Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Educación química
versión impresa ISSN 0187-893X
Educ. quím vol.19 no.4 Ciudad de México oct. 2008
De aniversario
Teaching and Learning with Chemistry in Context
Lucy Pryde-Eubanks*
* Department of Chemistry, Clemson University, Clemson, South Carolina, USA. E-mail: elucy@clemson.edu
Abstract
Chemistry in Context is an issues-based curriculum model developed by the American Chemical Society for non-science majors at the college level. Through six editions, the goal has been to establish chemical principles on a "need-to-know" basis, always within a framework of significant issues at the science-society interface. Global warming, air and water quality, acid rain, energy choices, and genetic engineering are just some of the topics that our students explore, helped by appropriate use of web-based materials. Allowing these students the opportunity to develop the skills and knowledge necessary for becoming responsible citizens is essential if we are successfully to address global challenges. This paper will discuss features of this successful curricular approach and how it changes both students and faculty.
Key words: Non-science majors, issues-based curriculum, science-society interface.
Resumen
Chemistry in Context es un modelo curricular con base en problemas, desarrollado por la Sociedad Americana de Química para el nivel universitario en carreras que no sean de ciencias. A través de seis ediciones, la meta ha sido establecer los principios de la química sobre una base de "necesidad-de-conocer", siempre dentro de un marco de aspectos significativos en la interfase ciencia-sociedad. Calentamiento global, calidad del aire y el agua, lluvia ácida, selección de energías e ingeniería genética son solamente algunos de los tópicos que el estudiante explora, ayudado con el uso apropiado de materiales basados en la Web. Resulta esencial permitir a los estudiantes la oportunidad de desarrollar las habilidades y el conocimiento necesarios para volverse ciudadanos responsables, si vamos a dirigirnos exitosamente hacia cambios globales. Este artículo debatirá algunos aspectos de esta aproximación curricular y cómo cambia tanto a los estudiantes como a los profesores.
The American Chemical Society's Chemistry in Context: Applying Chemistry to Society has set the standard for teaching through , the use of societal issues to develop the underlying chemical principles. The fifth edition of Chemistry in Context (Eubanks et al., 2006} and the recently released sixth edition (Eubanks et al., 2009) are being used by students across the United States, retaining Chemistry in Context's unique position as the market leader for non-science majors. The project includes a text, a lab manual, and extensive web-based supporting materials for an undergraduate college-level chemistry course targeted specifically for this important audience.
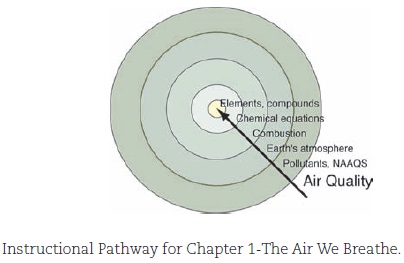
Every chemist is aware of the critical need to improve public perception and understanding of chemistry among the general public. What better place to start than with college students majoring in disciplines outside of the natural sciences? Through all editions of Chemistry in Context, the consistent plan has been to motivate student learning by placing chemical principles with a contextual framework of significant social, political, economic, and ethical issues. This is the chemistry that our non-science major students will need as future voters, parents, and citizens of the world. Each chapter opens with a science-technology-society issue that introduces the context. The issues have been chosen by the author team because of their potential to interest and engage students, but also because chemistry is needed for the discussion. As students explore each issue, chemical principles are introduced on a "need-to-know" basis. For example, consider the instructional pathway for the all-important first content chapter in the text. It sets the pattern for starting with the real-world issue and going back into the discipline of chemistry. Presenting instructional pathways to learning in this format was originated and rendered by Chemistry in Context co-author Catherine H. Middlecamp and are used with her permission.
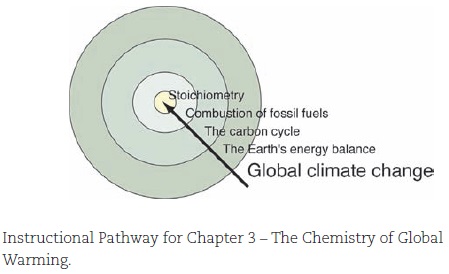
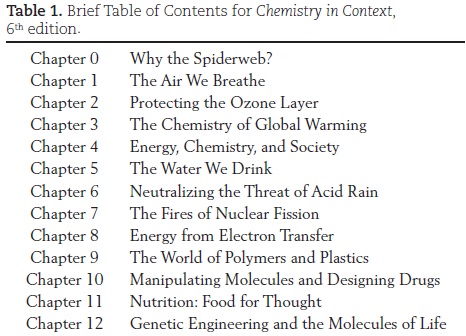
The chapter titles clearly reflect that this is not a traditional chemistry textbook (Table 1). You will not find the usual chapters on chemical structure and bonding, acids and bases, or even the much-dreaded stoichiometry (although that term is never used in Chemistry in Context). These and many other "real" chemistry topics are in the text, but always woven into the context established early in each chapter. The instructional pathway for Chapter 3, The Chemistry of Global Warming, illustrates how students are introduced to quantitative chemistry because the skill is needed to understand the issue at hand, not just because chemists deem it an essential topic.
You will note that there is a Chapter 0, an unusual feature in a textbook. Chapter 0 was first introduced in the fifth edition (Eubanks, 2006) to walk students through the process of using all the resources available to them and understanding the basic philosophy of the text. Much of the information that was found in the preface in previous editions of Chemistry in Context is now in this introductory chapter. Knowing that most students (and probably fewer instructors) read the preface of any book, the author team decided to write directly to the students. Yes, there is still a traditional preface for faculty, but this student-friendly chapter explains the pedagogy, problem-solving opportunities, and many of the media resources of the sixth edition. Students have responded favorably to this approach, commenting that they felt more comfortable after understanding the overall design of each chapter and how to learn from this non-traditional textbook and its resources in the most effective manner.
Chapter 0 also explains the symbolism of the spiderweb, an image that many students and faculty now associate with use of the Worldwide Web for both learning and entertainment. However, when the first edition of Chemistry in Context was published in 1994, few college courses used the resources of the Web. Rather, the title itself provides the clue to the choice of the spiderweb image. The word context derives from the Latin word meaning "to weave." The spiderweb reminds us that this text emphasizes the strong and complex connections that exist among chemistry, societal needs, and personal concerns.
After the introductory Chapter 0, the next four to six chapters are considered core chapters in which issues such as air quality, the ozone layer, global warming, fossil fuel energy, water quality, and acid rain are used to introduce and expand upon basic chemical principles. These chapters provide a coherent strand of issues focusing on a single theme, the environment. Within them, a foundation of necessary chemical concepts is developed from which other chemical principles are derived in subsequent chapters. Later chapters explore non-fossil fuel energy sources such as nuclear power, batteries, fuel cells, and the hydrogen economy. The emphases in the remaining four chapters are carbon-based issues and chemical principles related to polymers, drugs, nutrition, and genetic engineering. Thus, a third of the text has a distinct organic/biochemistry flavor. Most instructors teach seven to nine chapters in a typical one-semester course. However, others find that Chemistry in Context contains ample material for a two-semester course.
A coherent plan and lofty goals for our non-science major students are certainly laudable, but one has the responsibility to ask how such goals can be realized. Within Chemistry in Context, several strategies to enable student learning and to facilitate teaching are embedded in each chapter. The most straightforward of these are the Your Turn exercises, providing students ample opportunity to practice new skills or calculations. They often relate to a figure, table, or other information just introduced in the text, as in this example (Figure 2.1) from Chapter 2, Protecting the Ozone Layer. Answers are often given following the exercise or in an appendix to allow students to check their progress easily.
Every chapter also has many Consider This activities, giving students a chance to use what they are learning to make informed decisions. These may require consideration of opposing viewpoints, constructing a risk-benefit analysis, predicting the consequences of a particular action, or making and defending a personal decision. These activities may require additional research, as is the case with this example from Chapter 2 (Consider this 2.19). The Online Learning Center provides quick access to many of the Web sites useful for these Consider This and the Sceptical Chymist activities described next. Note the use of the Web icon, alerting students that they will need to access other resources, most conveniently from the Web.


The third type of activity found in the chapters is called the Sceptical Chymist. Students are required to marshal analytical skills to respond to various statements and assertions. The unusual spelling comes from an influential book written in 1661 by Robert Boyle, the early investigator studying the properties of air. His experimentation challenged some of the "conventional wisdom" of the time, which is what students will do in these activities. Here is an example (Sceptical Chymist 3.34) from Chapter 3, The Chemistry of Global Warming.

Figures Alive! animations and activities for each chapter are on the Web at the McGraw-Hill Online Learning Center (www.mhhe.com/cic). The animations bring textbook figures "alive" through the use of graphics and include interactive questions to guide the student to practice chapter essentials, better develop chapter concepts, and explore extensions of chapter material. Students are encouraged to visit the Figures Alive! resource early and often to gain the maximum benefit. For example, Figure 2.6 from Chapter 2 "comes alive" to enable students to learn more about relationships in the electromagnetic spectrum. Note the use of the Figures Alive! icon.

There is one more important icon used in Chemistry in Context, 6th Ed, one used to represent green chemistry topics. These topics have become increasingly important as each new edition has been written and brought up to date. Both green chemistry and the more recent emphasis on sustain-ability will be further developed in the next edition, already launched under the leadership of Catherine Middlecamp. For example, here is a green chemistry topic in the 6th edition from Chapter 8, Energy from Electron Transfer. This example follows an activity in which students identify the region of the electromagnetic spectrum in which the wavelengths with enough energy to split water into hydrogen and oxygen are found. Note that this particular Green Chemistry example provides a linking of Chapter 8 back to what has been learned in Chapter 2, and forward to genetic engineering to be discussed in Chapter 12. Such integration of learning by looking back and looking forward is an intentional design feature of this text.

There are several features at the end of each chapter to help students focus their learning. The first is a Conclusion to bring together the general theme of that particular chapter and place it into a larger perspective. Each conclusion provides a balanced assessment of the science-society issue under discussion and aims to link the issue with what has been previously learned and what will be examined in the chapters to follow. Here is an example, this one from Chapter 6, Neutralizing the Threat of Acid Rain. This chapter, found mid-way in Chemistry in Context, bridges the earlier topics of air quality, global warming, and energy from fossil fuels to the topics of nuclear energy and alternative energy that follow.
Conclusion
If you have learned anything from this chapter, we hope it has been skepticism, prudence, and the recognition that complex problems cannot be solved by simple or simplistic strategies. "Acid rain" is not the dire plague once described by environmentalists and journalists. Nor is it a matter to be ignored. It is sufficiently serious that federal legislation, the Clean Air Act Amendments of 1990, has been enacted to reduce SO2 and NOx emissions, precursors to acid deposition. In addition, nitrogen emissions are tied to many issues other than acid rain. The release of reactive forms of nitrogen in our environment has caused a cascading set of problems.
Any failure to acknowledge the intertwined relationships involving the combustion of coal and gasoline, the production of sulfur and nitrogen oxides, and the reduced pH of fog and precipitation is to deny some fundamental facts of chemistry. Knowledge of ecology and biological systems is needed as well, so that acid deposition can be understood in the context of entire ecosystems, a task that requires that experts from several disciplines collaborate.
Public health also is at issue. Economic analyses reveal that allocating funds to reduce sulfur and nitrogen emissions will have a huge payoff in terms of lower mortality rates, fewer illnesses, and higher quality of living.
One response that we as individuals and as a society might make to the problems of acid precipitation has hardly been mentioned in this chapter, yet it is potentially one of the most powerful. It is to conserve energy. Sulfur dioxide and nitrogen oxides are by-products of our voracious demand for energy, especially for electricity and transportation. Carbon dioxide, of course, is an even more plentiful product. If our personal, national, and global appetite for fossil fuels continues to grow unchecked, our environment may well become a good deal warmer and a good deal more acidic. Moreover, the problem may be intensified as petroleum and low-sulfur coals are consumed and we become even more reliant on high-sulfur coal. There are other sources of energy —nuclear fission, water and wind, renewable biomass, and the Sun itself. All currently are being utilized, and this no doubt will increase. We explore nuclear fission in the next chapter. But we conclude this chapter with the modest suggestion that, for a multitude of reasons, the conservation of energy by industry and collectively by individuals could have profoundly beneficial effects on our environment.
The Chapter Summary is the next feature at the end of each chapter. The summary items identify the expectations for learning for the students, with each item keyed to a specific section in the chapter. Students are often urged to use the summary items as a way to guide their learning and to review before moving on to another chapter. Items range from the specifics of chemical content to a more global perspective, requiring students to synthesize the chemical, political, and economic aspects of the topic. This set of summary items is again from Chapter 6, Neutralizing the Threat of Acid Rain.
Chapter Summary
Having studied this chapter, you should be able to:
Define the terms acid and base and apply these definitions (6.1-6.3)
• Use chemical equations to represent the dissociation (ionization) of acids and bases (6.1-6.2).
• Write neutralization reactions for acids and bases (6.3).
• Describe solutions as acidic, basic, or neutral based on their pH or concentrations of H+ and OH- (6.3-6.4).
• Calculate pH values given hydrogen or hydroxide ion in whole-number concentrations (6.4).
• Describe the differences between the pH of water, the pH of ordinary rain, and the pH of acid rain, and locate on a map of the United States where the most acidic rain falls (6.4-6.5).
• Explain the role of sulfur oxides and nitrogen oxides in causing acid rain (6.7-6.8).
• List the different sources of NOx and of SO2 and explain the variations in the levels of these pollutants over the past 30 years (6.9).
• Explain the production of acidic aerosols and their effects on building materials and human health (6.10-6. 11).
• Explain why N2 is a relatively inert element. Describe different forms of reactive nitrogen and how they are produced both naturally and by humans. Use the nitrogen cycle to explain the cascading effects of reactive nitrogen. (6.12).
• Describe how the industrial production of ammonia and the acidic deposition of nitrates both contribute to the build-up of reactive nitrogen on our planet (6.12).
• Describe nitrogen saturation and its consequences for lakes (6.13).
• Discuss the 1990 Clean Air Act Amendments and the cap and trade program. Describe the impact these continue to have on SO2 emissions (6.13-6.14).
• Describe how NOx emissions have been controlled differently from SO2 emissions (6.13).
• Outline different ways to control acid rain, noting the cost-benefit considerations involved (6.13-6.14).
• Explain why acid rain control is an exceedingly wise investment in terms of the benefits to human health (6.15).
The next resource for students is the end-of-chapter questions. They are grouped into three categories parallel to the in-chapter activities. Emphasizing Essentials give the opportunity to practice fundamental skills such as those practiced in the Your Turn activities within the chapter. Many questions are based on chemistry knowledge developed in this or earlier chapters. Some questions refer students to a Figure or Table to find their answer. Each question in this category has answers that can be easily judged as being "right" or "wrong" and answers for selected questions are given in an appendix, allowing students to check their understanding quickly. These Emphasizing Essentials questions are but a subset of the available questions and are again chosen from Chapter 6. You will be able to see how the questions relate to both the conclusion and summary items for this chapter.
Questions
Emphasizing Essentials
6. Write a balanced chemical equation for each acid-base reaction.
a. Potassium hydroxide is neutralized by nitric acid.
b. Hydrochloric acid is neutralized by barium hydroxide.
c. Sulfuric acid is neutralized by ammonium hydroxide.
13. Suppose you have a new mountain bike and accidentally spilled a can of carbonated cola on the metallic handle bars and paint.
a. Soft drinks are more acidic than acid rain. About how many times more acidic? Hint: Consult Figure 6.6.
b. In spite of the higher acidity, this spill is unlikely to damage your handle bars and paint (although the sugar probably isn't great on your gears). Why is damage unlikely?
27. The Clean Air Act was discussed both in this chapter and in Chapter 1, the Montreal Protocol in Chapter 2, and the Kyoto Accord in Chapter 3.
a. What principal issue does each of these address?
b. Place all three on a time-line.
End-of-Chapter questions in the next category, Concentrating on Concepts, ask students to integrate and apply the chemical concepts developed in this and earlier chapters and to relate the concepts to the "real world." Questions in this category parallel many of the Consider This activities in the text. Students are often asked to explain their answers and, as was the case with some of the questions in the Essentials category, to use figures or tables within this or other chapters for reference. Many answers to questions in this category do not have relatively simple "right" or "wrong" answers, but often involve making an informed decision based on what has been learned. Here are some selected Concentrating on Concepts questions, again from Chapter 6.
Questions
Concentrating on Concepts
33. Which of these has the lowest concentration of hydrogen ions: 0.1 M HCl, 0.1 M NaOH, 0.1 M H2SO4, pure water? Explain your answer.
35. As mentioned in Section 6.6, rain samples currently are being analyzed for acidity in the Central Analytical Laboratory in Illinois instead of out in the field.
a. The pH values tended to be slightly higher in the lab. Did the acidity increase or decrease?
b. Speculate on the causes of the pH increase.
47. Discuss the validity of the statement, "Photochemical smog is a local issue, acid rain is a regional one, and the enhanced greenhouse effect is a global one." Describe the chemistry behind each issue. Do you agree that the magnitude of the problem is really so different in scope?
The last category of end-of-chapter questions is Exploring Extensions. Like the Sceptical Chymist activities within each chapter, these questions challenge students to go beyond the information presented in the text and to extend and integrate the facts, concepts, and use their communication skills. Often additional research is required, usually indicated by the web icon. Here are some Exploring Extension questions from Chapter 6.
Questions
Exploring Extensions
53. Equation 6.18 shows that energy (in the form of a hot engine or other source of heat) must be added to get N2 and O2 to react to form NO. A Sceptical Chymist wants to check this assertion and determine how much energy is required. Show the Sceptical Chymist how this can be done. Hint: Draw the Lewis structures for the reactants and products, noting that NO does not have an octet of electrons. The bond energy is 607 kJ/mol for the N-to-N double bond.
54. The text describes a green chemistry solution to reducing NO emissions for glass manufacturers.
a. Identify the strategy.
b. Use the Web to research what other industries might use this green chemistry strategy. Write a report to summarize your findings.
58. Why are developing countries likely to emit an increasingly higher percentage of the global amount of SO2? Pick a nation, research its current emissions of SO2, and calculate its percent of global emissions. Are emissions likely to continue to increase in the future? Make a prediction and give your reasoning.
With all of these teaching and learning opportunities built into the text, students still ask the same age-old (and valid) question: "Will it be on the test?" The Online Learning Center provides two sets of multiple-choice quiz questions. Students can check their understanding and receive instant feedback, giving them some confidence in their progress in learning the fundamentals. Although this type of question does not represent the complete assessment strategy in most courses, it is a confidence builder for students to be able to practice their mastery in a non-threatening environment.
How do we know that the Chemistry in Context approach to teaching and learning leads to success for students? There are many indicators that I can see from my own teaching experience and feedback from the chemistry education community. The intimidation barrier involved in learning the "hard sciences" is significantly lowered, judging from attitude and belief surveys that students have taken before, during, and after course completion. The number of students enrolled in our courses grows as the word gets out about any issues-based course, whether it is based on Chemistry in Context or on other materials developed using this model. Students are observed to be engaged and voice their enthusiasm about what they are learning. Students talk about what they are learning to each other, to their peers, and even to their parents. Students are encouraged to explore local, regional, and global connections, sometimes for the first time in their education. They perform well not only on well-written multiple-choice questions, but develop skills in writing reasoned reports on activities and answers on essay-type questions. Many of these students stay in touch with faculty after the completion of the course and relate how important what they have learned has been to them. There is an interesting effect on the chemistry majors as well, with a demand for more relevance in their courses being heard. While much of this is admittedly anecdotal evidence, there are many of us that believe that any formal evaluation plan for the project would validate these observations and that students undergo significant change in both knowledge and attitude.
Faculty, too, are changed by the experience of teaching non-majors using Chemistry in Context. This is not the way most of us have been taught. Faculty who "buy in" to the issues-based approach find their teaching is changed in all their courses, not just those for non-science majors. Nevertheless, gathering current issues-based material can be time-consuming and challenging, even for faculty who want to make significant curricular change. To help meet their needs, the Online Learning Center supports not only student learning, but also for effective teaching of Chemistry in Context as well. In the password-protected center for instructors, one can find an Instructor's Resource Guide containing a chemical topic matrix, answers for suggested responses to many of the open-ended questions in the Consider This activities, and the solutions to the in-chapter and end-of-chapter activities and questions. There is also an instructor's guide for the lab manual, together with some additional experiments not found in the lab manual.
A new feature of the instructor's center for the 6th edition includes the McGraw-Hill Presentation Center. This is a multimedia collection of visual resources allowing instructors to utilize artwork from the text in multiple formats to create customized classroom presentations, visually based tests and quizzes, dynamic course Web course content, or attractive printer support materials. Very helpful indeed is the Instructor's Testing and Resources Online section containing an extensive Test Bank formatted for easy integration into common course management systems. Further exploration of these teaching resources is always an essential part of any workshop offered at local, regional, and national meetings or through specially arranged workshops for instructors or potential instructors using Chemistry in Context.
Many colleges and universities are currently re-examining their chemistry curricula to move towards integration of science disciplines and also to include more societal connections in their courses. Within chemistry departments, curriculum reform efforts are often focused on science majors, an understandable decision. However, the number of non-science majors within our colleges and universities is huge (approximately 100,000 in the United States alone) and we have a responsibility to these students as well. For all students, faculty are urged to "Engage the Big Questions", to "Connect Knowledge with Choices and Action", and to "Foster Civic Intercultural, and Ethical Learning" in every field of study (American Association of Colleges and Universities, 2007). By being a leader in urging and implementing such ideas, the American Chemical Society's Chemistry in Context project not only has had a major impact on teaching and learning in the United States, but has demonstrated an international influence as well. It has already been translated into Korean and Chinese and contracts have been signed to translate the materials into Japanese, Arabic, and Spanish. Thus, the convergence of Chemistry in Context with the need for science-literate global citizens cannot be underestimated.
References
American Association of Colleges and Universities, College Learning for the New Global Century, 2007 http://www.aacu.org/advocacy/leap/documents/GlobalCentury_final.pdf., p. 26, consulted the last time on June 5, 2008.
Eubanks, L., Middlecamp, C., Heltzel, C., Keller, S., Chemistry in Context, 6th Ed., McGraw-Hill, Dubuque, IA, 2009. [ Links ] Graphics in this article, with exception of the two learning pathways, are used with permission of McGraw-Hill Higher Education.
Eubanks, L., Middlecamp, C., Pienta, N., Weaver, G., Heltzel, C., Chemistry in Context, 5th Ed., McGraw-Hill, Dubuque, IA, 2006. [ Links ]