Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista del Instituto Nacional de Enfermedades Respiratorias
versión impresa ISSN 0187-7585
Rev. Inst. Nal. Enf. Resp. Mex. vol.20 no.1 México ene./mar. 2007
Estado del arte
Role of sublobar resection (segmentectomy and wedge resection) in the surgical management of non–small cell lung cancer
Papel de la resección sublobar (segmentectomía y resección en cuña) en el manejo quirúrgico del cáncer broncogénico de células no pequeñas
Brian Pettiford* Rodney J. Landreneau*
* Heart, Lung, and Esophageal Surgery Institute University of Pittsburgh Medical Center Pittsburgh, Pennsylvania
Address correspondence to:
Rodney Landreneau, M.D.
Heart, Lung and Esophageal Surgery Institute
University of Pittsburgh Medical Center UPMC Shadyside Medical Center,
Suite 715 5200 Centre Avenue Pittsburgh, Pennsylvania
15232 Phone: 412–623–2030
e–mail: landreneaurj@upmc.edu
Trabajo recibido: 13–XII–2006
Aceptado: 23–1–2007
INTRODUCTION
Most thoracic surgeons regard pulmonary resection less than lobectomy as inadequate for the management of lung cancers anatomically confined to a single lobe of the lung. Accordingly, sublobar resection is considered by many surgeons as a "compromise operation" that should be only employed for the management of small peripheral lung cancers present in patients with significant impairment in cardiopulmonary reserve, who cannot withstand the physiologic rigors of lobectomy.
High resolution computed tomography (CT) has resulted in the increasingly common finding of new sub–centimeter malignant lesions. Surveillance CT chest scanning efforts have led many surgeons to reassess the need for total lobectomy in the management of smaller peripheral non–small cell lung cancers. Anatomic segmentectomy or extended non–anatomic wedge resection has been considered as an adequate surgical cure of early–stage lung cancer. We review the clinical information available today in formulating an opinion regarding the appropriate use of sublobar resection for the small peripherally located NSCLC.
HISTORICAL PERSPECTIVE
Pulmonary segmentectomy was originally utilized for the resection of focal bronchiectasis and tuberculosis. Both of these pulmonary disease processes are commonly anatomically localized to discrete bronchopulmonary segments and the common bilateral involvement encourages the use of parenchymal sparing resection techniques.
The first reported use of segmentectomy for the management of bronchiectasis is credited to Churchill and Belsey in 19391. Kent and Blades' advocacy of individual ligation of bronchial and vascular hilar structures coupled with Overholt and Langer's 1947 description of the technique for resection of each bronchopulmonary segment in the treatment of bronchiectasis established the use of anatomic segmentectomy for discrete sublobar pathology2,3.
Interestingly, total pneumonectomy was still regarded as the only appropriate surgical option for the treatment of primary lung cancer during this period4. The dreadfully high mortality associated with pneumonectomy (40%) at that time led to the use of lobectomy as the preferred approach to resection of peripheral lung cancers5.
The use of anatomic segmentectomy for the management of peripheral lung cancers was explored by some thoracic surgeons6–10, however, the relative complexity of the operative approach compared to lobectomy and the increased morbidity related to prolonged air leak and local recurrence deterred the enthusiasm of most surgeons for this approach to lung cancer11. The use of segmentectomy, and sublobar resection in general, was relegated as a "compromise procedure" for the management of patients with significant impairment in cardiopulmonary reserve having peripheral lung lesions confined within segmental anatomic boundaries10–15.
An increasing body of evidence is emerging suggesting that sublobar resection with accurate nodal staging may be an adequate resection for the small peripheral non–small cell lung cancer. Surgical marginal status following sublobar resection continues to be an important concern, and measures to enhance the marginal clearance continue to be explored16–32.
CONTROVERSIES REGARDING THE USE OF SUBLOBAR RESECTION
The use of anatomic segmentectomy is generally accepted for the management of benign disease processes and metastatic carcinoma to the lung confined to an anatomic segment. The use of sublobar resection and segmentectomy in particular has been accepted as a reasonable approach for resection of patients with significant impairment in cardiopulmonary reserve. The primary controversy over the years among thoracic surgeons has been with the use of segmentectomy as primary management of peripheral primary non–small cell lung cancer for patients who are physiologically fit to undergo lobectomy.
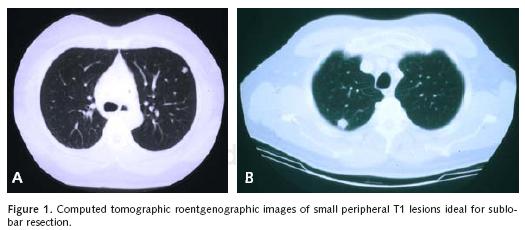
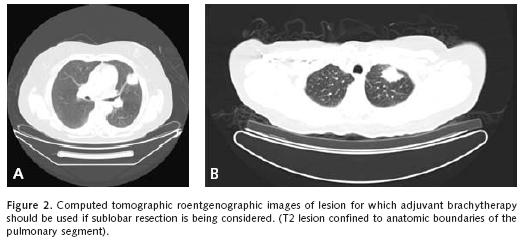
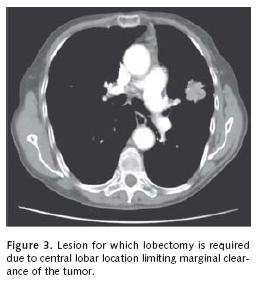
We will review representative pertinent clinical investigations addressing the appropriateness of sublobar resection in the primary management of non–small cell lung cancer. We must first clarify that we believe sublobar resection is inappropriate for the management of most clinical non–small cell lung cancer beyond that of stage I disease. For the most part, we favor the use of segmentectomy without adjuvant local therapy for small stage IA disease without endobronchi–al extension within anatomic segmental boundaries that are less than 2 centimeters in diameter (Figure 1a–b). A surgical margin of at least 2 centimeters should be readily achieved with lesions of this size. Larger lesions will necessarily be associated with anatomic margins of resection that are more likely to recur locally unless adjuvant therapeutic measures to be discussed are considered (Figure 2a–b and Figure 3).
Patient survival with sublobar resection
The argument of use of sublobar resection for stage I non–small cell lung cancer attracted international attention with the initiation of the randomized trial of sublobar resection versus lobectomy for good risk patients with stage IA disease conducted by the now defunct North American "Lung Cancer Study Group" during the late 1980's and early 1990's11. This study was inspired by the survival results seen among women undergoing less than total mastectomy for small primary breast cancers and Erret's 1986 reporting of equivalent survival results among stage I Non–small cell lung cancer patients with impaired cardio–pulmonary reserve undergoing sublobar resection compared to physiologically fit stage I patients undergoing lobectomy33,34.
The results of the Lung Cancer Study Group's efforts were reported in 1995. Primary findings of this study were that survival between sublobar resection and lobectomy were not significantly different but local recurrence was three times greater when sublobar resection was utilized. As an aside observation, they also stated that they found no difference in the loss of pulmonary functionality between lobectomy and sublobar resection patients when assessed one year following surgery. This important conclusion regarding postoperative functionality certainly caught the attention of many thoracic surgeons already convinced that lobectomy was the superior operation for even small stage I non–small cell lung cancers. Interestingly, this conclusion regarding postoperative physiologic equivalency between resection approaches was made despite the fact that over one third of the patients in the study were not available for pulmonary function testing at the one year postoperative mark. Subsequent analyses of the late effects of relative pulmonary functional preservation with segmentectomy compared to lobectomy have countered the conclusions of this study18'19. Furthermore, the survival and local recurrence differences were based on a one–tailed analysis designed to demonstrate a statistically significant difference at a higher p–value threshold. Lastly, one–third of the sublobar resection patients underwent wedge resection, which is not an anatomical resection and has well–documented local recurrence rates. Regardless of these findings, many thoracic surgeons continue to regard lobectomy as the gold standard treatment for early–stage non–small cell lung cancer.
In Japan, large computed tomographic (CT) radiologic screening programs in place for well over a decade have exposed an increased number of small, peripheral, early stage lung cancers.35 Programs utilizing "fast" CT scanners screening high–risk populations (older patients with significant smoking history and impairment in pulmonary function) are underway now in North America and Europe36. Renewed interests in sublobar resection and emerging enthusiasm with non–surgical percutaneous management of small peripheral lung cancers identified through these efforts are now seen18–25,29–32,37–40.
Interestingly, analyses that have compared sublobar resection with lobectomy identify patient age and the size of the tumor resected as the primary determinants of survival. Mery et al, examined the effect of age and type of surgery on survival in patients with early–stage non–small cell lung cancer.20 Their analysis of the Surveillance, Epidemiology, and End Results Database categorized the survival following resection of stage I non–small cell lung cancer in three age groups: <65 years, 65–74 years, and >75 years. A statistically greater number of elderly patients underwent limited resections, which included wedge resection. Two years following surgery, better survival was shown in young patients undergoing lobectomy as opposed to sublobar resection. No such survival time difference was demonstrated in the elderly population. Furthermore, the statistically significant long–term survival advantage favoring lobectomy for younger patients was lost compared to sublobar resection among patients greater than 70 years of age.
Okada et al, conducted a retrospective analysis of 1,272 consecutive patients who underwent complete resection with complete lymph node staging of non–small cell lung cancer stratifying individuals in 4 groups according to tumor size: 10 mm or less, 11–20 mm, 21–30 mm and >30 mm21. The cancer–specific 5–year survivals were 100%, 83.5%, 76.5%, and 57.9%, respectively for the 4 groups. Furthermore, no difference in cancer–specific survival was seen between patients undergoing lobectomy compared to segmentectomy for cancers smaller than 30 mm in diameter. The authors identified tumor size as an independent prognostic factor and suggested that segmentectomy with systematic nodal staging to avoid "stage shift" bias, be considered as primary therapy for tumors 20 mm or less in size.
El–sherif et al, evaluated a 13–year experience in the management of resectable stage I non–small cell lung cancer at their institution25. The recurrence patterns and survival of 784 patients (577 lobectomies, 207 sublobar resections that were primarily wedges) who underwent resection of stage I NSCLC were evaluated. No significant differences were observed in disease–free survival between sublobar resection and lobectomy for patients with stage IA disease, however a slightly worse disease–free survival was seen for stage IB patients undergoing sublobar resection compared to lobectomy (58% vs 50% 5 year disease–free survival). Sublobar resection was also associated with a lower overall 5–year survival compared to lobectomy (40% vs54%). The authors suggested that this reduced overall survival following sublobar resection might have been related to the generally poorer functional status and co–morbidities of the patients chosen for this resection rather than lobectomy at their institution.
Local recurrence concerns following sublobar resection
Although disease free survival remains the most critical parameter in the assessment of any treatment related modality for lung cancer, local recurrence is following primary therapy is also an important concern. Local recurrence can result in significant morbidity such as invasion into the chest wall or vital mediastinal structures, malignant pleural effusion, and problems related to airway obstruction and hemoptysis.
It is generally established that the risk of local recurrence is increased with the use of sublobar resection for the management of stage I non–small cell lung cancer. Primary factors related to local recurrence are the surgical marginal distance of resection and the related presence of microscopic extension of disease or "in transit" local metastases. Recent investigations have noted that the molecular immunohistochemical assessment of the tissue margin may assist in predicting the risk for local recurrence following sublobar resection17. Such pathologic assessment of surgical margins may aid in decision–making regarding local adjuvant treatment measures or re–resection to obtain clearance of disease. The present recommendations for sublobar resection are to establish a margin at least that of the diameter of the pulmonary lesion resected16.
Adjuvant radiotherapy has been considered as a possible option used in conjunction with sublobar resection to possibly reduce local recurrence. Miller and Hatcher reported a significant decrease in local recurrence in follow–up of a small group of sublobar resection patients undergoing postoperative focal external beam "postage stamp" radiation compared to an earlier group of patients they managed with sublobar resection alone12. Unfortunately, radiation treatment planning problems created by the unpredictable three dimensional course of staple lines, the risk of local radiation injury to the treated remaining lobar segments of the lung, and the transportation difficulties associated with a several week course of radiation therapy following thoracic surgery led to little enthusiasm with adjuvant external beam radiation therapy.
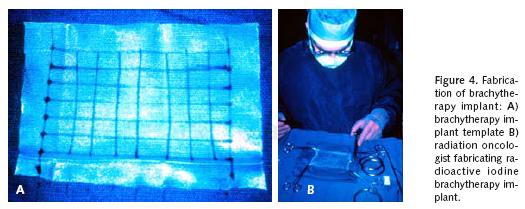
Others have utilized intraoperative brachytherapy as an adjunctive local control measure following lung resection associated with close or positive margins of resection. This use of adjuvant intraoperative brachytherapy was primarily used in the setting of locally advanced lung cancer where margins could not be reliably sterilized, and in an effort to provide immediate potential salvage therapy without the intrinsic delays associated with initiation of external beam radiotherapy after thoracotomy26. d'Amato and colleagues initially reported the use of intraoperative 125I brachytherapy as a local measure following sublobar resection of peripheral stage I lung cancers when pathologically clear surgical margins had been obtained27. These investigators described the fabrication of a radiation implant based upon a polyglycolate hernia mesh template in which polyglycolate suture having 125I pellets incorporated at one centimeter intervals along the length of the suture are woven into the mesh to create a treatment grid with pellets at 1 cm2 intervals (Figure 4a–b). This mesh template was then introduced into the chest and sutured onto the lung parenchymal surface at the suture line of resection to provide at least two centimeters of lateral margin coverage from the staple line. Usually 40 to 60 125I pellets within 4 to 5 lines of suture material were used with each brachytherapy implant. The total delivered radiation dose to the local tissues was calculated to be 10,000cGY at a 1 cm depth. In essence, this very high intensity local therapy effectively extended the margin of resection by another centimeter. Santos et al, subsequently reported a longitudinal follow–up of the use of intraoperative 125I brachytherapy from the same institution identifying that local recurrence following sublobar resection appeared to be significantly reduced compared to historical control sublobar resection cases28. On this further analysis, no local important radiation fibrosis, implant migration, nor unexpected decline in postoperative pulmonary function was noticed among patients receiving intraoperative 125I brachytherapy.
Other retrospective reports of the use of intraoperative 125I brachytherapy utilized with sublobar resection have been encouraging and have helped to further define the use of this adjuvant therapy approach aimed at reducing local recurrence. Fernando and associates reported a retrospective multi–center analysis of 291 patients, which compared outcomes after lobectomy (n = 167) with those following sublobar resection (n = 124). Nearly one–half of the sublobar resection group (n = 60) received adjuvant 125I brachytherapy. The local recurrence rate in the sublobar resection group was decreased from 17.2% to 3.3% with the use of adjuvant 125I brachytherapy29. Finally Birdas et al, retrospectively compared the outcomes of sublobar resection with brachytherapy to lobectomy for patients with pathologic stage IB non–small cell lung cancer. A total of 167 stage IB patients (126 patients undergoing lobectomy, 41 patients undergoing sublobar resection) were evaluated for local recurrence, disease–free survival and overall survival. Local recurrence seen in the sublobar resection with 125I brachytherapy group (4.8%) was similar to the lobectomy group (3.2%). There was no statistically significant difference in disease–free survival and overall survival between the two groups30. These investigators concluded that sublobar resection with intraoperative 125I brachytherapy provided equivalent local control, disease–free and overall survival outcomes similar to lobectomy for IB non–small lung cancer patients. In accordance with other investigators' concern for close surgical margins leading to increased risk for local recurrence16, they emphasized the potential utility of intraoperative 125I brachytherapy in theoretically "extending" the margin of resection when larger (T2) tumors are chosen for sublobar resection.
We recently presented outcomes of a retrospective analysis of 147 segmental resections performed over nearly a four–year period. All patients had either Stage IA (n = 86) or IB (n=61) NSCLC with a mean age of 70 years. Twenty–eight percent of the patients underwent VATS segmentectomy (n=41). Although complication rates were similar between the open (n = 106) and VATS groups, a higher number of major complications occurred in patients undergoing an open procedure. Overall survival was 76.9% with a local recurrence rate of 16%. Of note, 83% of the recurrences were observed when the surgical margin was less than 2 cm. A margin: tumor ratio <1 was associated with a higher recurrence rate. The authors concluded that segmentectomy is feasible by an open or VATS method with mobidity, mortality, recurrence and survival rates similar to lobectomy. The latter is recommended, however when margins >1.5 cm cannot be achieved.
Future investigative efforts regarding the utility of sublobar resection
Much of the information provided here has been the product of retrospective reviews of collected clinical experiences with sublobar resection. Randomized studies in progress or in conception will do much to define the role of sublobar resection and the use of adjuvant local control measures following these resections. Presently, the American College of Surgeons Oncology Group (ACOSOG) is conducting a randomized trial (Z04042) of sublobar resection (by anatomic segmentectomy or extended wedge resection) alone to similar sublobar resection with intraoperative 125I brachytherapy for stage IA non–small cell lung cancers. Over 200 patients are to be accrued for study. Cancer and Leukemia Group B (CALGB) is in the final phase of preparation of a randomized investigation of surgical resection of small (less than 2 centimeter in diameter) Stage IA non–small cell lung cancers by either lobectomy or sublobar resection by segmentectomy or wedge resection. Over 800 patients will be enrolled in this later study. The results of these studies should aid in establishing the role of sublobar resection in the future management of stage I non–small cell lung cancer.
PREOPERATIVE EVALUATION PRIOR TO SUBLOBAR RESECTION
Patients being considered for non–anatomic extended wedge resection or anatomic segmentectomy for the primary management of stage I non–small cell lung cancers have usually been those with potentially important impairment in cardiopulmonary reserve. As mentioned, this may include borderline or marginal pulmonary functional physiology that prohibits formal lobectomy. Predicted postoperative pulmonary function based upon the volume of functional pulmonary parenchyma to be resected may dictate the resection options for the patient with underlying pulmonary impairment.
Most patients will present to the thoracic surgeon with an abnormal chest CT scan. This CT scan should include imaging of the liver and adrenal glands. Special attention should be focused on the mediastinum and the size and segmental location of the pulmonary parenchymal lesion in question. Mediastinoscopy is performed if indicated based on enlarged mediastinal lymph nodes (greater than 1 centimeter in diameter) or Fluo–ro–Deoxy–Glucose avidity on Positron Emission Tomographic scanning.
The thoracic surgeon must also carefully evaluate the anatomic characteristics of lesion being considered for sublobar resection, as manifested through preoperative CTscan imaging and pre–operative bronchoscopic evaluation. It is generally appreciated that lesions chosen for sublobar resection by extended wedge resection done either by open thoracotomy or VATS approaches should be located within the outer third of the lung parenchyma, measure less than 3 cm in diameter, and have no evidence of endobronchial involvement. These concepts should be honored to prevent deep application of stapling devices during the course of a non–anatomic wedge resection. This approach will minimize the possibility of inadequate resection margins, staple line dehiscence and associated air leak/bleeding problems related to the distortion of the remaining lung parenchyma.
A complete physiologic evaluation of the patient being chosen for sublobar resection is important. One must remember that the individual usually chosen for sublobar resection, as definitive management of a peripheral malignant lesion is usually one whose cardiopulmonary functionality is in question. Thorough pulmonary functional evaluation is indicated. This must include formal pulmonary spirometry, arterial blood gas analysis, and carbon monoxide diffusion capacity assessment. Split–lung ventilation/perfusion nuclear scintigraphic assessment and maximum oxygen consumption exercise testing may be selectively considered prior to surgery based upon general functional concerns. Cardiac functional assessment using standard or pharmacological stress testing and 2–D echocardiography with estimates of pulmonary arterial pressures should be considered.
Once the patient has been deemed a candidate for anesthesia and sublobar resection, Radiation Oncology consultation is obtained if adjuvant intraoperative 125I brachytherapy is being considered as part of the treatment plan. In general, a tissue diagnosis of high PET avidity is required before radiation oncology evaluation.
OPERATIVE CONSIDERATIONS FOR SUBLOBAR RESECTION
Anesthetic airway control and thoracotomy incision
At the time of operation, laterally is marked. After intubation with a single–lumen endotracheal tube, a flexible bronchoscopy is performed in the standard fashion to insure the absence of endobronchial extension of the malignant process precluding the use of sublobar resection. A double–lumen tube is then inserted and the patient positioned laterally. It is important that the thoracic surgeon confirm proper positioning of the endotracheal tube after initial placement and after patient lateral positioning to insure the adequacy of selective contralateral lung ventilation and ipsilateral pulmonary atelectasis for the procedure. These actions can reduce the occurrence of time consuming intraoperative problems associated with inadequate selective airway control with double lumen intubation.
Whenever possible, we prefer the use of the VATS approach for the performance of sublobar resection. When the appropriate lesion is selected for this approach we find the VATS approach to be advantageous with regard to reduction in operative time, perioperative blood loss, hospital stay and postoperative morbidity. This technique is also equivalent in utility compared to open thoracotomy approaches41–45.
Lesion identification and VATS sublobar resection approach
Some important strategies for lesion localization and intercostal access for VATS instrumentation should be discussed when diagnostic sublobar resection of suspicious indeterminant nodules (prior to anatomic resection) or sublobar resection as definitive management of peripheral non–small cell lung cancers is being considered.
The intercostals access orientation of the tho–racoscope and instrumentation established should facilitate exploration of the entire cavity and identification of "target" lung pathology as determined through careful preoperative review of the chest radiograph and computed tomographic (CT) images. Generally, the initial intercostal access is achieved for the thoracoscope at the seventh intercostal space along the anterior superior iliac spine. Endosurgical instrumentation and the stapling device are introduced through a 1 cm intercostal access at the fifth or sixth interspace along the posterior axillary line, and a 1 cm vertical incision in the third interspace along the mid–axillary line. These intercostal access locations provide panoramic video imaging and permit triangulation of most lesions being considered for VATS wedge resection (Figure 5). Furthermore, the vertical mid–axillary incision can be extended if conversion to thoracotomy is indicated. The position of the thoracoscope and endoscopic grasper/endostapler may be alternated as needed to facilitate exposure to important visual angles and application of the surgical stapler to facilitate wedge resection.
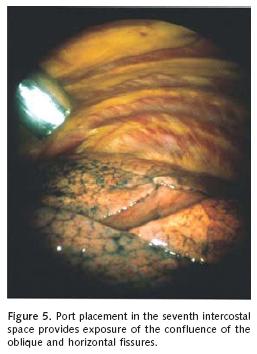
Careful examination of the surface of the lung in the region of the suspected lesion identified by preoperative CT scanning of the chest will often reveal local visceral pleural scarring or retraction in the case of malignancy. Once full atelectasis of the lung in the region of the lesion is obtained, localization can usually be achieved through effacement of the nodule against the surrounding collapsed lung. Gentle palpation of the lung in the area in question with a sponge forceps or endoscopic grasper can also identify the lesion when videoscopic inspection is less rewarding.
Issues related to the resection of the indeterminant pulmonary nodule necessarily apply to that of the known small peripheral lung cancer chosen for primary VATS sublobar resection. Certainly all efforts should be centered in obtaining a clear surgical margin of resection. This does require a conscientious effort on the part of the surgeon through each step of the endosurgical stapled extended wedge resection. Once the lesion is identified, it is important to assess and estimate the thickness of the parenchymal margin of resection. For small subpleural lesions, a simple wedge resection can be accomplished with adequate surgical margins. The visceral lung surface is grasped with forceps and an initial firing of the endostapler is applied beneath the elevated, atelectatic lung tissue deep to the subpleural lesion. The staple line is inspected for parenchymal closure and then the endostapler is introduced through an alternate intercostal access site to complete a standard "V" wedge resection with adequate margins. After completion of the resection, the lung is inspected for adequate pneumostasis.
For deeper lesions, the surgeon must determine if the required depth and thickness of the resection will preclude a safe and effective wedge resection of the lesion. This can be assessed by grasping the lung parenchymal surface near the lung lesion and then applying an endoscopic grasping forceps (Masher Forceps, Starr Medical, NY) across the line of possible parenchymal resection. If the tissue can be "thinned" beneath the lesion without compromising resection margins as determined by this tissue thickness approximating technique, the 45 mm or 60 mm length endostapler with thick tissue staplers (3.5 to 4.8 mm staple height) is used to transect the parenchymal tissue for the wedge resection. The relationship of the pulmonary lesion to the line of resection is assessed again before each subsequent stapler application with the aid of the "Masher" forceps estimation of the lung parenchymal thickness. The resection is continued with successive applications of the endostapler along the waist of pulmonary parenchyma deemed adequate for a clear resection margin.
Several techniques have been described for localization of pulmonary nodules that are not easily identified at the time of VATS sublobar resection. Beyond the careful preoperative assessment of the CT images of the chest to determine thesegmental location of the lesion, preoperative injection of methylene blue and or a needle localization techniques to identify the "soft" small, subpleural nodule have been described. Intraop–erative ultrasonography has also been utilized with variable success46
At completion of the sublobar resection, the pulmonary specimen is removed from the chest in a specimen retrieval bag, commercially available through a number of endosurgical companies, to avoid chest wall contamination by a potentially malignant lesion within the specimen. Alternatively, a sterile operating room latex glove can be introduced into the chest through an intercostal access site to be used as a retrieval bag. Following removal of the specimen, the lung is partially expanded, and the resection staple line margin is reexamined for hemostasis and air–leak control. A single chest tube is inserted through one of the lower intercostal access sites and positioned under thoracoscopic guidance toward a posterior apical position. The other intercostal access sites are closed primarily. The chest tube is removed when drainage is minimal and the air leak has resolved. This is customarily accomplished on the 2nd or 3rd postoperative day.
Mini–thoracotomy approach to sublobar resection
We do prefer to use a vertical axillary muscle sparing mini–thoracotomy incision when the VATS approach is not chosen for most sublobar pulmonary resections47. When performing upper lobe and middle lobe sublobar resections, the incision is placed at the lower border of the axillary hairline in the mid–axillary plane and extended dis–tally for 8 cm. The pectoralis minor muscle is reflected anteriorly. The muscle fibers of the serratus anterior muscle overlying the third rib are split. The lateral aspect of the third rib is resected sub–periosteally and a 2 cm portion of the 4th rib is also resected to further enhance mini–thoracotomy exposure. Two pediatric rib spreaders are inserted and positioned at right angles to each other for exposure.
For lower lobe sublobar mini–thoracotomy resections, the skin incision is begun approximately 3 cm inferior to the axillary hairline along the posterior axillary line, near the anterior border of the latissimus dorsi muscle and a similar 8 cm inci–sional length utilized for thoracotomy. The latissimus dorsi\s reflected posteriorly and the serratus anteriohs detached along a segment of its inferior muscular origin. The 4th rib is resected sub–periosteally, and a 2 cm portion of the 5th rib is removed. We have found that these vertical approaches allow for adequate exposure through a "mini–thoracotomy" incision with minimal chest wall trauma48. The vertical incisional approach also increases versatility in accessing a number of segments of the lung, and it is relatively cosmetically appealing compared to lateral thoracotomy.
In a small number of patients who may have had prior external beam irradiation or chemotherapy and are diagnosed with a second primary lung cancer, we prefer an anterior thoracotomy, particularly for sublobar resections in the upper lobes. A transverse incision is made over the bed of the ipsilateral third rib, a segment of which is resected subperiosteally. We have found that this method provides better access to the hilar structrures than does a more lateral approach.
A final concern regarding the performance of sublobar resection relates to the handling of pa–renchymal staple lines of resection when dividing severely emphysematous or indurated pulmonary tissues. The most important consideration is careful handling of the pulmonary parenchymal tissue and gentle manipulation of the open or endostapling devices across the pulmonary parenchyma. Staple line bolstering material and or topical sealants may be helpful in obtaining pneumostasis when the pulmonary parenchyma is emphysematous or indurated49,50.
Intraoperative brachytherapy
To reduce the likelihood of local recurrence, we commonly employ adjuvant 125I brachytherapy in patients we have performed anatomic segmentec–tomy or extended wedge resections as definitive management of non–small cell lung cancer. We conform to the technical details for creation of the brachytherapy implant and insertion described by d'Amato et al, detailed earlier in this chapter28. An alternative approach described by Lee et al, involves direct suturing of the polyglycolic acid suture with the incorporated 125I pellets within it's length directly to the lung surface without the utilization of the polyglycolic acid hernia mesh template50. The relative merits of these approaches will be one of the points of analysis in the ACOSOG Z04032 study mentioned earlier.
Thoracoscopic approach to formal segment–ectomy
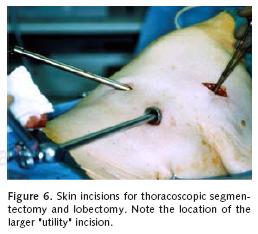
The Video–Assisted Thoracoscopic Surgical(VATS) approach for segmental resections is similar to that for VATS lobectomy. Port placement is similar to that used during VATS wedge resection. In cases of formal anatomic segmentectomy or lobectomy, an additional 2.5 to 3 cm incision is made in the fourth or fifth interspace on the anterior axillary line (Figure 6). The performance of VATS segmentectomy adds a new dimension to the hilar dissection that is not appreciated with VATS lobectomy. The application of a brachytherapy implant is also easily accomplished with the VATS approach when this adjuvant measure is felt to an important compliment for local control of the lung cancer28.
CONCLUSION
Segmentectomy demands a thorough knowledge of the three–dimensional bronchovascular anatomy of the lung. This anatomic detail makes segmentectomy significantly more challenging than lobectomy. Several principles must be applied when performing segmental lung resection: 1. the surgeon should avoid dissection in a poorly developed fissure, 2. use the transected bronchus as the base of the segmental resection during the division of the lung parenchymal in the intersegmental plane, 3. consider the use of endostapler division of the pulmonary parenchyma in order to reduce the air leak complications related to "finger fracture" dissection of the intersegmental plane, 4. consider the use of adjuvant 125I brachytherapy as a means of reducing local recurrence following sublobar resection.
Increasing evidence supports the use of anatomic segmentectomy in the treatment of primary lung cancer for appropriately selected patients. This resection approach appears most appropriate in the management of the small (less than 2 cm in diameter) peripheral stage I non–small cell lung cancer in which a generous margin of resection can be obtained. Accurate intraoperative nodal staging is important to estimate the relative utility of these approaches compared to more aggressive resection and to determine the need for adjuvant systemic therapy if metastatic lymphadenopathy is identified. Future investigations comparing the results of sublobar resection compared to lobectomy will more clearly define the role of segmentectomy among good risk patients with clinically stage I non–small cell lung cancer. At the present time, it appears that sublobar resection is an appropriate therapy for the management of stage I non–small cell lung cancer identified in the elderly patient, those individuals with significant cardiopulmonary dysfunction, and for the management of peripheral, low volume, metastatic disease to the lung. As the primary disadvantage of sublobar resection is that of local recurrence, intraoperative adjuvant 125I brachy–therapy may be considered to minimize this local recurrence risk.
REFERENCES
1. Churchill ED, Belsey R. Segmental pneumonectomy in bronchiectasis: the lingula segment of the left upper lobe. Ann Surg 1939;109:481–499. [ Links ]
2. Kent EM, Blades B. The anatomic approach to pulmonary resection. Ann Surg 1942;116:782–794. [ Links ]
3. Overholt RH, Langer L. A new technique for pulmonary segmental resection, its application in the treatment of bronchiectasis. Surg Gynecol Obstet 1947;84: 257. [ Links ]
4. Oschner A, DeBakey M. Primary pulmonary malignancy. Treatment by total pneumonectomy. Analysis of 79 collected cases and presentation of 7 personal cases. Surg Gynecol Obstet 1939;68:435–441. [ Links ]
5. Churchill ED, Sweet RH, Soutter L, Scannell JG. The surgical management of carcinoma of the lung; a study of cases treated at the Massachusetts General Hospital from 1930 to 1950. J Thorac Surg 1950; 20:349–365. [ Links ]
6. Churchill ED, Sweet RH, Scannell JG, Wilkins EW Jr. Further studies in the surgical management of carcinoma of the lung; a further study of the cases treated at the Massachusetts General Hospital from 1950 to 1957. J Thorac Surg 1958;36:301–308. [ Links ]
7. Bonfils–Roberts EA, Clagett OT. Contemporary indication for pulmonary segmental resections. J Thoracic Cardiovasc Surg 1972;63:433–438. [ Links ]
8. Jensik RJ, Faber LP, Milloy FJ, Monson DO. Segmental resection for lung cancer. A fifteen–year experience. J Thorac Cardiovasc Surg 1973;66:563–572. [ Links ]
9. Read RC, Yoder G, Schaeffer RC. Survival after conservative resection for T1 NO MO non–small cell lung cancer. Ann Thorac Surg 1990; 49:391–398. [ Links ]
10. Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five–year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg 1993 ;107:1087–1094. [ Links ]
11. Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy vs limited resection for T1 NO non–small cell lung cancer. Lung Cancer Study Group. Ann Thoracic Surg 1995;60:615–623. [ Links ]
12. Miller Jl, Hatcher CR Jr. Limited resection of bronchogenic carcinoma in the patient with marked impairment of pulmonary function. Ann Thorac Surg 1987; 44:340–343. [ Links ]
13. Landreneau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1 NO MO) non–small cell lung cancer. J Thorac Cardiovasc Surg 1997;113:691–698. [ Links ]
14. Shennib HA, Landreneau RJ, Mulder DS, Mack MJ. Video–assisted thoracoscopic wedge resection of T1 lung cancer in high–risk patients. Ann Surg 1993; 218:555–558. [ Links ]
15. Lewis RJ. The role of video–assisted thoracic surgery for carcinoma of the lung: wedge resection to lobectomy by simultaneous individual stapling. Ann Thorac Surg 1993;56:762–768. [ Links ]
16. Sawabata N, Ohta M, Matsumura A, et al; Thoracic Surgery Study Group of Osaka University. Optimal distance of malignant negative margin in excision of non–small cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415–420. [ Links ]
17. Masasyesva BG, Tong BC, Brock MV, et al. Molecular margin analysis predicts local recurrence after sublobar resection of lung cancer. Int J Cancer 2005; 113:1022–1025. [ Links ]
18. Keenan RJ, Landreneau RJ, Maley RH Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228–233. [ Links ]
19. Harada H, Okada M, Sakamoto T, Matsuoka H, Tsubota N. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041–2045. [ Links ]
20. Mery CM, Pappas AN, Bueno R, et al. Similar long–term survival of elderly patients with non–small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest 2005;128:237–245. [ Links ]
21. Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non–small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 2005;129:87–93. [ Links ]
22. Yoshikawa K, Tsubota N, Kodoma K, Ayabe H, Taki T, Mori T. Prospective study of extended segmentectomy for small lung tumors: the final report. Ann Thorac Surg 2002;73:1055–1058. [ Links ]
23. Koike T, Yamato Y, Yoshiya K, Shimoyama T, Suzuki R. Intentional limited pulmonary resection for peripheral T1 NO MO small–sized lung cancer. J Thorac Cardiovasc Surg 2003;125:924–928. [ Links ]
24. Okada M, Yoshikawa K, Hatta T, Tsubota N. Is segmentectomy with lymph node assessment an alternative to lobectomy for non–small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 2001 ;71: 956–960. [ Links ]
25. El–Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non–small cell lung cancer: a 13–year analysis. Ann Thorac Surg 2006;82:408–415. [ Links ]
26. Nori D, Li X, Pugkhem T. Intraoperative brachytherapy using Celfoam radioactive plaque implants for resected stage III non–small cell lung cancer with positive margin: a pilot study. J Surg Oncol 1995;60:257–261. [ Links ]
27. d'Amato TA, Galloway M, Szydlowski G, Chen A, Landreneau RJ. Intraoperative brachytherapy following thoracoscopic wedge resection of stage I lung cancer. Chest 1998;114:1112–1115. [ Links ]
28. Santos R, Colonias A, Parda D, et al. Comparison between sublobar resection and 125lodine brachytherapy after sublobar resection in high–risk patients with stage I non–small–cell lung cancer. Surgery 2003;134:691–697. [ Links ]
29. Fernando HC, Santos RS, Benfield JR, et al. Lobar and sublobar resection with and without brachytherapy for small stage IA non–small cell lung cancer. J Thorac Cardiovasc Surg 2005;129:261–267. [ Links ]
30. Birdas TJ, Koehler RP, Colonias A, et al. Sublobar resection with brachytherapy versus lobectomy for stage Ib non small cell lung cancer. Ann Thorac Surg 2006;81:434–438. [ Links ]
31. Patel AN, Santos RS, De Hoyos A, Luketich JD, Landreneau RJ. Clinical trials of peripheral stage I (T1N0M0) non–small cell lung cancer. Semin Thorac Cardiovasc Surg 2003;15:421–430. [ Links ]
32. Jones DR, Stiles BM, Denlinger CE, Antippa P, Daniel TM. Pulmonary segmentectomy: results and complications. Ann Thorac Surg 2003;76:343–348. [ Links ]
33. Fisher B, Anderson S, Bryant J, et al. Twenty–year follow–up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233–1241. [ Links ]
34. Errett LE, Wilson J, Chiu RC, Munro DD. Wedge resection as an alternative procedure for peripheral bronchogenic carcinomas in poor–risk patients. J Thorac Cardiovasc Surg 1985;90:656–661. [ Links ]
35. lkeda N, Hayashi A, Miura Y, et al. Present strategy of lung cancer screening and surgical management. Ann Thorac Cardiovasc Surg 2005;11:363–366. [ Links ]
36. Henschke Cl; I–ELCAP Investigators. CT screening for lung cancer: update 2005. Surg Oncol Clin N Am 2005;14:761–776. [ Links ]
37. Uematsu M. Stereotactic radiation therapy for non small cell lung cancer. Nippon Geka Gakkai Zasshi 2002;103:256–257. [ Links ]
38. Fernando HC, de Hoyos A, Landreneau RJ, et al. Radiofrequency ablation for the treatment of non–small cell lung cancer in marginal surgical candidates. J Thorac Cardiovasc Surg 2005;129:639–644. [ Links ]
39. El–Sherif A, Luketich JD, Landreneau RJ, Fernando HC. New therapeutic approaches for early stage non–small cell lung cancer. Surg Oncol 2005;14:27–32. [ Links ]
40. Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Cy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005;63:1427–1431. [ Links ]
41. Landreneau RJ, Mack MJ, Hazelrigg SR, et al. Video–assisted thoracic surgery: basic technical concepts and intercostal approach strategies. Ann Thorac Surg 1992;54:800–807. [ Links ]
42. Landreneau RJ, Mack MJ, Keenan RJ, Hazelrigg SR, Dowling RD, Ferson PF. Strategic planning for video–assisted thoracic surgery. Ann Thorac Surg 1993; 56:615–619. [ Links ]
43. Landreneau RJ, Hazelrigg SR, Mack MJ, et al. Postoperative pain–related morbidity: video–assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 1993;56:1285–1289. [ Links ]
44. Landreneau RJ, Mack MJ, Hazelrigg SR, Naunheim KS, Keenan RJ, Ferson PF. Video–assisted thoracic surgery: a minimally invasive approach to thoracic oncology. In: DeVita V, Hellman S, Rosenberg S, editors. Cancer: principles and practice of oncology – "Update series". Philadelphia: Lippincott Raven; 1994;8:1–14. [ Links ]
45. Mack MJ, Shennib H, Landreneau RJ, Hazelrigg SR. Techniques for localization of pulmonary nodules for thoracoscopic resection. J Thorac Cardiovasc Surg 1993;106:550–553. [ Links ]
46. Noirclerc M. Muscle–sparing thoracotomy. Ann Thorac Surg 1989;47:330. [ Links ]
47. Szwerc MF, Landreneau RJ, Santos RS, Keenan RJ, Murray GF. Minithoracotomy combined with mechanically stapled bronchial and vascular ligation for anatomical lung resection. Ann Thorac Surg 2004;77:1904–1909. [ Links ]
48. Miller Jl Jr, Landreneau RJ, Wright CE, Santucci TS, Sammons BH. A comparative study of buttressed versus nonbuttressed staple line in pulmonary resections. Ann Thorac Surg 2001 ;71:319–322. [ Links ]
49. Gagarine A, Urschel JD, Miller JD, Bennett WF, Young JE. Effect of fibrin glue on air leak and length of hospital stay after pulmonary lobectomy. J Cardiovasc Surg (Torino) 2003;44:771–773. [ Links ]
50. Lee W, Daly BD, DiPetrillo TA, et al. Limited resection for non–small cell lung cancer: observed local control with implantation of 1–125 brachytherapy seeds. Ann Thorac Surg 2003;75:237–242. [ Links ]














