Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Educación química
Print version ISSN 0187-893X
Educ. quím vol.19 n.2 Ciudad de México Apr. 2008
Experiencias y Cátedra
Chemistry of Alkali Metals 2. On the Reactions of Alkali Metals with Liquid NO2 and the Nature of the Product
Química de los metales alcalinos. 2. Sobre las reacciones de los metales alcalinos con NO2 y la naturaleza del producto
Vladimir M. Petrusěvski and Keti Risteska1
1 Institute of Chemistry Faculty of Natural Sciences & Mathematics, Ss. Cyril & Methodius University, Arhimedova 5, 1001 Skopje, Republic of Macedonia. Correo electrónico: vladop@iunona.pmf.ukim.edu.mk
Recibido: 31 de agosto de 2007;
Aceptado: 31 de enero de 2008.
Abstract
The reactions of sodium and potassium with liquid nitrogen(IV) oxide were performed to check the nature of the product, that was claimed (Nekrasov, 1976) to be MNO3 (M = Na, K). Both classical chemical reactions and FT-IR spectrometry were used in the course of its analysis. It was proved that the product was indeed nitrate, sometimes containing nitrites as impurities.
Key words: alkali metals, nitrogen(IV) oxide, nitrogen dioxide, dinitrogen tetroxide, alkali nitrates, alkali nitrites, IR spectroscopy.
Resumen
Las reacciones de sodio y potasio con óxido de nitrógeno(IV) líquido fueron realizadas para confirmar la naturaleza del producto, que fue predicha por Nekrasov (1976) como MNO3 (M = Na, K). Tanto reacciones químicas clásicas como espectrometría FT-IR fueron empleadas durante el análisis. Se demostró que el producto era nitrato, con nitrito como impureza, en ocasiones.
Palabras clave: Metales alcalinos, óxido de nitrógeno(IV), dióxido de nitrógeno, tetróxido de dinitrógeno, nitratos alcalinos, nitritos alcalinos, espectrometría IR.
Introduction
An old general chemistry textbook (Nekrasov, 1976) contained the information that upon the action of liquefied NO2 (i.e. N2O4) on sodium, a slow reaction occurred, resulting in formation of NaNO3. This seemed as an interesting assertion, as one could write at least two equations between NO2 and metallic sodium. Thus, the formation of NaNO3 can be justified by:

However, since NO2 is electron deficient, it is also possible that a nitrite be formed, as the equation

also seems to be a feasible one.
As the mentioned textbook is a rather old one, it seemed reasonable to allow that few pieces of information it contains might not necessarily be correct. Therefore, one had to check the chemical nature of the product in order to prove whether eq. (1) or eq. (2) or perhaps both are operative in this particular case. In the present paper we discuss our approach to the above problem.
Objectives
This is a paper that describes a research experiment, suitable for university students. Research experiments are experiments whose results are novelty for the students, although these results are not necessarily a novelty in an absolute sense of the word (Monkovic et al., 2007). The important objective of experiments of the above type is to teach students to be critical when faced with statements that are not supported by sound arguments. The source of information (Nekrasov, 1976) is a textbook that has been written in the middle of the 20th century. It may contain true information, but not necessarily. In particular, when it becomes evident that more than one chemical equation may be written for the reaction in question, it might be a challenge to make an identity check of the product obtained. Another alternative would be to perform a thorough literature survey.
Both simple classical methods (characteristic reactions for the presence of nitrous acid and nitrites) and sophisticated ones (FT-IR spectroscopy) have been employed. In the course of this, the instructor could stress and explain the following to the audience:
a) The reaction between alkali metals and nitrogen(IV) oxide might be considered as a reaction between Usanovich base and Usanovich acid. This classification is, in a way, a generalization of the Lewis acid-base concept. Few details for it are given elsewhere (Brandis, 2006).
• The Usanovich base (an electron donor) is the alkali metal.
• The Usanovich acid (electron deficient compound) is nitrogen(IV) oxide.
• As in other cases where reaction between acid and base occurs (a neutralization reaction), the product (MNOx) is salt.
b) Theoretically, more than one product might be obtained. It might be of interest to check the nature of the product and confirm (or refute) the information given in the literature.
c) Nitrous acid (nitrite in acidified medium) is by far superior oxidation agent than nitric acid (nitrate in acidified medium) of the same concentration (for some reason, the latter proves to be very far from a well-known fact; students usually have the wrong impression that nitric acid and nitrates are superior oxidants).
d) IR spectroscopy is being used as a low level, but positive proof of products identity. It is enough to know that the IR spectrum is a "fingerprint" of certain compound (and for solids, even a fingerprint for a given polymorphic modification). There are no two compounds (no two polymorphs) with the same IR spectrum. Identical IR spectra point to identical composition of the samples.
Safety Tips & Disposal
All nitrogen oxides are toxic (SIRI MSDS Index, 2008). When working with them, one is supposed to work in a hood (fumy-cupboard). Aqueous solutions of the water soluble oxides (those that are water soluble react with water giving HNO3 and/or HNO2) can be disposed under the drain with plenty of water. In the event of poisoning, call for physician immediately.
Alkali metals are highly reactive & corrosive substances (DOE Handbook, 1994). Always wear a face shield and gloves when working with them. Never experiment with large pieces of alkali metals, unless you know exactly what you are doing and are aware of all consequences.
Always destroy small pieces of alkali metals that are left after the experiment. For that purpose, the easiest and cheapest alternative would be to use a large beaker half-filled with water and to destroy one small piece at a time. As pointed by one of the reviewers, a safer (albeit a bit more expensive variant) would be to destroy the traces of alkali metals with isopropanol. Be prepared for very vigorous reaction if the metals are allowed to react with water (potassium pieces always catch fire; sodium ones as a rule don't).
Experimental 1: Generation and Liquefaction of Nitrogen(IV) Oxide
Nitrogen dioxide was generated by thermal decomposition of dry Pb(NO3)2. This was performed in a test-tube (containing a mixture of the lead salt and some dry sand—the latter is useful for making the reaction mixture more porous, and also for allowing a side-reaction of the PbO product with SiO2 resulting in formation of PbSiO3). The test-tube was corked and heated electrically. A long glass tube passed through the cork and was connected to a large test-tube, placed in ice bath containing a mixture of table salt and ice for attaining temperatures lower than 0 °C (theoretically down to -21 °C; in our case the temperature was kept just below 0 °C). The latter is important for decreasing the rate of evaporation of the liquefied nitrogen(IV) oxide. The setup for the experiment is given in Figure 1.

One proceeds with the heating until 1-2 mL of liquefied nitrogen(IV) oxide were collected (this is, actually, an equilibrium mixture of N2O4 and NO2; at the temperature of the experiment, the N2O4 is by far the dominant species and only traces of NO2 are present). The color of the liquid phase is pale yellowish-brown; that of the gas phase is somewhat darker (cf. Fig. 2). This result is easily interpreted in terms of the NO2 enriched gas phase with respect to the composition of the liquid phase.

One of the reviewers suggested "an easier" method for NO2/N2O4 generation (Mattson, 1997). While this is certainly possible, we have some doubts whether this is really an easier method for generation of medium quantities of the equilibrium NO2/N2O4 mixture (the above method was, originally, recommended for microscale gas chemistry experiments).
Experimental 2: Reaction of Alkali Metals with Liquid Nitrogen(IV) Oxide
Few pieces of either potassium or sodium (each of the size of half a match-head) were added to the liquid, while the test-tube was held in the ice bath. A slow reaction was soon evident. The bottom of the test-tube was covered with a microcrystal-line yellowish covering (cf. Fig. 3a), and slow gas evolution was evident. The gas evolution seems to be in favor of eq. (1). Alternatively, it could result from slow evaporation of the liquid nitrogen(IV) oxide, the initial bubbles being formed on the source of heterogeneity (i.e. the pieces of alkali metal subsequently covered by the reaction product). It would be difficult to tell the difference between NO gas evolution and N2O4 gas evaporation, because at the temperature of the experiment (close to 0 °C) both are expected to be practically colorless. Curiously, the reaction with potassium does not seem to be significantly (if at all) faster than that with sodium.

After 15-20 minutes the test-tube is removed from the ice bath and moderate stream of air is passed through it. In few minutes the color of the crystals (probably resulting from adsorbed/absorbed NO2) disappears (cf. Fig. 3b).
Experimental 3: Examining the Nature of the Product
The white microcrystals could be due to MNO3, MNO2 or a mixture of both, as mentioned above (where M stands for either Na or K). One way to detect the presence of nitrites is the reaction with acidified solution of permanganate (Fowles, 1959). An instantaneous discoloration occurs due to oxidation to nitrates, the Mn(VII) being reduced to Mn(II). Also, the reaction with acidified aqueous solution of potassium iodide is instantaneous, and gives rise to intense brown color of elemental iodine, unlike the reaction with nitrates that needs some time and gives rise to only light brown color (Fowles, 1959). The results of the check for nitrites with the residue obtained from potassium are given in Fig. 4 (the results for sodium are similar, but for some unknown reason, these seemed to contain slightly more nitrite impurities).

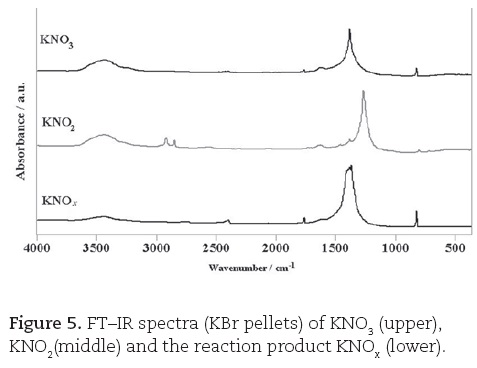
The FT-IR spectra (from KBr pellets) were also acquired from the product (labelled KNOx), obtained in the reaction of nitrogen(IV) oxide with potassium metal and were compared with the corresponding spectra of both KNO3 and KNO2 crystals (cf. Fig. 5).
Interpretation of the Experimental Results & Discussion
The reaction of the product with KMnO4 solution indicates that only traces of nitrite ions are present (cf. Fig. 4, left). Namely, the color of the solution, after the addition of KNOx(aq) slowly fades with time. This experiment suggests that the white crystalline solid obtained as a product is practically pure KNO3.
The parallel reaction of both KNOx(aq) and KNO3(aq) with KI(aq) supports the above conclusion. After immediate mixing of the solutions (cf. Fig. 4, middle and right, upper row), no color due to elemental iodine could be registered, hence no measurable quantities of nitrite ions are present. Upon standing overnight, pale yellowish-brown color is seen in both beakers (cf. Fig. 4, middle and right, lower row). Again, the color intensity seems to be the same in both beakers. If nitrite ions are present at all, these must be present in minor quantities, below the detection point.
The final confirmation of this conclusion comes from the analysis of the FT-IR spectra. There is a clear-cut distinction between the spectra of KNO2 and KNO3. The spectrum of the product (cf. Fig. 5) matches almost perfectly the spectrum of KNO3 (the only difference is that the concentration of the samples is unequal). The bands in the spectra could be easily assigned to the fundamental vibrations of the NO3- (i.e. NO2-) ions (Nakamoto, 1973), but this is actually not necessary, since a simple comparison (without a priori knowing any details about the vibrational spectra) proves that the sample of KNOx is almost pure KNO3.
An important question that has to be answered regards the differences in the behavior of sodium and potassium, in reaction with the liquid. As we mentioned, it seems that sodium produces more nitrite impurities than potassium. While the reasons for this finding remain unclear, it might be speculated that it has something to do with the reactivity with potassium which is superior to that of sodium. Another (much more trivial) explanation might be offered in terms of adsorbed NO2 on the particles of MNO3 formed during the reaction. If this is not thoroughly "splashed" with air (presumably in the case of NaNO3), then it might give the same reactions as HNO2, or NO2- ions and may, consequently, lead to erroneous conclusions. The latter option seems to be strengthened by the fact that nitrites never form in reactions of metals with NO2 (Addison, 1980). Further, discussions with an electrochemistry expert (Gulaboski, 2007) confirm the notion that the formation of nitrites is quite unlikely, judging from the values of the redox potentials for nitrite and for nitrate formation.
Conclusion
The experiment shows that the product is indeed sodium (potassium) nitrate, as stated in the textbook (Nekrasov, 1976). While this finding adds some value to the old textbook, it is by no means a proof that "everything that we find in the literature must be true". It is a good practice to be cautious, and from time to time to check statements given in literature, particularly in those cases where it seems that there exists some doubt.
Acknowledgements
We are indebted to the reviewers for pointing to several weaknesses in the first draft of the manuscript, and also for pointing to a more than relevant publication devoted to the area covering the above problem (Addison, 1980).
References
Addison, C. C., Dinitrogen Tetroxide, Nitric Acid and Their Mixtures as Media for Inorganic Reactions, Chem. Rev., 80, 21-39, 1980. [ Links ]
Brandis, K., Acid-Base physiology, 2006. Consulted in the following URL http://www.anaesthesiamcq.com/AcidBaseBook/ab1_2.php (accessed January 8th, 2008). [ Links ]
DOE Handbook: Department of Energy. Office of Nuclear Safety and Environment. Alkali Metals Sodium, Potassium, NaK, and Lithium, 1994. Consulted in the URL http://hss.energy.gov/NuclearSafety/techstds/standard/hdbk1081/hbk1081d.html (accessed January 8th, 2008). [ Links ]
Gulaboski, R., Private communication, 2007. [ Links ]
Fowles, G., Lecture Experiments in Chemistry, 5th edition, Bell & Sons Ltd., London, pp. 170-172, 1959. [ Links ]
Mattson, Bruce. Creighton University, Omaha, Nebraska. Microscale Gas Chemistry. Experiments with Nitrogen Oxides, 1997. Consulted in the following URL: http://mattson.creighton.edu/NOx/index.html (accessed January 8th, 2008). [ Links ]
Monkovic, M., Ivanovski, V., Petrusěvski, V. M., Aust. J. Ed. Chem., 68, 17-19 (2007). [ Links ]
Nakamoto, K., Infrared and Raman Spectra of Inorganic and Coordination Compounds, 3rd edition, John Wiley & Sons, New York, 1978. [ Links ]
Nekrasov, B. V., General Chemistry Course, 6th edition, Naucna knjiga, Beograd, p. 283, 1976 (translation from Russian into Serbian). [ Links ]
SIRI MSDS Index (Safety Information Resources, Inc., Material Safety Data Sheets Index). International Chemical Safety Cards. Nitrogen Dioxide, 2008. Consulted in the following URL http://hazard.com/msds/mf/cards/file/0930.html (Accessed January 8th, 2008). [ Links ]














