Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista del Instituto Nacional de Enfermedades Respiratorias
versión impresa ISSN 0187-7585
Rev. Inst. Nal. Enf. Resp. Mex. vol.20 no.1 México ene./mar. 2007
Conferencia magistral
Endobronchial stents and bronchial sparing surgery in the management of lung cancer
"Stents" endobronquiales y cirugía preservadora de bronquios en el manejo del cáncer broncogénico
Brian Pettiford* Rodney J. Landreneau*
* Heart, Lung, and Esophageal Surgery Institute University of Pittsburgh Medical Center Pittsburgh, Pennsylvania
Address correspondence to:
Rodney Landreneau, M.D.
Heart, Lung and Esophageal Surgery Institute
University of Pittsburgh Medical Center UPMC Shadyside Medical Center,
Suite 715 5200 Centre Avenue Pittsburgh, Pennsylvania
15232 Phone: 412–623–2030
e–mail: landreneaurj@upmc.edu
Trabajo recibido: 13–XII–2006
Aceptado: 29–I–2007
As the leading cause of cancer deaths, lung cancer accounts for an estimated 1.3 million deaths worldwide each year1. Tobacco abuse accounts for 80–90% of lung cancer. Non–small cell lung cancer accounts for 80% of lung cancer cases. Most lung cancers are parenchymal or hilar in location. Approximately 30% of lung cancer patients may present with central airway obstructive symptoms2. This presentation is often the result of an endobronchial primary or endobronchial spread of a locally advanced malignant process. Symptoms may include unexplained progressive dyspnea, frequent dry cough, bronchospasm, or hemoptysis. A fever and productive cough may also signal a postobstructive pneumonia from a postobstructive partial or complete airway collapse secondary to endobronchial tumor. Central airway cancer may also represent tumor recurrence in cases of surgically resected disease. Endobronchial tumor may represent too a benign disease process.
The approach to patients with endobronchial tumors should always begin with a thorough history and physical examination. The history should focus on symptoms of dyspnea, cough, oxygen requirement, weight–loss, and hoarseness. These patients may already have a well–documented history of a centrally located lung cancer. These symptoms may very well represent local progression, or indicate the failure of therapeutic modalities such as external beam radiation or chemotherapy. In cases of hemoptysis, it is important to determine the frequency and volume of expectoration. Furthermore, the use of anticoagulant medication should be investigated. Physical examination should include but not be limited to an evaluation of the patients overall condition including respiratory status, breath sounds and end organ perfusion. The presence of tachypnea, accessory respiratory muscle use and confusion indicate decompensation and the need for urgent intervention.
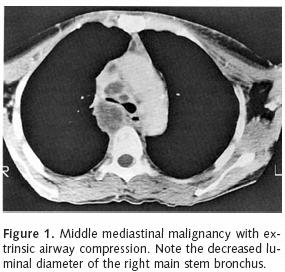
Workup of patients should include basic laboratory indices. A coagulation profile including PT, PTT, and platelet count are of paramount importance, especially when endobronchial therapy is being considered. An ABG and pulmonary function tests are also useful to evaluate baseline oxygenation, ventilation and respiratory mechanics. They may be used as a point of reference throughout a patient's course of therapy. Radiographic investigation should begin with a chest X–ray. Plain films may show lobar collapse, consolidation and infiltrative processes. A contrast–enhanced chest CT is useful in cases of a locally advanced central tumor with endobronchial involvement (Figure 1). This study can demonstrate the relationship between the tumor and bronchovascular structures. Furthermore, chest CT can show the presence of endobronchial tumor. A lung window image can also identify the presence of aerated lung tissue distal to a site of bronchial obstruction. This information is paramount to determining treatment feasibility. Lastly, a cardiac assessment should be performed, as many of these patients will likely have concomitant cardiac dysfunction.
Parenchymal sparing surgical techniques are useful in the management of resectable lung cancer with limited bronchial involvement. Right upper lobe sleeve lobectomy is a well–described technique for management of tumor that extends within and proximal to the right upper lobe orifice. The relatively long length of the left main stem bronchus also lends itself to bronchus–sparing techniques. It is imperative to map the proximal and distal extent of gross disease at the time of bronchoscopy. Our preference is to perform the vascular division and fissure separation prior to bronchotomy. Bronchial division is begun proximal to the tumor. Bronchotomy is made at a right angle to the bronchial long axis with a cold knife. As the distal end of the bronchus is divided, a 0.5 to 1 cm portion of redundant posterior membranous bronchial tissue is preserved. This manoeuver is key to correcting the size discrepancy between the proximal and distal bronchi. We use interrupted 3–0 PDS to fashion the anastomosis. The medial aspect of the closure is performed initially as this area is the least accessible to the surgeon. The posterior wall is completed first. During this time period, any additional size differences between the proximal and distal airways are gradually corrected. After completion, the anastomosis integrity is confirmed under water seal. A pedicled pericardial fat pad, intercostal muscle bundle or (in the case right sided bronchoplasty) an azygous vein flap is used to provide coverage to the anastomosis. Following chest closure, a repeat flexible bronchoscopy is performed to assess the anastomosis and evacuate secretions. Repeat toilet endoscopies are performed based upon the patient's clinical status, including physical exam and chest X–ray. We have a low threshold for repeat bronchoscopy in this patient population. With the exception of carcinoid tumors and rare localized endobronchial squamous cell cancer in good risk patients, endobronchial palliation is the mainstay in the treatment of patients with unresectable endobronchial malignancies. Bronchoscopic intervention may include laser ablation, brachytherapy, photodynamic therapy and endobronchial stent placement. The two primary bronchoscopic approaches include flexible and rigid bronchoscopy (Figure 2). Rigid bronchoscopy, which requires general anesthesia, is effective for bulky lesions located within the central airway. This modality provides airway ventilatory control and allows for tumor debulking. Rigid bronchoscopy is also effective in localizing and tamponading airway hemorrhage. Airway stent deployment and balloon bronchoplasty may be performed using the rigid scope. Contraindications to rigid bronchoscopy include bleeding diathesis, cervical spine injury or severe degenerative cervical disease with a poor range of motion. Lesions located distally along the airway are generally not amenable to rigid bronchscopy.
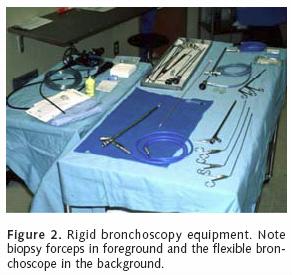
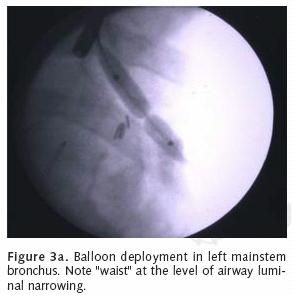
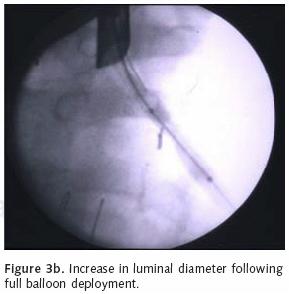
Flexible bronchoscopy can be useful for the application of YAG laser therapy, photodynamic therapy, and to localize bleeding site within the airway. Unlike rigid bronchoscopy, the flexible approach provides accessibility to the more distal airway including the segmental level. Subsegmental access can even be gained via fluoro–scopic guidance. The flexible scope can be inserted through the rigid bronchoscope to reach the distal left main stem and upper lobe orifices. Like rigid bronchoscopy, the flexible scope can be used to deploy bronchial stents or perform balloon bronchoplasty under fluoroscopic guidance (Figures 3a, b). Flexible bronchoscopy can also be performed using topical anesthesia, conscious sedation or general anesthesia. The authors prefer general anesthesia for most cases of endobronchial therapy because of the greater degree of airway control and the rapidity of operation that is allowed. A major limitation of flexible bronchoscopy is tumor debulking given the small luminal diameter of the instrument.
SPECIFIC APPLICATIONS
Nd:YAG (neodymium–ytrium–aluminum–garnet) Laser
Early reports of laser therapy of endobronchial cancer began in the late seventies and early eighties3–7. The rigid bronchoscope was used most often. YAG laser therapy demonstrated a high cure rate for benign masses as well as carcinoid tumors. Cavaliere et al, reported the results of a five–year experience in 1,000 patients. They reported that squamous cell cancer was the most common malignant cell type, accounting for nearly seventy percent of malignant endobronchial tumors. Improved results were more demonstrable in lesions within the trachea and main stem bronchi. Benign tumors were particularly amenable to laser therapy secondary to a mostly polypoid and localized tendency. The authors also reported the use of the laser for tracheal stenosis and granulomas. Although laser therapy for tracheal stenosis always resulted in immediate improvement, these lesions had a tendency to recur within months after therapy. Laser therapy can be curative in the treatment of granulomas if the source of mechanical irritation is addressed as well.
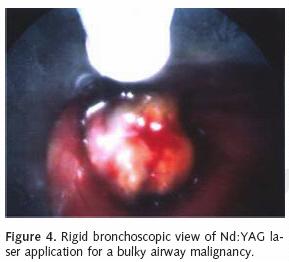
The Nd:YAG laser with a wavelength of 1,064 nm delivers near infrared radiation via a flexible quartz fiber. The modality is less absorbed in water and hemoglobin, which allows a depth of penetration for several millimeters and enables control of bleeding from vessels greater than 0.5 mm in size. The Nd:YAG laser can therefore effectively photocoagulate small blood vessels and tumor tissue. This approach can subsequently allow for mechanical tumor debulking with forceps or with the tip of the bronchoscope. As mentioned, the laser may be used with either the flexible or rigid scope. Using the laser in combination with the rigid scope allows for effective tumor debulking and relatively rapid airway restoration (Figure 4). It is important to use a rigid scope with a wide tube to allow for laser application, ventilation and extraction of secretions and tumor bulk. The flexible endoscope can be inserted through the rigid scope to allow for precise coagulation of more distally located tumors. The photocoagulated tissue may then be debrided with the tip of the flexible instrument. This approach may require several treatments to achieve a successful thermal necrosis of central and distal airway tumors. Our preference is to place the laser on a continuous setting with 0.5 to 1 second pulses at 30–35 watts. Most power settings range from 25–35 watts in pulses up to 5 seconds.
Use of the Nd:YAG laser is not without risks. Major complications include airway fire, hypoxemia, airway perforation, exsanguinating hemorrhage and pneumothorax. One complication is airway fire. It is imperative that the fraction of inspired oxygen (FIO2) is decreased prior to laser application. Our practice is to decrease the FIO2 to room air after obtaining an adequate pulse oxygen saturation level. At least 30 seconds to one minute is allowed to elapse prior to engaging the laser. During this time period, the laser is kept in the "disarmed" mode. When using the flexible scope, the laser tip is positioned just beyond the distal end of the scope. This allows for precise coagulation when the laser is engaged and fired. It is also recommended that the power is kept below 40 watts and the exposure time is limited to less than 3 seconds8.
There were early reports of catastrophic systemic air embolism during Nd:YAG laser operations9–11. A high coolant flow rate when gas–cooled laser fibers are in contact with tumor may result in air entry into the pulmonary venous system through disrupted capillaries. This mechanism has resulted in cardiovascular collapse manifested by arrhythmias, hypotension and even cardiac arrest in some cases. This complication can be avoided by using the endobronchial laser in the noncontact mode or by using a fluid coolant.
Despite the potential complications associated with endobronchial Nd:YAG laser therapy, this modality has proven useful in the management of endobronchial tumors. Special attention to detail and judicious use of the laser will avoid untoward events in most cases.
ENDOBRONCHIAL STENTS
Endobronchial stents are particularly useful in the management of unresectable lung cancer with direct endobronchial ingrowth or extrinsic compression. Stents may also prove invaluable in the management of esophageal cancer complicated by the development of tracheoesophageal fistula.
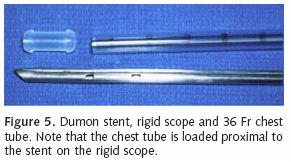
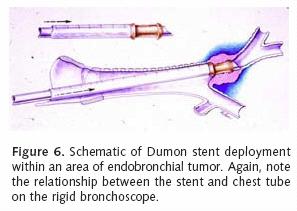
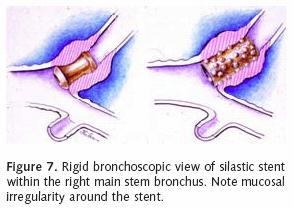
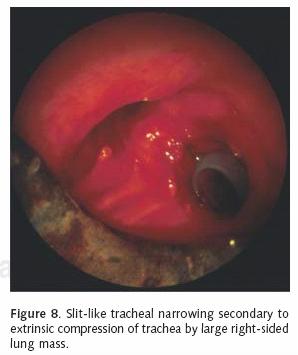
Most patients who receive endobronchial therapy for lung cancer have received maximal medical treatment including external beam radiation therapy. Stent insertion is considered a means of palliation. T–tube stent insertion to manage airway malignancy was initially used by Montgomery to temporize patients with primary tracheal malignancies12. In the 1990s, the Dumon stent was considered the gold standard prosthesis for airway management in patients with endobronchial malignancies13. This stent, composed of silicon requires rigid bronchoscopy for insertion (Figure 5). Our practice is to liberally lubricate the shaft of the rigid bronchoscope. A 36 French chest tube is then placed along the shaft14. The stent is positioned at the tip of the rigid scope, just beyond the chest tube (Figure 6). Under direct vision, the rigid scope places the stent within the affected area. The chest tube is then grasped and held stationary with a Kocher clamp or other heavy hemostat while the rigid scope is withdrawn. The chest tube effectively keeps the silicon stent in position while it is disengaged from the rigid scope (Figure 7). A major advantage of silicon stents is that they are less irritating and subsequently result in less granulation tissue formation. This decreased reactivity makes silicon stents easy to extract if necessary. They are particularly useful for maintenance of airway lumen in cases of extrinsic compression (Figure 8). The inherently thick stent structure of silicon stents significantly compromise luminal diameter, however.
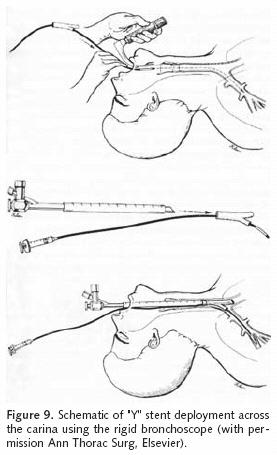
The "Y" stent is appropriate for carinal lesions with associated main stem bronchial involvement15. Sonett14 et al, described a technique for precise Y stent positioning. A 7/14 Fogarty catheter is passed through the short limb of the stent. The proximal end of the stent and a 36 Fr chest tube are loaded onto the rigid scope as described above. The Fogarty catheter is subsequently placed into the distal right airway within a segmental bronchus and inflated to secure the catheter as shown in Figure 9. The long end of the stent is then positioned inside the left main stem as described above for Dumon stent placement. The Y stent is held into place by a biopsy forceps as the rigid scope is withdrawn.
Self–expanding metallic stent (SEMS) have become increasingly popular in the management of endobronchial malignancy. This is the most commonly used stent in our practice.
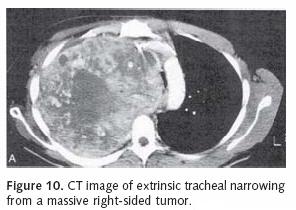
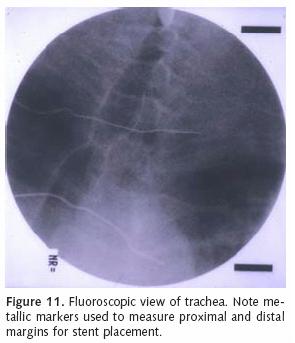
We use the Ultraflex tracheobronchial covered stent to treat endobronchial malignancies when stent deployment is indicated (Figure 10). The covered portion is placed in contact with the tumor to prevent tumor ingrowth within the stent interstices. We accomplish stent deployment with a flexible bronchoscope in the operating room under general anesthesia and with the assistance of fluoroscopy. While the lesion is visualized with the flexible scope, metallic markers are placed on the patient's chest along the proximal and distal margins of involvement (Figure 11). Fluoroscopy is used to guide marker positioning. A guide wire is then inserted through the working port of the endoscope and positioned across the target area. The appropriately sized stent, based upon length and luminal diameter, is then advanced over the wire and positioned under fluoroscopic guidance. The prosthesis is slowly deployed under fluoroscopy. Stent position may be adjusted prior to complete deployment. It is imperative to achieve precise stent placement, as SEMS are not easily repositioned following deployment. Furthermore, aggressive attempts at stent repositioning may result in airway injury and mucosal hemorrhage.
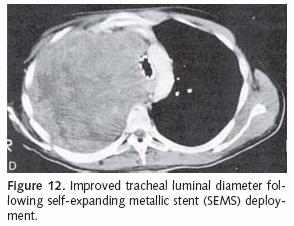
Stent placement for endobronchial cancer management is quite useful for restoring airway patency in the setting of extrinsic compression or endobronchial tumor (Figure 12). An airway stent may help decompress a postobstructive pneumonia or resolve an atelectatic lobe or segment. Even so, such therapy should only be employed when there is patent airway distal to the proposed area of stent deployment. This may be suggested by aerated lung or patent bronchial passages on CT scan. Often stent placement is preceded by tumor debulking using the Nd:YAG laser and rigid bronchoscope. Photodynamic therapy (PDT) (which will be discussed later in this chapter) may also be used to reestablish an endobronchial lumen prior to stent insertion. Again, proximal stent placement in the setting of complete airway occlusion serves no useful purpose and should be avoided.
PHOTODYNAMIC THERAPY
Photodynamic therapy (PDT) has become increasingly popular in the treatment of endobronchial malignancies. This therapy, introduced by Dougherty et al, demonstrated necrosis in various tumor types16,17. Although we use this modality more often for endoluminal control of unresectable esophageal cancer, it can certainly be applied to advanced lung cancer with airway involvement. The FDA approved the use of photofrin for palliation of symptoms in patients with obstructing endobronchial non–small cell lung cancer in 1998. PDT is based upon the reactivity of a photosensitizing agent, porfimer sodium (Photophrin), when exposed to laser light at 630 nm wavelength. The porfimer then becomes excited and generates a series of radical reactions. This activity produces singlet oxygen, which subsequently generates superoxide and hydroxyl radicals. This series of photochemical reactions causes cell death. This agent is concentrated mainly in skin epithelial cells, the reticuloendothelial system, and tumor cells.
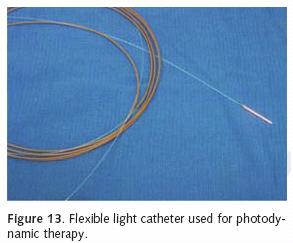
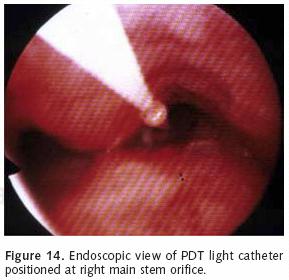
Photofrin is administered as 2 mg/kg intravenously. The agent reaches the highest concentration within 36–48 hours. The patient is sequestered from direct sunlight and bright indoor light during this time period. The patient then undergoes a flexible bronchoscopy at which time a 1 cm–1.5 cm optic diffuser catheter is placed within the area of tumor involvement (Figure 13). Approximately 200 joules of light energy is administered into the tumor (Figure 14). Our practice is to perform a repeat endoscopy after 24–48 hours. This will allow for the development of tumor necrosis.
At the time of repeat bronchoscopy, the response to therapy can be assessed and a mechanical de–bridement can be performed. Debridement may require the use of the rigid bronchoscope. We typically perform 2–3 treatments over a four to five–day time period.
For patients undergoing external beam radiation therapy following PDT, a 2–4 week convalescent period is recommended to allow the associated inflammatory response to resolve. A 4–week interval is recommended before beginning PDT following external beam radiotherapy. Additional precautions relate to the accumulation of the photosensitizer in normal tissues. As mentioned above, Photofrin is sequestered in the retina, skin and reticuloendothelial system, including the liver and spleen. Accordingly, patients are at significant risk for retinal injury and severe sunburn manifested by erythema and blisters. We therefore recommend that patients avoid direct skin and eye exposure to sunlight for at least 30 days following injection. Ambient indoor light exposure is recommended to assist with inactivation of the remaining drug. Sunscreens are ineffective in protecting against phototoxicity.
LIFE BRONCHOSCOPY
Endobronchial carcinoma in situ has a reported incidence up to 15% in patients with NSCLC. Second primary lung cancer can occur at a rate of 1–3% per patient year. These lesions are quite formidable when diagnosed–as only 50% are resectable at the time of diagnosis. For patients who have undergone therapy for endobronchial tumor, surveillance is necessary to identify recurrent disease early. Fiber optic bronchoscopy is the mainstay in the detection of recurrent endobronchial tumor; however white light bronchoscopy is limited in its ability to localize occult tracheobronchial recurrences. Light energy may also be used for diagnosis in this setting. This approach takes advantage of the fact that normal bronchial mucosa emits green fluorescence when illuminated with light in a 440 nm wavelength18,19. In a single–center prospective study, Kurie et al, demonstrated dyspla–sia and metaplasia from sites with an abnormal appearance on LIFE bronchoscopy. They could not demonstrate any improvement in the detection of histologic abnormalities over white light bronchoscopy20. Weigel et al, demonstrated a three–fold increase in the sensitivity of screening when adding LIFE bronchoscopy to white light bronchoscopy21. In a subsequent paper, LIFE bronchoscopy surveillance identified carcinoma in situ in 6% of postoperative patients who were felt to have been disease free by other surveillance measures. Squamous cell carcinoma was identified in 75% of the lesions detected in the study population22.
Although LIFE broncoscopy shows some potential in the early detection of second primaries and recurrent tracheobronchial malignancy, large prospective randomized trials are need to fully explore its utility in patients with lung cancer.
CONCLUSION
The management of endobronchial malignancy is a challenging problem. A variety of therapeutic modalities exist to treat patients with tracheobronchial disease. Effective treatment requires experience with flexible and rigid bronchoscopy. Furthermore, careful analysis of radiographs is essential when approaching these patients. Self–expanding metal stents have become quite popular for maintaining airway patency. The use of SEMS alone or in combination with Nd:YAG laser may provide adequate and relatively long–term palliation for patients with unresectable disease. Photodynamic therapy, while relatively new in the treatment of tracheobronchial cancer, can reestablish and maintain airway luminal integrity with minimal morbidity and negligible mortality. While LIFE bronchoscopy shows promise in detection of carcinoma in situ in patients with a history of lung cancer, additional trials are likely needed to definitively demonstrate its applicability.
REFERENCES
1. WHO. Cancer: Facts about cancer. WHO website. 2006. [ Links ]
2. Cavaliere S, Venuta F, Foccoli P, Toninelli C, La Face B. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest 1996; 110:1536–1542. [ Links ]
3. Toty L, Personne CL, Hertzog P, et al. Utilisation d'un faisceau laser (YAG) a conducteur souple, pour le traitement endoscopique de certaines lesions tracheo–bronchiques. Rev Fr Mal Respir 1979; 7:57–60. [ Links ]
4. Dumon JF, Reboud E, Garbe L, Aucomte F, Meric B. Treatment of tracheobronchial lesions by laser photo–resection. Chest 1982; 81:278–284. [ Links ]
5. Bourcereau J, Gharbi N, Lescot B, Marchal M. YAG laser in bronchial endoscopy. Laser Med Microchirurg 1982; 1:35–36. [ Links ]
6. Oho K, Ogawa I, Amemiya R, et al. Indications for endoscopic Nd–YAG laser surgery in the trachea and bronchus. Endoscopy 1983; 15:302–306. [ Links ]
7. McDougall JC, Cortese DA. Neodymium–YAG laser therapy of malignant airway obstruction. A preliminary report. Mayo Clin Proc 1983; 58:35–39. [ Links ]
8. LoCicero J. Endoluminal management of malignant airways disease. In: Shields TW, LoCicero J, Ponn RB, editors. General thoracic surgery. 5th ed. Philadelphia: Lippincott, Williams & Wilkins; 2000.p. 1357–1368. [ Links ]
9. Dumon JF, Shapshay S, Bourcereau J, et al. Principles for safety in application of neodymium–YAG laser in bronchology. Chest 1984; 86:163–168. [ Links ]
10. Shapshay SM, Beamis JF Jr. Safety precautions for bronchoscopic Nd–YAG laser surgery. Otolaryngol Head Neck Surg 1986; 94:175–180. [ Links ]
11. Tellides G, Ugurlu BS, Kim RW, Hammond GL. Pathogenesis of systemic air embolism during bronchoscopic Nd:YAG laser operations. Ann Thorac Surg 1998; 65:930–934. [ Links ]
12. Montgomery WW. T–tube tracheal stent. Arch Otolaryngol 1965;82:320–321. [ Links ]
13. Dumon JF, Cavaliere S, Diaz–Jimenez JP, et al. Seven–year experience with the Dumon prosthesis. J Bronchol 1996; 3:6–10. [ Links ]
14. Sonett JR, Keenan RJ, Ferson PF, Griffith BP, Landreneau RJ. Endobronchial management of benign, malignant, and lung transplantation airway stenoses. Ann Thorac Surg 1995; 59:1417–1422. [ Links ]
15. Freitag L, Tekolf E, Stamatis G, Greschuchna D. Clinical evaluation of a new bifurcated dynamic airway stent: a 5–year experience with 135 patients. Thorac Cardiovasc Surg 1997; 45:6–12. [ Links ]
16.Dougherty TJ, Lawrence G, Kaufman JH, Boyle D, Weishaupt KR, Goldfarb A. Photoradiation in the treatment of recurrent breast carcinoma. J Natl Cancer Inst 1979; 62:231–237. [ Links ]
17. Dougherty TJ. Photoradiation therapy for cutaneous and subcutaneous malignancies. J Invest Dermatol 1981; 77:122–124. [ Links ]
18. Lam S, Kennedy T, Unger M, et al. Localization of bronchial intraepithelial neoplastic lesions by fluorescence bronchoscopy. Chest 1998; 113:696–702. [ Links ]
19. Hung J, Lam S, LeRiche JC, Palcic B. Autofluorescence of normal and malignant bronchial tissue. Lasers Surg Med 1991; 11:99–105. [ Links ]
20. Kurie JM, Lee JS, Morice RC, et al. Autofluorescence bronchoscopy in the detection of squamous metaplasia and dysplasia in current and former smokers. J Natl Cancer Inst 1998; 90:991–995. [ Links ]
21. Weigel TL, Yousem S, Dacic S, Kosco PJ, Siegfried J, Luketich JD. Fluorescence bronchoscopic surveillance after curative surgical resection for non–small–cell lung cancer. Ann Surg Oncol 2000; 7:176–180. [ Links ]
22. Weigel TL, Kosco PJ, Dacic S, Rusch VW, Ginsberg RJ, Luketich JD. Postoperative fluorescence bronchoscopic surveillance in non–small cell lung cancer patients. Ann Thorac Surg 2001; 71:967–970. [ Links ]














