen in Uraninite and the Composition of Uraninite in General, Am. J. Sc., 40, 385-394, 1892. [ Links ]
Kellas, A. M., Ramsay, W., Examination of Gases from Certain Mineral Water, Proc. Roy. Soc., 59, 68-69, 1895. [ Links ]
Lord Rayleigh, Density of Nitrogen, Nature, 46, 512-513, 1892. [ Links ]
Lord Rayleigh, Ramsay, W., Argon a New Constituent of the Atmosphere, The Society, London, 1895. [ Links ]
Moore, R.B., Sir William Ramsay, J. Franklin Inst., 186, 29-55, 1918. [ Links ]
Olszewski, K., The Liquefaction and Solidification of Argon, Phil. Trans., 186, 253-259, 1885. [ Links ]
Ramsay, W., Preliminary Note on Antimony Phosphide, fier., 6, 1362, 1874a.
Ramsay, W., On Hydrogen Persulphide, J. Chem. Soc., 12, 857-860, 1874b. [ Links ]
Ramsay, W., On Sodium Ethyl Thiosulphate, J. Chem. Soc., 13, 687-688, 1875. [ Links ]
Ramsay, W., On Bismuthiferrous Tesseral Pyrites, J. Chem. Soc., 1, 153-154, 1876a. [ Links ]
Ramsay, W., On Picoline and its Derivatives, Phil. Mag., 2, 269-281, 1876b. [ Links ]
Ramsay, W., On the Dehydration of Hydrates by the Lime Method, J. Chem. Soc., 2, 395-399, 1877. [ Links ]
Ramsay, W., On the Volumes of Liquids at their Boiling Points, Obtainable from Unit Volumes of their Gases, J. Chem. Soc. Trans., 35, 463-474, 1879a. [ Links ]
Ramsay, W., On the Heat of Formation of Aniline, Picoline, Toluidine, Lutidine, Pyridine, Dipicoline, Pyrrol, Glycerin and Furfurol, J. Chem. Soc., Trans. , 35, 696-703, 1879b. [ Links ]
Ramsay, W., Volumes of Some Compounds of Benzene, Naphthalene, Anthracene and Phenanthrene Series, J. Chem. Soc., 39, 63-66, 1881a. [ Links ]
Ramsay, W., On the Atomic Volume of Nitrogen, J. Chem. Soc., Trans., 39, 66-68, 1881b. [ Links ]
Ramsay, W., On the Critical State of Gases, Proc. Roy. Soc., 30, 323-329, 1882. [ Links ]
Ramsay, W., A System of Inorganic Chemistry, J. & A. Churchill, London, 1891b. [ Links ]
Ramsay, W., Pedectic Motion in Relation to Colloidal Solutions, J. Chem. Soc., Proc., 8, 17-19, 1892. [ Links ]
Ramsay, W., Discovery of Helium in Cléivite, J. Chem. Soc., Trans., 1107-1108, 1895a. [ Links ]
Ramsay, W., On a Gas Showing the Spectrum of Helium, the Reputed Cause of D3, one of the Lines in the Coronal Spectrum, Proc. Roy. Soc., 58, 65-67, 1895b. [ Links ]
Ramsay, W., Helium, a Gaseous Constituent of Certain Minerals, Part 1. Proc. Roy. Soc., 58, 81-89, 1895c. [ Links ]
Ramsay, W., Helium, a Gaseous Constituent of Certain Minerals, Part II. Density, Proc. Roy. Soc., 59, 325-330, 1896. [ Links ]
Ramsay, W., Theoretical Modern Chemistry, Dent, London, 1900a. [ Links ]
Ramsay, W., Introduction to the Study of Physical Chemistry, Longmans and Green, London, 1900b. [ Links ]
Ramsay, W., Émanation du Radium (Exradio), ses Propriétés et ses Changements, Compt. Rendus, 138, 1388-1394, 1904. [ Links ]
Ramsay, W., Decomposition of Water by Radium, Meddel. K. Vet. Ahd. Nobelinst., I, 909-911, 1905. [ Links ]
Ramsay, W., The Chemical Action of Radium-Emanation, Part I. Action on Distilled Water, J. Chem. Soc., Trans., 91, 931-942, 1907. [ Links ]
Ramsay, W., Stoichiometry, Longmans and Green, London, 1908. [ Links ]
Ramsay, W., The Formation of Neon a Product of Radio-Active Change, J. Chem. Soc., Trans. , 1367-1370, 1912. [ Links ]
Ramsay, W., Aston, E. A., Molecular Surface Energy of Mixtures of Non-associating Liquids, Proc. Roy. Soc., 56, 182-191, 1894a. [ Links ]
Ramsay, W., Aston, E. A., Molecular Surface Energy of the Esters, Showing its Variation with Chemical Constitution, Proc. Roy. Soc., 56, 162-170, 1894b. [ Links ]
Ramsay, W., Aston, E. A., Molecular Surface Energy of Some Liquid Mixtures, Trans. Roy. Irish Acad., 32, 93-100, 1902. [ Links ]
Ramsay, W., Dobbie, J. J., On the Decomposition Products of Quinine; Part I, Oxidation with Permanganate, J. Chem. Soc., 33, 102-104, 1878. [ Links ]
Ramsay, W., Masson, Volumes of Sodium, Bromine and Phosphorus, J. Chem. Soc., Trans., 39, 50, 1881. [ Links ]
Ramsay, W., Masson, Analysis of the Waters of the Thermal Springs of Bath, J. Chem. Soc., 1370, 1912. [ Links ]
Ramsay, W., Shields, J., The Molecular Complexity of Liquids, J. Chem. Soc., Trans., 63, 1089-1109, 1893. [ Links ]
Ramsay, W., Soddy, F., Experiments in Radioactivity and the Production of Helium from Radium, Proc. Roy. Soc., 72, 204-207, 1903. [ Links ]
Ramsay, W., Soddy, F., Further Experiments on the Production of Helium from Radium, Proc. Roy. Soc., 73, 346-358, 1904a. [ Links ]
Ramsay, W., Soddy, F., Experiments in Radio-Activity and the Production of Helium from Radium, Washington, 1904b. [ Links ]
Ramsay, W., Travers, M. W., The Gaseous Constituents of Certain Mineral Substances and Natural Waters, Proc. Roy. Soc., 60, 442-448, 1897. [ Links ]
Ramsay, W., Whytlaw-Gray, R., Physical Properties of Radium Emanation, J. Chem. Soc., Trans., 1073, 1909a. [ Links ]
Ramsay, W., Whytlaw-Gray, R., Liquid and Solid Radium Emanation, J. Chem. Soc., 82, 1909b. [ Links ]
Ramsay, W., Young, S., Decomposition of Ammonia by Heat, J. Chem. Soc., 45, 88-93, 1884a. [ Links ]
Ramsay, W., Young, S., On the Vapour Pressure of a Substance in the Solid and Liquid States at the Same Temperature, Brit. Assoc. Rep., 675-676, 1884b. [ Links ]
Ramsay, W., Young, S., The Influence of Pressure on the Temperature of Volatilisation of Solids, Phil Trans., 175, 37-48, 1884c. [ Links ]
Ramsay, W., Young, S., Influence of Change of Condition From the Liquid to the Solid State, Phil Trans., 175, 461478, 1884d. [ Links ]
Ramsay, W., Young, S., The Decomposition of Ammonia by Heat, J. Chem. Soc., 45, 88-93, 1884e. [ Links ]
Ramsay, W., Young, S., On a New Method of Determining the Vapour Pressures of Solids and Liquids, and on the Vapour Pressure of Acetic Acid, J. Chem. Soc., 42-45, 1885a. [ Links ]
Ramsay, W., Young, S., Vapour Pressure of Mercury, J. Chem. Soc., Proc., 115, 1885b. [ Links ]
Ramsay, W., Young, S., Influence of Change of Condition from the Liquid to the Solid State on Vapour Pressure, Proc. Roy. Soc., 36, 499-500, 1885c. [ Links ]
Ramsay, W., Young, S., Method of Obtaining Constant Temperatures, J. Chem. Soc., 47, 640-657, 1885d. [ Links ]
Ramsay, W., Young, S., Some Thermodynamical Relations -Part I, Proc. Phys. Soc., 7, 289-306, 1885e. [ Links ]
Ramsay, W., Young, S., Some Thermodynamical Relations -Part II, Proc. Phys. Soc., 7, 307-326, 1885f. [ Links ]
Ramsay, W., Young, S., Some Thermodynamical Relations -Part III, Proc. Phys. Soc., 7, 327-334, 1885g. [ Links ]
Ramsay, W., Young, S., A Study of the Thermal Properties of Ethyl Alcohol, Proc. Roy. Soc., 38, 329-330, 1885h. [ Links ]
Ramsay, W., Young, S., Nature of Liquids as Shown by a Study of the Thermal Properties of Stable and Dissociable Bodies, J. Chem. Soc., Proc., 2, 226-227, 1886a. [ Links ]
Ramsay, W., Young, S., Evaporation and Dissociation (Acetic Acid), J. Chem. Soc., 49, 790-812, 1886b. [ Links ]
Ramsay, W., Young, S., On Evaporation and Dissociation -Part I, Phil. Trans., 177, 71-122, 1886c. [ Links ]
Ramsay, W., Young, S., Continuous Transition from the Liquid to the Gaseous State of Matter at all Temperatures, Phil. Mag., 23, 435-458, 1887a. [ Links ]
Ramsay, W., Young, S., Thermal Properties of Ether, Proc. Roy. Soc., 40, 381-382, 1887b. [ Links ]
Ramsay, W., Young, S., A Study of the Thermal Properties of Methyl Alcohol, Phil. Trans., 178, 313-334, 1887c. [ Links ]
Ramsay, W., Young, S., Some Thermodynamic Relations - Part VI. On the Continuous Change From the Gaseous to the Liquid State al all Temperatures, Phil. Mag., 24, 196-212, 1887d. [ Links ]
Regnault, V., Cours Elémentaire de Chimie, V. Mason, Paris, 1850. [ Links ]
Rutherford, E., Radioactivity Produced in Substances by the Action of Thorium Compounds, Phil. Mag., 49, 1-14, 161192, 1900. [ Links ]
Rutherford, E., Soddy, F., The Radioactivity of Thorium Compounds. I. An Investigation of Radioactive Emanation, J. Chem. Soc., Trans., 81, 321-50, 1902a. [ Links ]
Rutherford, E., Soddy, F., The Radioactivity of Thorium Compounds, II. The Cause and Nature of Radioactivity, J. Chem. Soc., Trans., 81, 321-50, 1902b. [ Links ]
Thomson, J., A Quantitative Investigation of Certain Relations Between the Gaseous, the Liquid, and the Solid States of Water-Substance, Proc. Roy. Soc., 22, 27-36, 1873. [ Links ]
Tilden, W. A., Sir William Ramsay, Macmillan, London 1918. [ Links ]
Travers, M. W., A Life of Sir William Ramsay, Arnold, London, 1956. [ Links ]
Whytlaw-Gray, R., Ramsay, W., Density of Niton and the Disintegration Theory, Proc. Roy. Soc., 84, 536-550, 1911. [ Links ]
Whytlaw-Gray, R., Ramsay, W., The Atomic Weight of Radium (determination of), Proc. Roy. Soc., 86, 270-290, 1913. [ Links ]
Wisniak, J., The Composition of Air: Discovery of Argon, Educ. quím., 18, 69-84, 2007. [ Links ]














